Numerical Modelling of Erythrocyte Sticking Mechanics
Abstract
:1. Introduction
2. Problem Formulation
3. Methodology
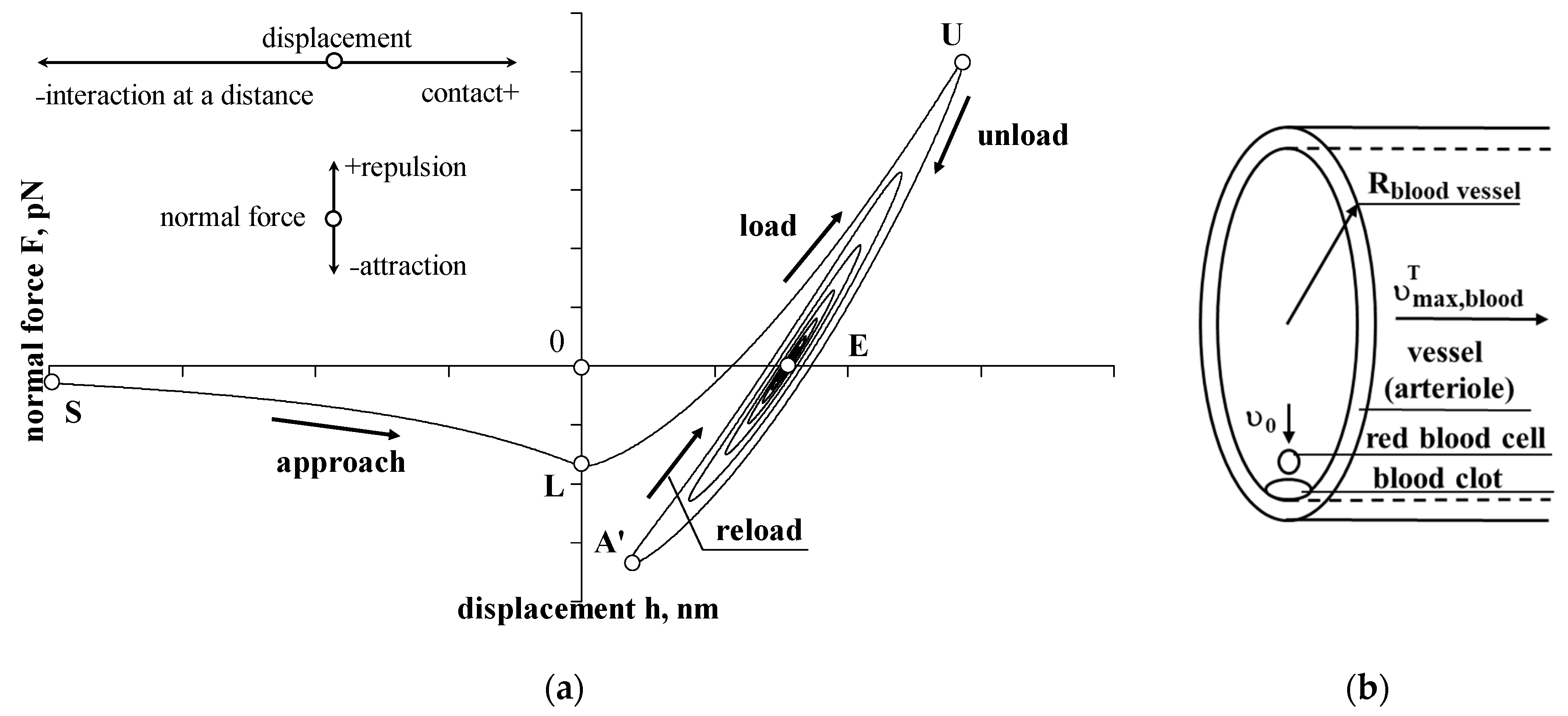
3.1. Approach, Interaction at a Distance
3.2. Load, Contact
3.3. Unload, Contact
3.4. Reload and Reunload, Contact
4. Numerical Experiment
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitz-Gerald, J.M. Implications of a theory of erythrocyte motion in narrow capillaries. J. Appl. Physiol. 1969, 27, 912–918. [Google Scholar] [CrossRef]
- Fischer, T.M.; Stöhr-Lissen, M.; Schmid-Schönbein, H. The red cell as a fluid droplet: Tank tread-like motion of the human erythrocyte membrane in shear flow. Science 1978, 202, 894–896. [Google Scholar] [CrossRef]
- Cokelet, G.R. Dynamics of erythrocyte motion in filtration tests and in vivo flow. Scand. J. Clin. Lab. Investig. 1981, 41, 77–82. [Google Scholar] [CrossRef]
- Shiga, T.; Maeda, N.; Kon, K. Erythrocyte rheology. Crit. Rev. Oncol. Hematol. 1990, 10, 9–48. [Google Scholar] [CrossRef]
- Maeda, N. Erythrocyte rheology in microcirculation. Jpn. J. Physiol. 1996, 46, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Petrig, B.L.; Qi, X.; Burns, S.A. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt. Express 2008, 16, 12746–12756. [Google Scholar] [CrossRef]
- Gu, B.; Wang, X.; Twa, M.D.; Tam, J.; Girkin, C.A.; Zhang, Y. Noninvasive in vivo characterization of erythrocyte motion in human retinal capillaries using high-speed adaptive optics near-confocal imaging. Biomed. Opt. Express 2018, 9, 3653–3677. [Google Scholar] [CrossRef]
- Rosendaal, F.R. Venous thrombosis: A multicausal disease. Lancet 1999, 353, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Prandoni, P.; Bilora, F.; Marchiori, A.; Bernardi, E.; Petrobelli, F.; Lensing, A.W.; Prins, M.H.; Girolami, A. An association between atherosclerosis and venous thrombosis. N. Engl. J. Med. 2003, 348, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Cutlip, D.E.; Baim, D.S.; Ho, K.K.L.; Popma, J.J.; Lansky, A.J.; Cohen, D.J.; Carrozza, J.P.; Chauhan, M.S.; Rodriguez, O.; Kuntz, R.E. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation 2001, 103, 1967–1971. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Erythrocyte aggregation: Basic aspects and clinical importance. Clin. Hemorheol. Microcirc. 2013, 53, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Farsaci, F.; Tellone, E.; Galtieri, A.; Ficarra, S. Is a dangerous blood clot formation a reversible process? Introduction of new characteristic parameter for thermodynamic clot blood characterization: Possible molecular mechanisms and pathophysiologic applications. J. Mol. Liq. 2018, 262, 345–353. [Google Scholar] [CrossRef]
- Wagner, C.; Steffen, P.; Svetina, S. Aggregation of red blood cells: From rouleaux to clot formation. Comptes Rendus Phys. 2013, 14, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Tutwiler, V.; Peshkova, A.D.; Le Minh, G.; Zaitsev, S.; Litvinov, R.I.; Cines, D.B.; Weisel, J.W. Blood clot contraction differentially influences internal and external fibrinolysis. J. Thromb. Haemost. 2019, 17, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Tzou, H.S.; Lee, H.-J.; Arnold, S.M. Smart materials, precision sensors/actuators, smart structures, and structronic systems. Mech. Adv. Mater. Struct. 2004, 11, 367–393. [Google Scholar] [CrossRef] [Green Version]
- Zhou, E.H.; Lim, C.T.; Quek, S.T. Finite element simulation of the micropipette aspiration of a living cell undergoing large viscoelastic deformation. Mech. Adv. Mater. Struct. 2005, 12, 501–512. [Google Scholar] [CrossRef]
- Marion, N.W.; Liang, W.; Liang, W.; Reilly, G.C.; Day, D.E.; Rahaman, M.N.; Mao, J.J. Borate glass supports the in vitro osteogenic differentiation of human mesenchymal stem cells. Mech. Adv. Mater. Struct. 2005, 12, 239–246. [Google Scholar] [CrossRef]
- Lin, S.T.; Bhattacharyya, D.; Fakirov, S.; Matthews, B.; Cornish, J. A novel microfibrillar composite approach towards manufacturing nanoporous tissue scaffolds. Mech. Adv. Mater. Struct. 2014, 21, 237–243. [Google Scholar] [CrossRef]
- Stano, P.; de Souza, T.P.; Carrara, P.; Altamura, E.; D’Aguanno, E.; Caputo, M.; Luisi, P.L.; Mavelli, F. Recent Biophysical Issues about the Preparation of Solute-Filled Lipid Vesicles. Mech. Adv. Mater. Struct. 2015, 22, 748–759. [Google Scholar] [CrossRef]
- Chanda, A.; Callaway, C.; Clifton, C.; Unnikrishnan, V. Biofidelic human brain tissue surrogates. Mech. Adv. Mater. Struct. 2018, 25, 1335–1341. [Google Scholar] [CrossRef] [Green Version]
- Abpeikar, Z.; Milan, P.B.; Moradi, L.; Anjomshoa, M.; Asadpour, S. Influence of pore sizes in 3D-scaffolds on mechanical properties of scaffolds and survival, distribution, and proliferation of human chondrocytes. Mech. Adv. Mater. Struct. 2021, 29, 4911–4922. [Google Scholar] [CrossRef]
- Korayem, M.H.; Rastegar, Z. Development of 3D manipulation of viscoelastic biological cells by AFM based on contact models and oscillatory drag. Mech. Adv. Mater. Struct. 2021, 28, 2572–2584. [Google Scholar] [CrossRef]
- Martynenko, A.; Zozulya, V.V. Mathematical modeling of the cardiac tissue. Mech. Adv. Mater. Struct. 2021, 29, 4506–4522. [Google Scholar] [CrossRef]
- Asadi, A.; Hedayat, D.; Ghofrani, S.; Mehrizi, A.A.; Shadalooyi, G.; Kadkhodapour, J.; Anaraki, A.P. Modification of hexachiral unit cell to enhance auxetic stent performance. Mech. Adv. Mater. Struct. 2022, 1–5. [Google Scholar] [CrossRef]
- Li, N.; Zhuo, Q.; Yu, K.; Dong, W.; Chen, D.; Zheng, R. Bionic bone structure: Establishment of joint model based on bone nanopores structure and its mechanical behavior and biocompatibility. Mech. Adv. Mater. Struct. 2022, 29, 1072–1079. [Google Scholar] [CrossRef]
- Gholampour, S.; Fatouraee, N. Boundary conditions investigation to improve computer simulation of cerebrospinal fluid dynamics in hydrocephalus patients. Commun. Biol. 2021, 4, 394. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, S.; Yamini, B.; Droessler, J.; Frim, D. A New Definition for Intracranial Compliance to Evaluate Adult Hydrocephalus After Shunting. Front. Bioeng. Biotechnol. 2022, 10, 900644. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, S.; Hajirayat, K. Minimizing thermal damage to vascular nerves while drilling of calcified plaque. BMC Res. Notes 2019, 12, 338. [Google Scholar] [CrossRef] [Green Version]
- Gholampour, S.; Balasundaram, H.; Thiyagarajan, P.; Droessler, J.; Yamini, B. A mathematical framework for the dynamic interaction of pulsatile blood, brain, and cerebrospinal fluid. Comput. Methods Programs Biomed. 2022, 107209. [Google Scholar] [CrossRef]
- Jasevičius, R. Numerical modeling of red blood cell interaction mechanics. Mech. Adv. Mater. Struct. 2022, 1–8. [Google Scholar] [CrossRef]
- Jasevičius, R. Numerical modeling of coronavirus interaction mechanics with a host human cell. Mech. Adv. Mater. Struct. 2022, 29, 2186–2196. [Google Scholar] [CrossRef]
- Tsuji, Y.; Tanaka, T.; Ishida, T. Lagrangian numerical simulation of plug flow of cohesionless particles in a horizontal pipe. Powder Technol. 1992, 71, 239–250. [Google Scholar] [CrossRef]
- Jasevičius, R.; Kruggel-Emden, H. Numerical modelling of the sticking process of a S. aureus bacterium. Int. J. Adhes. Adhesiv. 2017, 77, 15–28. [Google Scholar] [CrossRef]
- Turgeon, M.L. Clinical Hematology: Theory and Procedures, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; p. 570. [Google Scholar]
- MediaLab, Inc. Checked Online 2022-04-04. Available online: https://www.labce.com/spg469924_initial_evaluation_of_rbc_morphology_from_automate.aspx (accessed on 4 December 2022).
- Kumar, A.A.; Patton, M.R.; Hennek, J.W.; Lee, S.Y.R.; D’Alesio-Spina, G.; Yang, X.; Kanter, J.; Shevkoplyas, S.S.; Brugnara, C.; Whitesides, G.M. Density-based separation in multiphase systems provides a simple method to identify sickle cell disease. Proc. Natl. Acad. Sci. USA 2014, 111, 14864–14869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulińska, I.; Targosz, M.; Strojny, W.; Lekka, M.; Czuba, P.; Balwierz, W.; Szymoński, M. Stiffness of normal and pathological erythrocytes studied by means of atomic force microscopy. J. Biochem. Biophys. Methods 2006, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abay, A.; Simionato, G.; Chachanidze, R.; Bogdanova, A.; Hertz, L.; Bianchi, P.; Akker, E.V.D.; Von Lindern, M.; Leonetti, M.; Minetti, G.; et al. Glutaraldehyde—A subtle tool in the investigation of healthy and pathologic red blood cells. Front. Physiol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef]
- Rubenstein, D.A.; Yin, W.; Frame, M.D. Biofluid Mechanics: An Introduction to Fluid Mechanics, Macrocirculation, and Microcirculation, 2nd ed.; Academic Press: London, UK, 2016; p. 544. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Connell, S.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Tavares, A.; Ariëns, R.A.S.; Santos, N.C. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 2010, 4, 4609–4620. [Google Scholar] [CrossRef]
- Liu, W.; Carlisle, C.R.; Sparks, E.A.; Guthold, M. The mechanical properties of single fibrin fibers. J. Thromb. Haemost. 2010, 8, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Zhmurov, A.; Protopopova, A.D.; Litvinov, R.I.; Zhukov, P.; Weisel, J.W.; Barsegov, V. Atomic structural models of fibrin oligomers. Structure 2018, 26, 857–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.B.; Eaton, K.A.; Princiotta, S.M.; Rushin, C.A.; Valeri, C.R. Size dependent platelet subpopulations: Relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br. J. Haematol. 1982, 50, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, M.; Fritz, M.; Kacher, C.M.; Cleveland, J.; Hansma, P. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 1996, 70, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyoum, M.; Enawgaw, B.; Melku, M. Human blood platelets and viruses: Defense mechanism and role in the removal of viral pathogens. Thromb. J. 2018, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilschut, J. Membrane Fusion; CRC Press: Boca Raton, FL, USA, 1990; p. 938. [Google Scholar] [CrossRef]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH: Berlin, Germany, 2003; p. 495. [Google Scholar] [CrossRef]
- Nagaoka, T.; Yoshida, A. Noninvasive evaluation of wall shear stress on retinal microcirculation in humans. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1113–1119. [Google Scholar] [CrossRef]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. The behaviors of ferro-magnetic nano-particles in and around blood vessels under applied magnetic fields. J. Magn. Magn. Mater. 2011, 323, 651–668. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Mombouli, J.V.; Taylor, A.A.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization caused by bradykinin in human coronary arteries. J. Clin. Investig. 1993, 92, 2867–2871. [Google Scholar] [CrossRef]
- Tutwiler, V.; Litvinov, R.I.; Protopopova, A.; Nagaswami, C.; Villa, C.; Woods, E.; Abdulmalik, O.; Siegel, D.L.; Russell, J.E.; Muzykantov, V.R.; et al. Pathologically stiff erythrocytes impede contraction of blood clots. J. Thromb. Haemost. 2021, 19, 1990–2001. [Google Scholar] [CrossRef]
- Jasevičius, R. Numerical simulation of the mechanics of oblique interaction of a bacterium with a flat surface. Mech. Adv. Mater. Struct. 2022, 29, 2884–2894. [Google Scholar] [CrossRef]
- Jasevičius, R. Numerical modeling of the mechanics of the interaction of coronavirus with the lung epithelial cell. Mech. Adv. Mater. Struct. 2022, 29, 3030–3039. [Google Scholar] [CrossRef]


| Objects | Initial Parameters | Values | References, Sources |
|---|---|---|---|
| Erythrocyte (red blood cell) | Diameter, | Turgeon [35] | |
| Initial interaction distance, | Jasevičius [31] | ||
| Initial velocity, | Jasevičius [31] | ||
| Mass, | (picograms) | MediaLab, Inc. [36] | |
| Density, | Kumar et al. [37] | ||
| Young’s modulus, | Dulińska et al. [38] | ||
| Poisson’s ratio, | Abay et al. [39] | ||
| Surface potential, | Pei et al. [40] | ||
| Surface charge density | Rubenstein et al. [41] | ||
| The adhesion force at point A, (between erythrocyte and fibrin) | Carvalho et al. [42] | ||
| The adhesion force at point L, (erythrocyte–fibrin) | - | ||
| Adhesive dissipative energy, | - | ||
| Fibrin | Young’s modulus, Density, | 4 MPa | Liu et al. [43] Zhmurov et al. [44] |
| Endothelial cell | Surface charge density | Rubenstein [41] | |
| Platelet (thrombocyte) | Density, | Thompson et al. [45] | |
| Young’s modulus, | Radmacher et al. [46] | ||
| Poisson ratio, | Radmacher et al. [46] | ||
| Diameter, (non activated) | Seyoum et al. [47] | ||
| Blood | PH | Wilschut [48] | |
| Temperature, | 36.8 °C | Jasevičius [31] | |
| The permittivity of the free space, | Jasevičius [31] | ||
| Debye length, A dielectric constant of water, | 74.5 | Rubenstein [41] Butt et al. [49] | |
| Blood flow velocity, | Nagaoka and Yoshida [50] | ||
| Blood flow velocity at distance in normal direction, | Jasevičius [31] | ||
| Viscosity, | ) | Nacev et al. [51] | |
| Arteriole | Diameter, Surface potential, | 100 μm | Nagaoka and Yoshida [50] Nakashima et al. [52] |
| Blood clot | Poisson ratio, | Tutwiler et al. [53] | |
| Simulation | Time step | (picoseconds) | - |
| Parameter | Values at Certain Points | |||||
|---|---|---|---|---|---|---|
| S | L | U | A′ | E | ||
| Without drag force | , μs | 0 | 11.59687 | 15.120995 | 19.679315001 | ≈150 |
| , pN | −5.78593 | −34.27663 | 104.69451942 | −66.7513884 | 0 | |
| , nm | −20 | 0 | 14.3479743 | 1.806173644 | 7.71084038 | |
| , mm/s | −0.10783 | −4.6735475 | 0 | 0 | 0 | |
| With drag force | , μs | 0 | 107.64 | ≈250 | - | ≈250 |
| , pN | −5.78593 | −0.18155 | 0 | - | 0 | |
| , nm | −20 | 0 | 7.562478 | - | 7.562478 | |
| , mm/s | −0.10783 | −0.30232 | 0 | - | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasevičius, R. Numerical Modelling of Erythrocyte Sticking Mechanics. Appl. Sci. 2022, 12, 12576. https://doi.org/10.3390/app122412576
Jasevičius R. Numerical Modelling of Erythrocyte Sticking Mechanics. Applied Sciences. 2022; 12(24):12576. https://doi.org/10.3390/app122412576
Chicago/Turabian StyleJasevičius, Raimondas. 2022. "Numerical Modelling of Erythrocyte Sticking Mechanics" Applied Sciences 12, no. 24: 12576. https://doi.org/10.3390/app122412576







