Geometrical Effect of Active Material on Electrode Tortuosity in All-Solid-State Lithium Battery
Abstract
:1. Introduction
2. Theoretical Background
3. Experimental Details
3.1. Materials and Electrode Fabrication
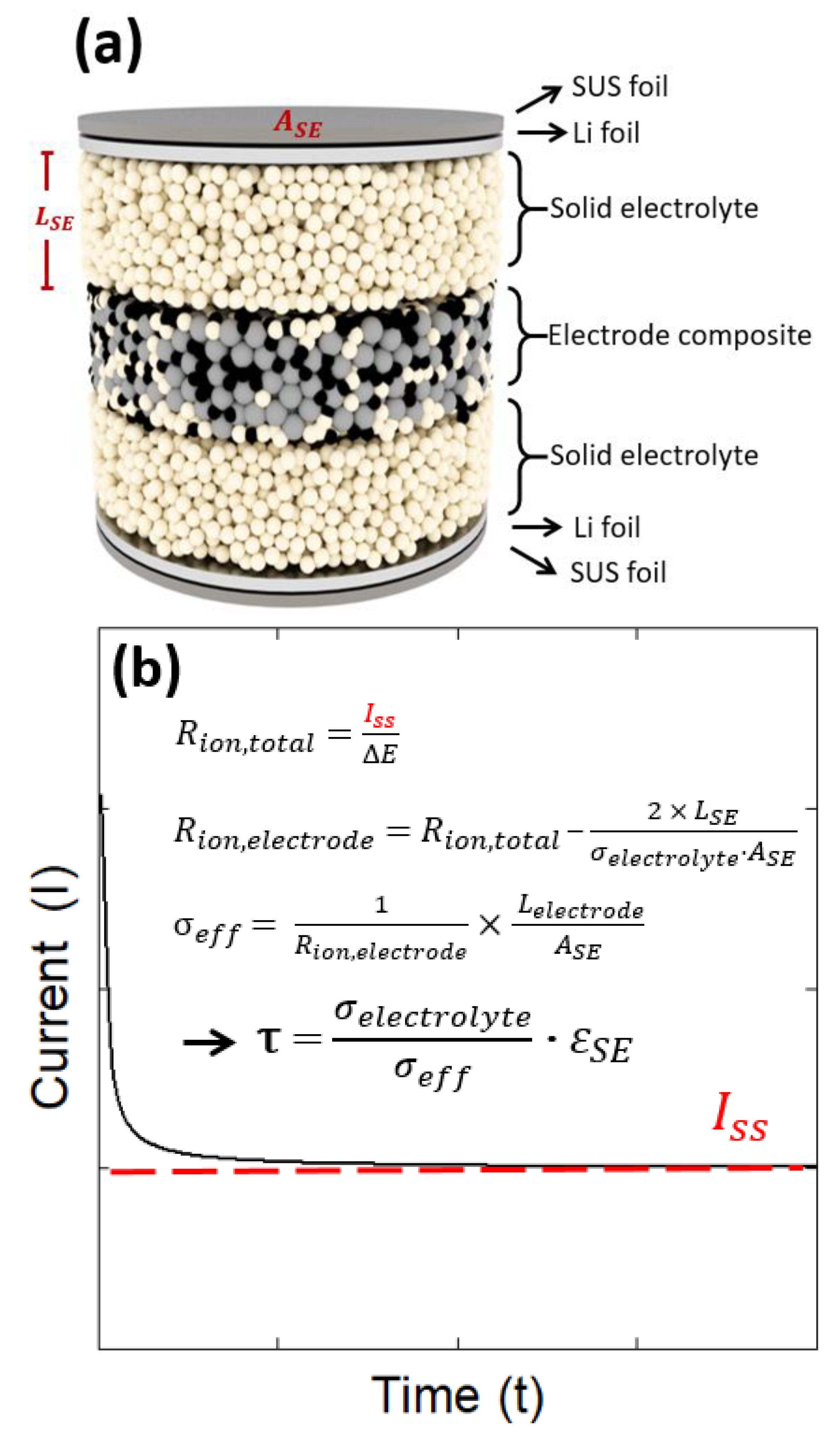
3.2. Cell Fabrication and Tortuosity Measurement
4. Results and Discussion
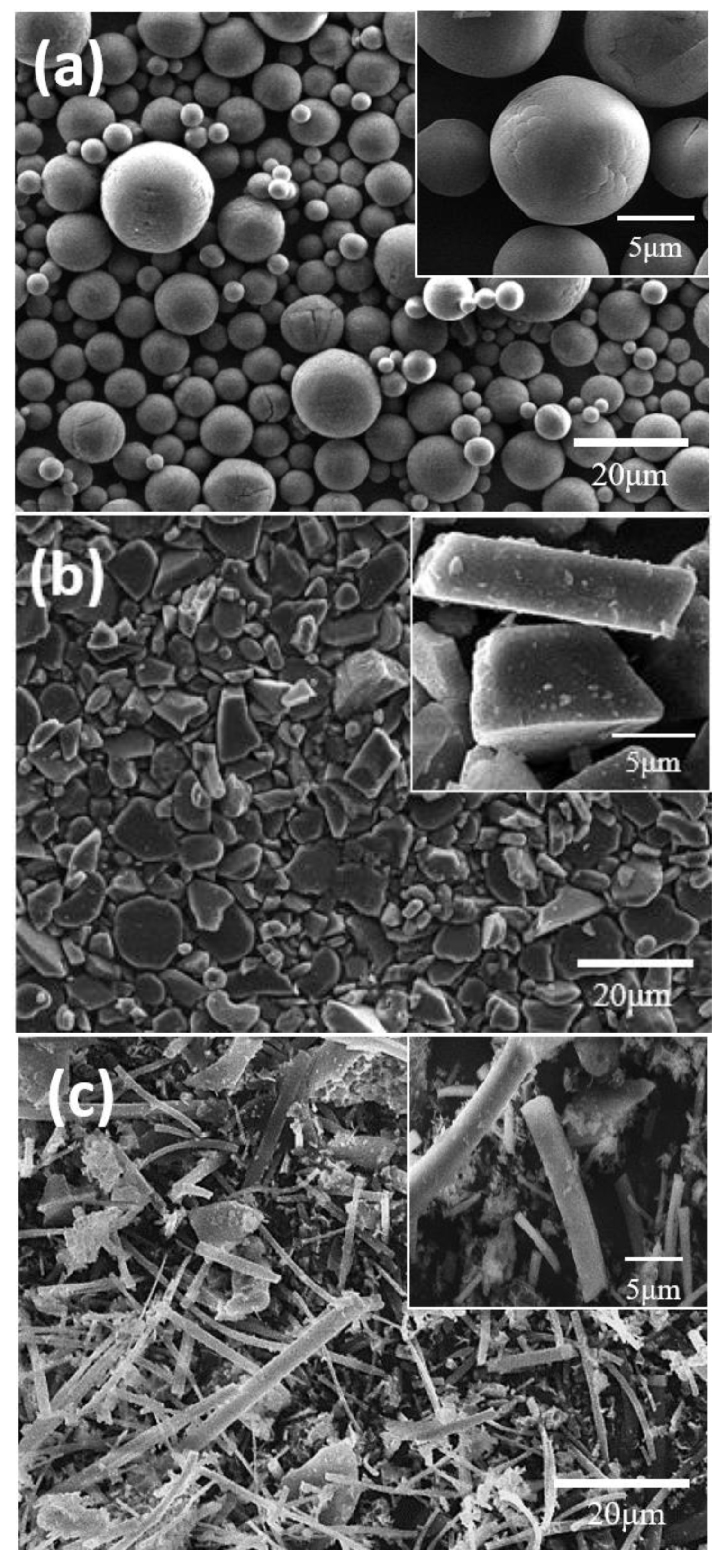
4.1. Shape Effect
4.2. Size Effect
4.3. Electrode Density (Pressure) Effect
5. Conclusions
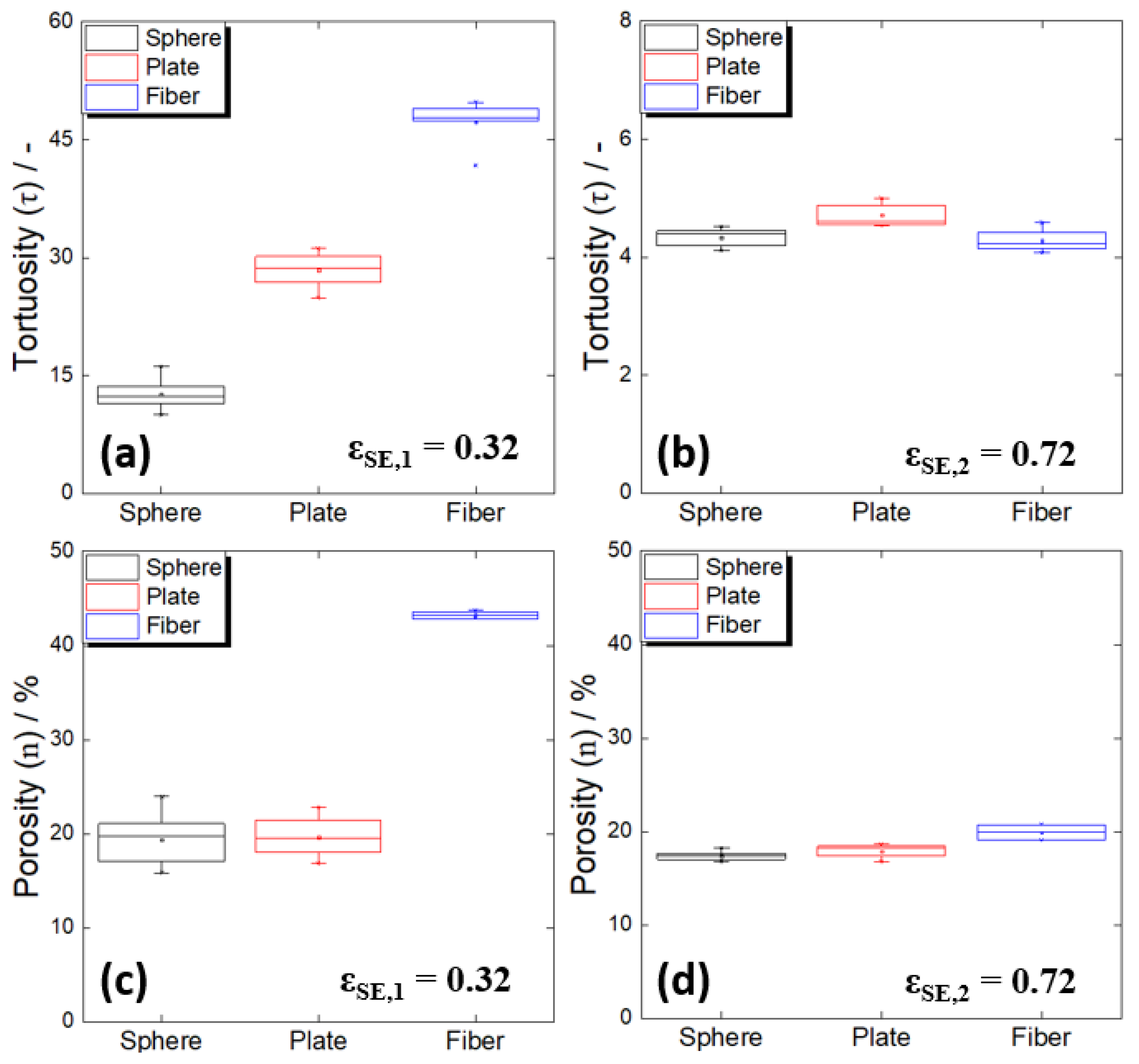
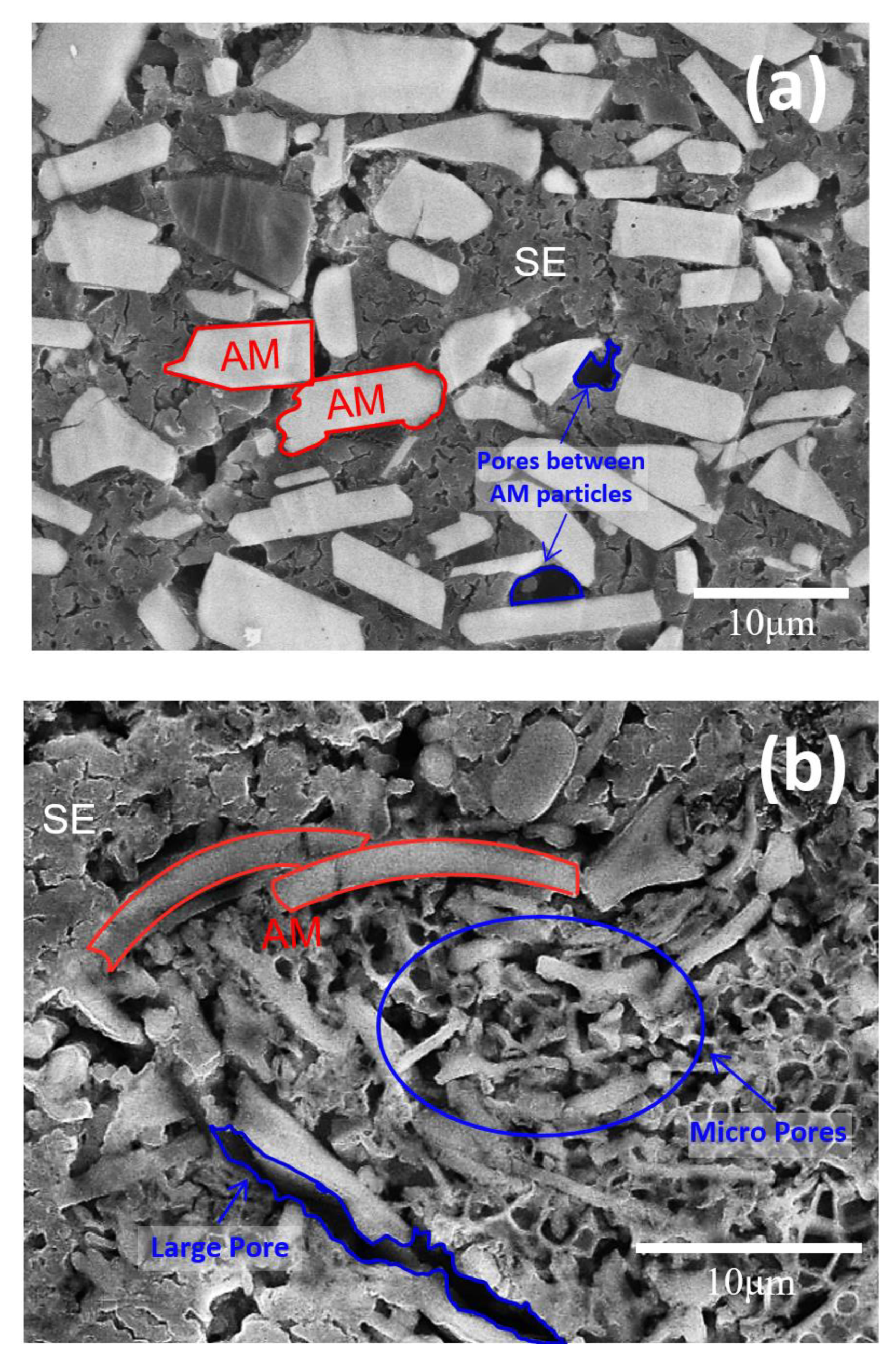
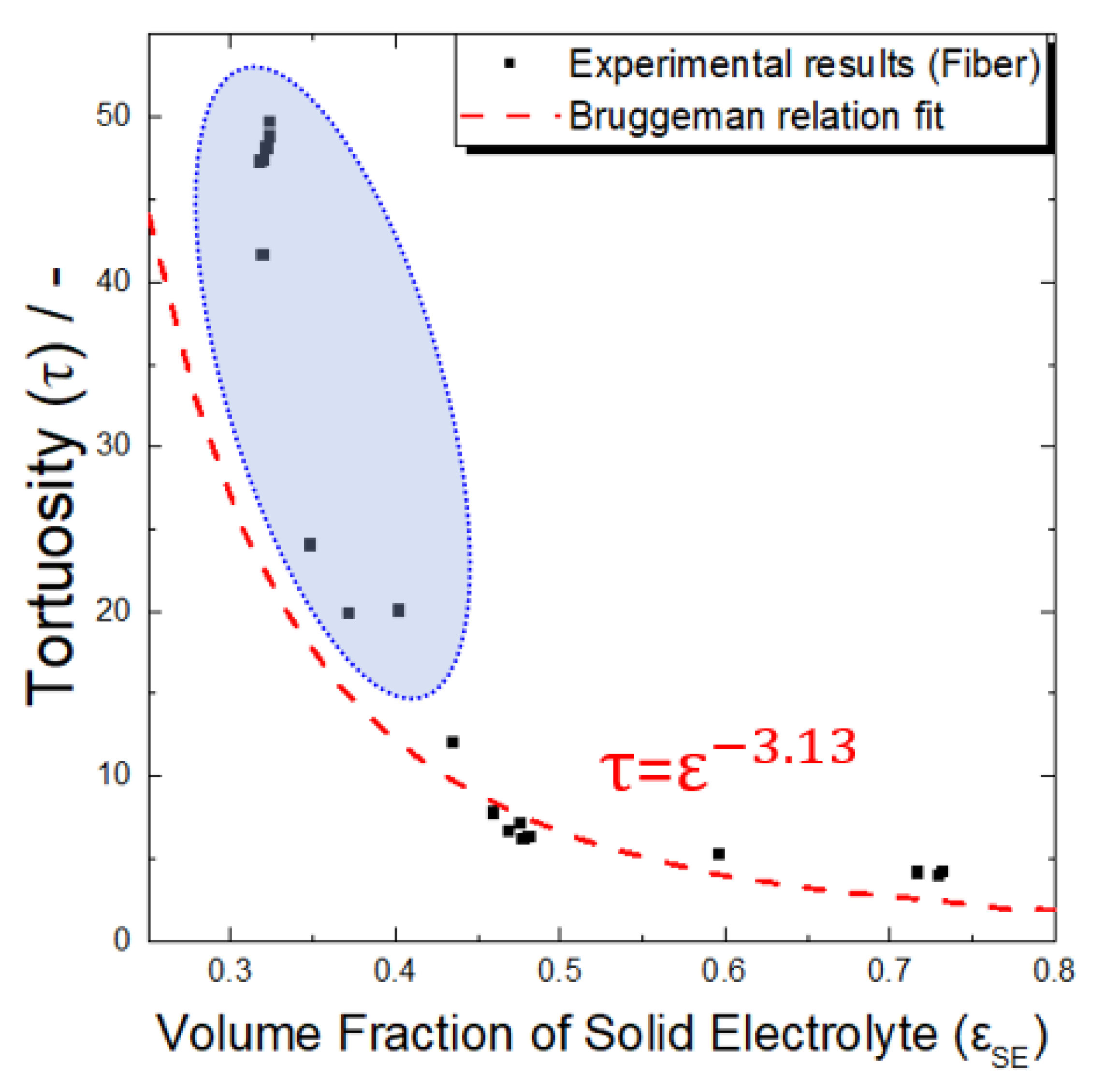
- In the electrodes with a high active material ratio, the tortuosity values increased in the order of spherical, plate-shaped, and fibrous active materials. Because the porosity values of the electrodes using the spherical and plate-shaped active materials were almost the same, the difference in tortuosity between the two shapes was due to the difference in the complexity of the solid electrolyte path resulting from a simple shape difference. The porosity of the electrode using the active fibrous material was approximately twice that of the others because the solid electrolyte did not sufficiently fill in the micropores formed by the aggregation of the material particles. In an all-solid-state battery electrode, when a high proportion of active fibrous material is used, special attention should be paid to the low filling factor of the solid electrolyte resulting from particle aggregation.
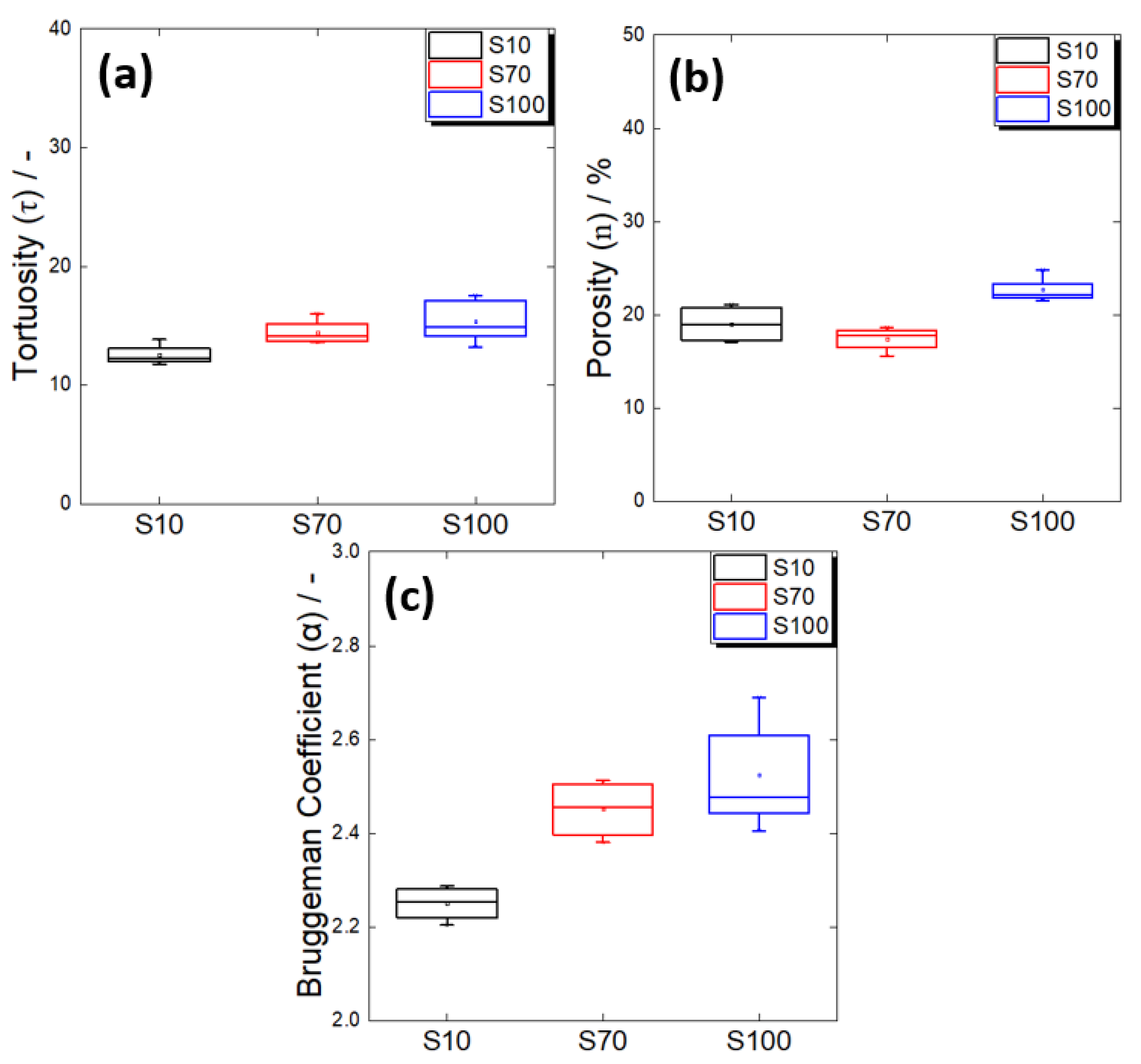
- As the particle size of the spherical active material increased, the degree of tortuosity also increased. When the particle sizes were 10 μm and 70 μm, the electrode porosity values were similar, suggesting that the 70 μm active material electrode had higher tortuosity due to the poor connectivity of the solid electrolyte in the large particle gaps. The electrode with the 100 μm active material showed a significant increase in porosity, implying that the increase in unfilled pores, along with the low solid electrolyte connectivity in the large particle gaps, additionally contributed to the increase in tortuosity. In other words, even if the particle size of the active material increases and the particle gaps increase under a high active material ratio, the connectivity between electrolyte particles may deteriorate as a result of the limited amount of solid electrolyte.
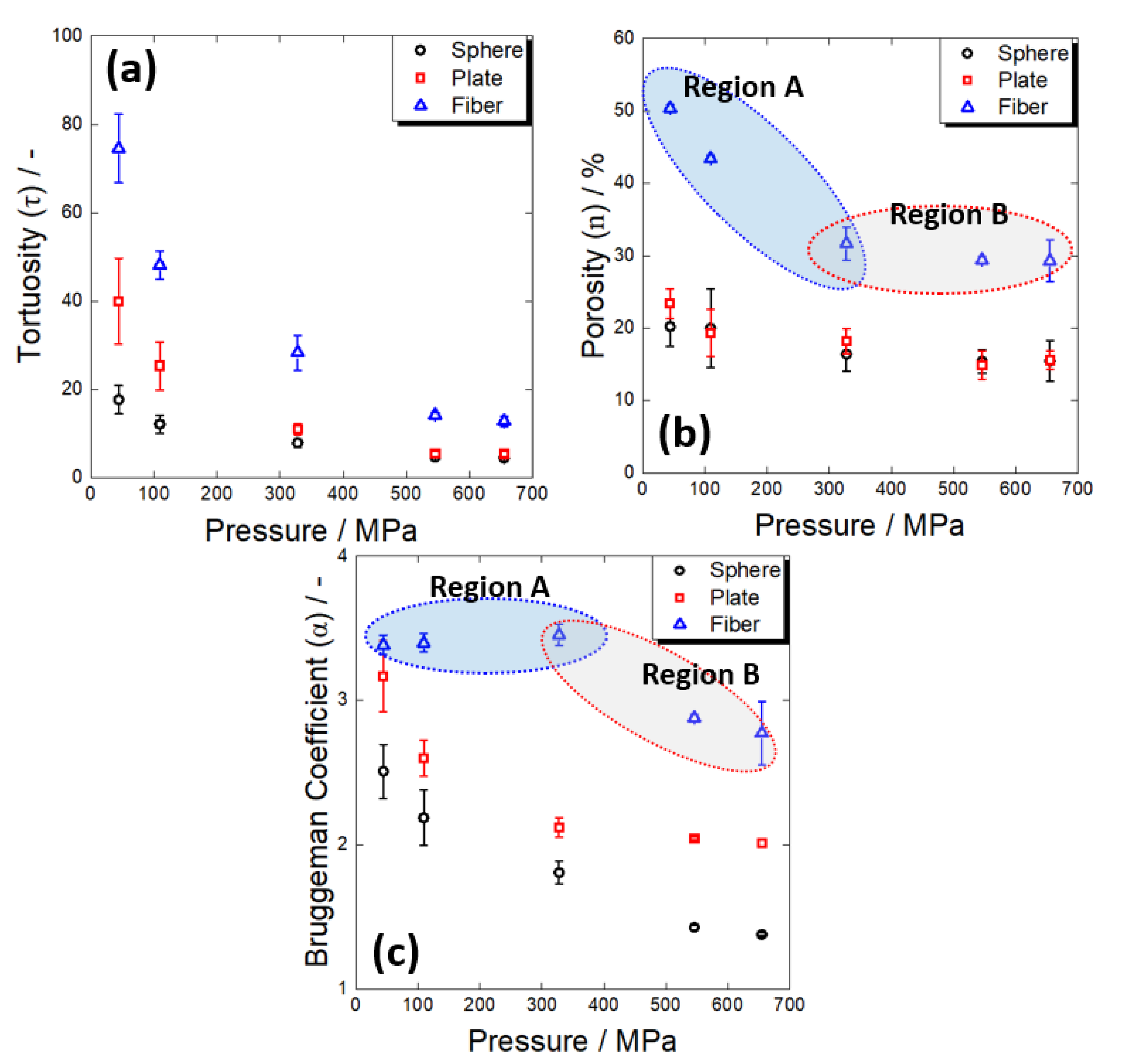
- Regardless of the material shape, the tortuosity decreased with pressing pressure, and the fibrous material showed a different porosity change pattern to the others. In the electrodes containing the spherical and plate-shaped materials, the porosity change was insignificant as the pressing pressure increased, suggesting that the tortuosity decrease with the pressing pressure was simply due to the improvement in the contact characteristics between the electrolyte particles. In the case of the fibrous material, the porosity considerably changed in the low-pressure region, but there was little change in the high-pressure region. It was conjectured that the electrolyte penetrated the fibrous aggregate and filled the micropores at low pressure, while the tortuosity further decreased at high pressure as the contact characteristics between the electrolyte particles were improved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yada, C.; Brasse, C. Better batteries with Solid-state instead of liquid-based electrolytes. ATZelektronik Worldw. 2014, 9, 10–15. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.-Y.; He, Z.-J.; Li, Y.-J.; Mao, J.; Dai, K.-H.; Yan, C.; Zheng, J.-C. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Dawson, J.A.; Canepa, P.; Famprikis, T.; Masquelier, C.; Islam, M.S. Atomic-scale influence of grain boundaries on Li-ion conduction in solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 2018, 140, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-J.; Zhang, Y.-B.; Liu, T.; Xu, B.-Q.; Lin, Y.-H.; Nan, C.-W.; Shen, Y. Addressing the interface issues in all-solid-state bulk-type lithium ion battery via an all-composite approach. ACS Appl. Mater. Interfaces 2017, 9, 9654–9661. [Google Scholar] [CrossRef]
- Pervez, S.A.; Cambaz, M.A.; Thangadurai, V.; Fichtner, M. Interface in solid-state lithium battery: Challenges, progress, and outlook. ACS Appl. Mater. Interfaces 2019, 11, 22029–22050. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Li, J.; An, C. Interfaces: Key issue to be solved for all solid-state lithium battery technologies. J. Electrochem. Soc. 2020, 167, 070541. [Google Scholar] [CrossRef]
- Deng, C.; Chen, N.; Hou, C.; Liu, H.; Zhou, Z.; Chen, R. Enhancing Interfacial Contact in Solid-State Batteries with a Gradient Composite Solid Electrolyte. Small 2021, 17, 2006578. [Google Scholar] [CrossRef]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.-W. Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, F.; Luo, J.; Zhang, L.; Sun, X. Recent development of lithium argyrodite solid-state electrolytes for solid-state batteries: Synthesis, structure, stability and dynamics. Nano Energy 2021, 83, 105858. [Google Scholar] [CrossRef]
- Wu, J.-F.; Pang, W.K.; Peterson, V.K.; Wei, L.; Guo, X. Garnet-type fast Li-ion conductors with high ionic conductivities for all-solid-state batteries. ACS Appl. Mater. Interfaces 2017, 9, 12461–12468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Adekoya, D.; Hencz, L.; Ma, J.; Chen, S.; Yan, C.; Zhao, H.; Cui, G.; Zhang, S. Stable seamless interfaces and rapid ionic conductivity of Ca–CeO2/LiTFSI/PEO composite electrolyte for high-rate and high-voltage all-solid-state battery. Adv. Energy Mater. 2020, 10, 2000049. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Fan, A.; Liu, X.; Wang, H.; Ma, W.; Zhu, L.; Zhang, X. A high energy and power all-solid-state lithium battery enabled by modified sulfide electrolyte film. J. Power Sources 2021, 485, 229325. [Google Scholar] [CrossRef]
- Newman, J. Optimization of porosity and thickness of a battery electrode by means of a reaction-zone model. J. Electrochem. Soc. 1995, 142, 97. [Google Scholar] [CrossRef]
- Patel, K.K.; Paulsen, J.M.; Desilvestro, J. Numerical simulation of porous networks in relation to battery electrodes and separators. J. Power Sources 2003, 122, 144–152. [Google Scholar] [CrossRef]
- Kehrwald, D.; Shearing, P.R.; Brandon, N.P.; Sinha, P.K.; Harris, S.J. Local tortuosity inhomogeneities in a lithium battery composite electrode. J. Electrochem. Soc. 2011, 158, A1393. [Google Scholar] [CrossRef]
- Bae, C.-J.; Erdonmez, C.K.; Halloran, J.W.; Chiang, Y.-M. Design of battery electrodes with dual-scale porosity to minimize tortuosity and maximize performance. Adv. Mater. 2013, 25, 1254–1258. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Janek, J. Modeling effective ionic conductivity and binder influence in composite cathodes for all-solid-state batteries. ACS Appl. Mater. Interfaces 2020, 12, 12821–12833. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Xu, X.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Low-Tortuosity Thick Electrodes with Active Materials Gradient Design for Enhanced Energy Storage. ACS Nano 2022, 16, 4805–4812. [Google Scholar] [CrossRef]
- Ebner, M.; Chung, D.-W.; García, R.E.; Wood, V. Tortuosity anisotropy in lithium-ion battery electrodes. Adv. Energy Mater. 2014, 4, 1301278. [Google Scholar] [CrossRef]
- Usseglio-Viretta, F.L.E.; Finegan, D.P.; Colclasure, A.M.; Heenan, T.M.M.; Abraham, D.P.; Shearing, P.R.; Smith, K. Quantitative relationships between pore tortuosity, pore topology, and solid particle morphology using a novel discrete particle size algorithm. J. Electrochem. Soc. 2020, 167, 100513. [Google Scholar] [CrossRef]
- So, M.; Inoue, G.; Hirate, R.; Nunoshita, K.; Ishikawa, S.; Tsuge, Y. Effect of mold pressure on compaction and ion conductivity of all-solid-state batteries revealed by the discrete element method. J. Power Sources 2021, 508, 230344. [Google Scholar] [CrossRef]
- Hlushkou, D.; Reising, A.E.; Kaiser, N.; Spannenberger, S.; Schlabach, S.; Kato, Y.; Roling, B.; Tallarek, U. The influence of void space on ion transport in a composite cathode for all-solid-state batteries. J. Power Sources 2018, 396, 363–370. [Google Scholar] [CrossRef]
- Vijayaraghavan, B.; Ely, D.R.; Chiang, Y.-M.; García-García, R.; Garcia, R.E. An analytical method to determine tortuosity in rechargeable battery electrodes. J. Electrochem. Soc. 2012, 159, A548. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Demortière, A.; Fleutot, B.; Delobel, B.; Delacourt, C.; Cooper, S.J. The electrode tortuosity factor: Why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. NPJ Comput. Mater. 2020, 6, 123. [Google Scholar] [CrossRef]
- Du, W.; Xue, N.; Shyy, W.; Martins, J.R.R.A. A surrogate-based multi-scale model for mass transport and electrochemical kinetics in lithium-ion battery electrodes. J. Electrochem. Soc. 2014, 161, E3086. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Cericola, D.; Spahr, M.E. Impedance spectroscopic studies of the porous structure of electrodes containing graphite materials with different particle size and shape. Electrochim. Acta 2016, 191, 558–566. [Google Scholar] [CrossRef]
- Malifarge, S.; Delobel, B.; Delacourt, C. Determination of tortuosity using impedance spectra analysis of symmetric cell. J. Electrochem. Soc. 2017, 164, E3329. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity determination of battery electrodes and separators by impedance spectroscopy. J. Electrochem. Soc. 2016, 163, A1373. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Kirkpatrick, K.; Mason, T.; Garboczi, E. Experimental limitations in impedance spectroscopy:: Part IV. Electrode contact effects. Solid State Ionics 1997, 98, 93–104. [Google Scholar] [CrossRef]
- Nara, H.; Mukoyama, D.; Shimizu, R.; Momma, T.; Osaka, T. Systematic analysis of interfacial resistance between the cathode layer and the current collector in lithium-ion batteries by electrochemical impedance spectroscopy. J. Power Sources 2019, 409, 139–147. [Google Scholar] [CrossRef]
- Dhillon, S.; Kant, R. Theory for electrochemical impedance spectroscopy of heterogeneous electrode with distributed capacitance and charge transfer resistance. J. Chem. Sci. 2017, 129, 1277–1292. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xu, K.; Jow, T. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Battistel, A.; Fan, M.; Stojadinović, J.; La Mantia, F. Analysis and mitigation of the artefacts in electrochemical impedance spectroscopy due to three-electrode geometry. Electrochim. Acta 2014, 135, 133–138. [Google Scholar] [CrossRef]
- Froboese, L.; Van Der Sichel, J.F.; Loellhoeffel, T.; Helmers, L.; Kwade, A. Effect of microstructure on the ionic conductivity of an all solid-state battery electrode. J. Electrochem. Soc. 2019, 166, A318. [Google Scholar] [CrossRef]
- Indrikova, M.; Grunwald, S.; Golks, F.; Netz, A.; Westphal, B.; Kwade, A. The morphology of battery electrodes with the focus of the conductive additives paths. J. Electrochem. Soc. 2015, 162, A2021. [Google Scholar] [CrossRef]
- Clennell, M.B. Tortuosity: A guide through the maze. Geol. Soc. Lond. Spec. Publ. 1997, 122, 299–344. [Google Scholar] [CrossRef]
- Kaiser, N.; Spannenberger, S.; Schmitt, M.; Cronau, M.; Kato, Y.; Roling, B. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte. J. Power Sources 2018, 396, 175–181. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- MacMullin, R.B.; Muccini, G.A. Characteristics of porous beds and structures. AIChE J. 1956, 2, 393–403. [Google Scholar] [CrossRef]
- Vadakkepatt, A.; Trembacki, B.; Mathur, S.R.; Murthy, J.Y. Bruggeman’s exponents for effective thermal conductivity of lithium-ion battery electrodes. J. Electrochem. Soc. 2015, 163, A119. [Google Scholar] [CrossRef]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire electrodes for electrochemical energy storage devices. Chem. Rev. 2014, 114, 11828–11862. [Google Scholar] [CrossRef] [PubMed]
- Pouraghajan, F.; Knight, H.; Wray, M.; Mazzeo, B.; Subbaraman, R.; Christensen, J.; Wheeler, D. Quantifying tortuosity of porous Li-ion battery electrodes: Comparing polarization-interrupt and blocking-electrolyte methods. J. Electrochem. Soc. 2018, 165, A2644. [Google Scholar] [CrossRef]
- Tu, S.; Lu, Z.; Zheng, M.; Chen, Z.; Wang, X.; Cai, Z.; Chen, C.; Wang, L.; Li, C.; Seh, Z.W. Single-Layer-Particle Electrode Design for Practical Fast-Charging Lithium-ion Batteries. Adv. Mater. 2022, 34, 2202892. [Google Scholar] [CrossRef]
- Doux, J.-M.; Yang, Y.; Tan, D.H.S.; Nguyen, H.; Wu, E.A.; Wang, X.; Banerjee, A.; Meng, Y.S. Pressure effects on sulfide electrolytes for all solid-state batteries. J. Mater. Chem. A 2020, 8, 5049–5055. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 2020, 474, 228601. [Google Scholar] [CrossRef]
- So, M.; Inoue, G.; Hirate, R.; Nunoshita, K.; Ishikawa, S.; Tsuge, Y. Simulation of fabrication and degradation of all-solid-state batteries with ductile particles. J. Electrochem. Soc. 2021, 168, 030538. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Jeong, J.; Shin, H.-C. Geometrical Effect of Active Material on Electrode Tortuosity in All-Solid-State Lithium Battery. Appl. Sci. 2022, 12, 12692. https://doi.org/10.3390/app122412692
Park S-Y, Jeong J, Shin H-C. Geometrical Effect of Active Material on Electrode Tortuosity in All-Solid-State Lithium Battery. Applied Sciences. 2022; 12(24):12692. https://doi.org/10.3390/app122412692
Chicago/Turabian StylePark, So-Yeon, Jiung Jeong, and Heon-Cheol Shin. 2022. "Geometrical Effect of Active Material on Electrode Tortuosity in All-Solid-State Lithium Battery" Applied Sciences 12, no. 24: 12692. https://doi.org/10.3390/app122412692



