Structure–Properties Relationship of Reprocessed Bionanocomposites of Plasticized Polylactide Reinforced with Nanofibrillated Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Nanofibrillated Cellulose (NFC)
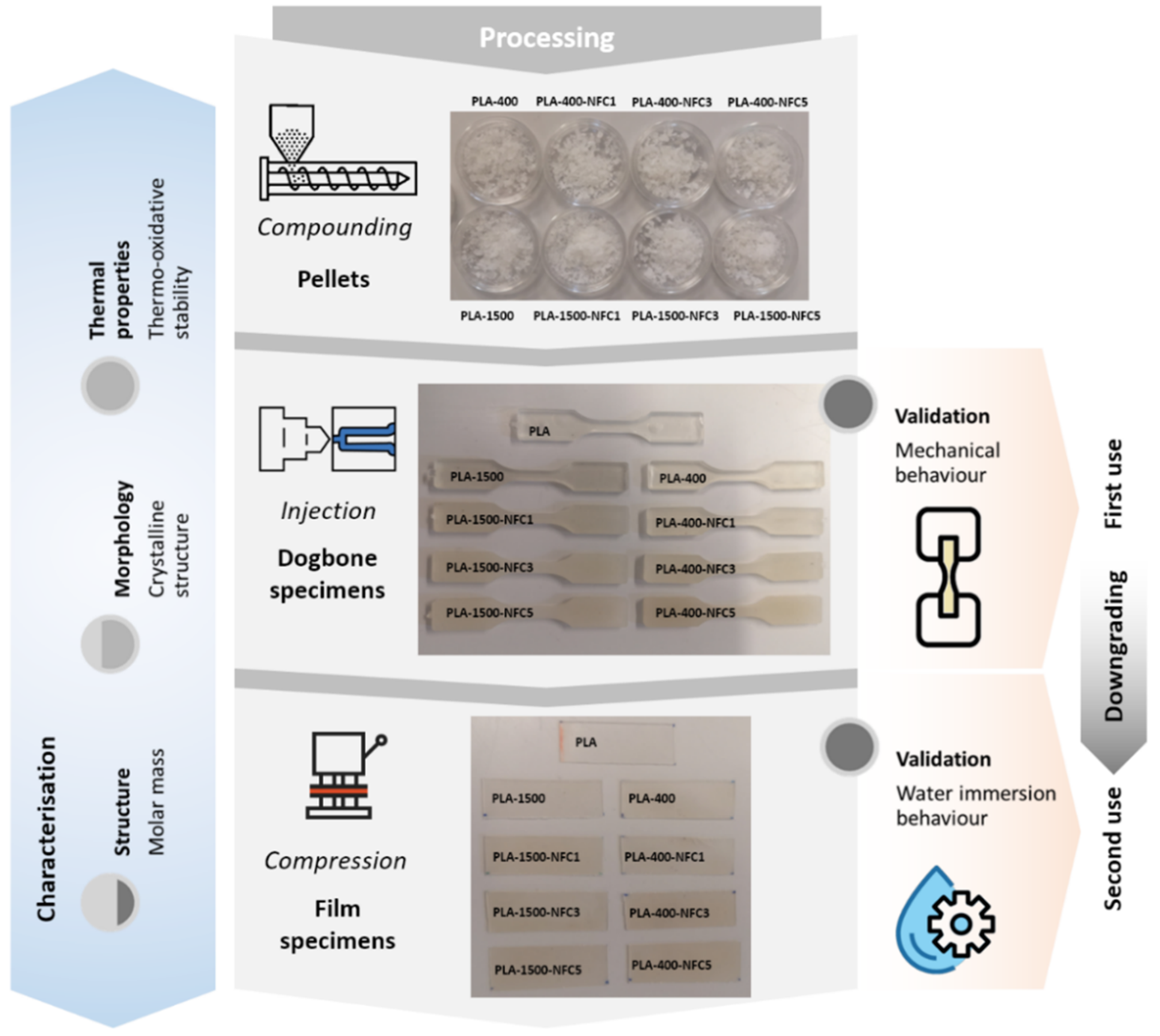
2.3. Compounding, Processing, and Recycling of Bionanocomposites
2.4. Gel Permeation Chromatography (GPC)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Thermogravimetric Analysis (TGA)
2.7. Field Emission Scanning Electron Microscopy (FE-SEM)
2.8. Tensile Test
2.9. Water Absorption Studies
3. Results and Discussion
3.1. Structural and Morphological Consequences of Processing
3.1.1. Molar Mass
3.1.2. Thermal Properties and Crystalline Structure
3.1.3. Thermo-Oxidative Stability
3.2. Validation of the Bionanocomposites
3.2.1. First Service Life: Mechanical Performance and Fracture Surface of Dog-Bone Specimens
3.2.2. Downgraded Second Service Life: Diffusion, Sorption, and Permeation of Water in Film Reprocessed Specimens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug. Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, T.-D. Biobased and Biodegradable Polymers Nanocomposites. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar] [CrossRef]
- Kfoury, G.; Raquez, J.M.; Hassouna, F.; Odent, J.; Toniazzo, V.; Ruch, D.; Dubois, P. Recent Advances in High Performance Poly(Lactide): From “Green” Plasticization to Super-Tough Materials via (Reactive) Compounding. Front. Chem. 2013, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Sun, G.; Chan, C.M. The Effects of the Low-Molecular-Weight Component on Banded Spherulites of Poly(l-Lactic Acid). Colloid. Polym. Sci. 2013, 291, 1495–1501. [Google Scholar] [CrossRef]
- Ljungberg, N. Tributyl Citrate Oligomers as Plasticizers for Poly (Lactic Acid): Thermo-Mechanical Film Properties and Aging. Polymer 2003, 44, 7679–7688. [Google Scholar] [CrossRef]
- Ljungberg, N. The Effects of Plasticizers on the Dynamic Mechanical and Thermal Properties of Poly(Lactic Acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and Mechanical Properties of Plasticized Poly(L-Lactic Acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Sepúlveda, F.A.; Rivera, F.; Loyo, C.; Canales, D.; Moreno-Serna, V.; Benavente, R.; Rivas, L.M.; Ulloa, M.T.; Gil-Castell, O.; Ribes-Greus, A.; et al. Poly (Lactic Acid)/D-Limonene/ZnO Bio-Nanocomposites with Antimicrobial Properties. J. Appl. Polym. Sci. 2022, 139, 51542. [Google Scholar] [CrossRef]
- Brüster, B.; Adjoua, Y.O.; Dieden, R.; Grysan, P.; Federico, C.E.; Berthé, V.; Addiego, F. Plasticization of Polylactide with Myrcene and Limonene as Bio-Based Plasticizers: Conventional vs. Reactive Extrusion. Polymers 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, Z.; Zhang, L.; Tang, Z.; Zhang, R.; Zhu, J. Isosorbide Dioctoate as a “Green” Plasticizer for Poly(Lactic Acid). Mater. Des. 2016, 91, 262–268. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Toughening of Biodegradable Polylactide/Poly(Butylene Succinate- Co -Adipate) Blends via in Situ Reactive Compatibilization. ACS. Appl. Mater. Interfaces. 2013, 5, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Dong, L.; Yang, Y. Dynamic Mechanical and Thermal Properties of Plasticized Poly(Lactic Acid). J. Appl. Polym. Sci. 2006, 101, 1583–1590. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased Additive Plasticizing Polylactic Acid (PLA). Polímeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and Techniques for Improving Heat Resistance and Mechanical Performances of Poly(Lactic Acid) (PLA) Biodegradable Materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F. Thermal Behavior of PLA Plasticized by Commercial and Cardanol-Derived Plasticizers and the Effect on the Mechanical Properties. J. Anal. Calorim. 2021, 146, 131–141. [Google Scholar] [CrossRef]
- Younes, H.; Cohn, D. Phase Separation in Poly(Ethylene Glycol)/Poly(Lactic Acid) Blends. Eur. Polym. J. 1988, 24, 765–773. [Google Scholar] [CrossRef]
- Hu, Y. Crystallization and Phase Separation in Blends of High Stereoregular Poly(Lactide) with Poly(Ethylene Glycol). Polymer 2003, 44, 5681–5689. [Google Scholar] [CrossRef]
- Hu, Y.; Rogunova, M.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of Poly(Lactide)/Poly(Ethylene Glycol) Blends. Part 1. Poly(Lactide) with Low Stereoregularity. Polymer 2003, 44, 5701–5710. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G. Plasticizing Polylactide? The Effect of Different Plasticizers on the Mechanical Properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Grohens, Y. Thermo-Mechanical Characterization of Plasticized PLA: Is the Miscibility the Only Significant Factor? Polymer 2006, 47, 4676–4682. [Google Scholar] [CrossRef]
- Sanyang, M.S.; Jawaid, M. Bio-Based Polymers and Nanocomposites; Springer International Publishing: Cham, Germany, 2019; ISBN 978-3-030-05824-1. [Google Scholar]
- Banerjee, R.; Ray, S.S. An Overview of the Recent Advances in Polylactide-Based Sustainable Nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Vatansever, E.; Arslan, D.; Nofar, M. Polylactide Cellulose-Based Nanocomposites. Int. J. Biol. Macromol. 2019, 137, 912–938. [Google Scholar] [CrossRef]
- Raisipour-Shirazi, A.; Ahmadi, Z.; Garmabi, H. Polylactic Acid Nanocomposites Toughened with Nanofibrillated Cellulose: Microstructure, Thermal, and Mechanical Properties. Iran. Polym. J. 2018, 27, 785–794. [Google Scholar] [CrossRef]
- Perić, M.; Putz, R.; Paulik, C. Influence of Nanofibrillated Cellulose on the Mechanical and Thermal Properties of Poly(Lactic Acid). Eur. Polym. J. 2019, 114, 426–433. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Zhao, R.; Zhou, Y.; Wang, Z. Preparation of Nanofibrillated Cellulose and Application in Reinforced PLA/Starch Nanocomposite Film. J. Polym. Env. 2019, 27, 728–738. [Google Scholar] [CrossRef]
- Perić, M.; Putz, R.; Paulik, C. 3D-Printed Pla Filaments Reinforced with Nanofibrillated Cellulose. J. Renew. Mater. 2020, 8, 759–772. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Reyes-Contreras, P.; Barra, P.A.; Mendonça, R.T.; Carrillo-Varela, I.; Badia, J.D.; Serra, A.; Ribes-Greus, A. The Role of Eucalyptus Species on the Structural and Thermal Performance of Cellulose Nanocrystals (CNCs) Isolated by Acid Hydrolysis. Polymers 2022, 14, 423. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Sugawara, E.; Nikaido, H. Polylactic Acid; Sin, B.S.T.L.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128144725. [Google Scholar]
- Akbar, M.U.; Rehman, F.U.; Ibrahim, M.; Barikani, M.; Mohammadi, M.; Sobhani, H.; Mohammadi, A.; Farrukh, M.A. Processing Methods of Bionanocomposites. In Bionanocomposites: Green Synthesis and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 87–104. ISBN 9780128167519. [Google Scholar]
- Badia, J.D.; Gil-Castell, Ó.; Teruel-Juanes, R.; Ribes-Greus, A. Recycling of Polylactide. Ref. Modul. Mater. Sci. Mater. Eng. 2019, 2, 282–295. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material Valorisation of Amorphous Polylactide. Influence of Thermo-Mechanical Degradation on the Morphology, Segmental Dynamics, Thermal and Mechanical Performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Badia, J.D.; Monreal, L.; Sáenz de Juano-Arbona, V.; Ribes-Greus, A.; Monreal-Mengual, L.; Sáenz de Juano, V.; Ribes-Greus, A. Dielectric Spectroscopy of Reprocessed Polylactide. Polym. Degrad. Stab. 2014, 107, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Ribes-Greus, A. Mechanical Recycling of Polylactide, Upgrading Trends and Combination of Valorization Techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Gil-Castell, O.; Badia, J.D.; Ribes-Greus, A. Suitability of Blends from Virgin and Reprocessed Polylactide: Performance and Energy Valorization Kinetics. J. Renew. Mater. 2018, 6, 370–382. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ingles-Mascaros, S.; Teruel-Juanes, R.; Serra, A.; Ribes-Greus, A. Polylactide-Based Self-Reinforced Composites Biodegradation: Individual and Combined Influence of Temperature, Water and Compost. Polym. Degrad. Stab. 2018, 158, 40–51. [Google Scholar] [CrossRef]
- Dreier, J.; Brütting, C.; Ruckdäschel, H.; Altstädt, V.; Bonten, C. Investigation of the Thermal and Hydrolytic Degradation of Polylactide during Autoclave Foaming. Polymers 2021, 13, 2624. [Google Scholar] [CrossRef]
- Cosate de Andrade, M.F.; Fonseca, G.; Morales, A.R.; Mei, L.H.I. Mechanical Recycling Simulation of Polylactide Using a Chain Extender. Adv. Polym. Technol. 2018, 37, 2053–2060. [Google Scholar] [CrossRef]
- Benvenuta-Tapia, J.J.; Vivaldo-Lima, E. Reduction of Molar Mass Loss and Enhancement of Thermal and Rheological Properties of Recycled Poly(Lactic Acid) by Using Chain Extenders Obtained from RAFT Chemistry. React. Funct. Polym. 2020, 153, 104628. [Google Scholar] [CrossRef]
- Kfoury, G.; Hassouna, F.; Raquez, J.M.; Toniazzo, V.; Ruch, D.; Dubois, P. Tunable and Durable Toughening of Polylactide Materials Via Reactive Extrusion. Macromol. Mater. Eng. 2014, 299, 583–595. [Google Scholar] [CrossRef]
- Scaffaro, R.; Morreale, M.; Mirabella, F.; La Mantia, F.P. Preparation and Recycling of Plasticized PLA. Macromol. Mater. Eng. 2011, 296, 141–150. [Google Scholar] [CrossRef]
- Pascual-Jose, B.; Badia, J.D.; Múgica, A.; Addiego, F.; Müller, A.J.; Ribes-Greus, A. Analysis of Plasticization and Reprocessing Effects on the Segmental Cooperativity of Polylactide by Dielectric Thermal Spectroscopy. Polymer 2021, 223, 123701. [Google Scholar] [CrossRef]
- Brüster, B.; Addiego, F.; Hassouna, F.; Ruch, D.; Raquez, J.M.; Dubois, P. Thermo-Mechanical Degradation of Plasticized Poly(Lactide) after Multiple Reprocessing to Simulate Recycling: Multi-Scale Analysis and Underlying Mechanisms. Polym. Degrad. Stab. 2016, 131, 132–144. [Google Scholar] [CrossRef]
- Brüster, B.; Montesinos, A.; Reumaux, P.; Pérez-Camargo, R.A.; Mugica, A.; Zubitur, M.; Müller, A.J.; Dubois, P.; Addiego, F. Crystallization Kinetics of Polylactide: Reactive Plasticization and Reprocessing Effects. Polym. Degrad. Stab. 2018, 148, 56–66. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Ortega, E.; Solvoll, A.M.; Lorenzo, V.; de la Orden, M.U.; Martínez Urreaga, J. Effects of Aging and Different Mechanical Recycling Processes on the Structure and Properties of Poly(Lactic Acid)-Clay Nanocomposites. J. Polym. Env. 2018, 26, 2142–2152. [Google Scholar] [CrossRef] [Green Version]
- Botta, L.; Scaffaro, R.; Sutera, F.; Mistretta, M. Reprocessing of PLA/Graphene Nanoplatelets Nanocomposites. Polymers 2017, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Sutera, F.; Mistretta, M.C.; Botta, L.; La Mantia, F.P. Structure-Properties Relationships in Melt Reprocessed PLA/Hydrotalcites Nanocomposites. Express. Polym. Lett. 2017, 11, 555–564. [Google Scholar] [CrossRef]
- Tesfaye, M.; Patwa, R.; Kommadath, R.; Kotecha, P.; Katiyar, V. Silk Nanocrystals Stabilized Melt Extruded Poly (Lactic Acid) Nanocomposite Films: Effect of Recycling on Thermal Degradation Kinetics and Optimization Studies. Thermochim. Acta 2016, 643, 41–52. [Google Scholar] [CrossRef]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally Recyclable Polylactic Acid/Cellulose Nanocrystal Films through Reactive Extrusion Process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Peinado, V.; Castell, P.; García, L.; Fernández, A. Effect of Extrusion on the Mechanical and Rheological Properties of a Reinforced Poly(Lactic Acid): Reprocessing and Recycling of Biobased Materials. Materials 2015, 8, 7106–7117. [Google Scholar] [CrossRef]
- Sharif, A.; Mondal, S.; Hoque, M.E. Polylactic Acid (PLA)-Based Nanocomposites: Processing and Properties. In Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 233–254. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Dedieu, I.; Peyron, S.; Gontard, N.; Aouf, C. The Thermo-Mechanical Recyclability Potential of Biodegradable Biopolyesters: Perspectives and Limits for Food Packaging Application. Polym. Test. 2022, 111, 107620. [Google Scholar] [CrossRef]
- Badia, J.D.; Gil-Castell, O.; Ribes-Greus, A. Long-Term Properties and End-of-Life of Polymers from Renewable Resources. Polym. Degrad. Stab. 2017, 137, 35–57. [Google Scholar] [CrossRef]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of Bioplastics: Routes and Benefits. J. Polym. Environ. 2020, 28, 2551–2571. [Google Scholar] [CrossRef]
- Filho, G.R.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.L.; Messadeq, Y. Synthesis and Characterization of Cellulose Acetate Produced from Recycled Newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Betancourt, F.; Mendonça, R.T.; Chinga-Carrasco, G.; Pereira, M. Relationship between Rheological and Morphological Characteristics of Cellulose Nanofibrils in Dilute Dispersions. Carbohydr. Polym. 2020, 230, 115588. [Google Scholar] [CrossRef] [PubMed]
- Yasim-Anuar, T.A.T.; Ariffin, H.; Norrrahim, M.N.F.; Hassan, M.A.; Andou, Y.; Tsukegi, T.; Nishida, H. Well-Dispersed Cellulose Nanofiber in Low Density Polyethylene Nanocomposite by Liquid-Assisted Extrusion. Polymers 2020, 12, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and Characterization of Modified Cellulose Nanofibers Reinforced Polylactic Acid Nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2014; Volume 8.
- Lauritzen, J.I.; Hoffman, J.D. Formation of Polymer Crystals with Folded Chains from Dilute Solution. J. Chem. Phys. 1959, 31, 1680–1681. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I., Jr. Crystallization of Bulk Polymers With Chain Folding: Theory of Growth of Lamellar Spherulites. J. Res. Natl. Bur. Stand.-A. Phys. Chem. 1961, 65, 1961. [Google Scholar] [CrossRef]
- Vasanthakumari, R.; Pennings, A.J. Crystallization Kinetics of Poly(l-Lactic Acid). Polymers 1983, 24, 175–178. [Google Scholar] [CrossRef]
- ISO 62:2008; Plastics—Determination of Water Absorption. International Organisation for Standarisation (ISO): Geneva, Switzerland, 2008.
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018.
- Gil-Castell, O.; Andres-Puche, R.; Dominguez, E.; Verdejo, E.; Monreal, L.; Ribes-Greus, A. Influence of Substrate and Temperature on the Biodegradation of Polyester-Based Materials: Polylactide and Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) as Model Cases. Polym. Degrad. Stab. 2020, 180, 109288. [Google Scholar] [CrossRef]
- ASTM D6400-04; Standard Specification for Compostable Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2004.
- ASTM D6868-03; Standard Specification for Biodegradable Plastics Used as Coatings on Paper and Other Compostbale Substrates. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2003.
- ASTM D5338-11; Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials under Controlled Composting Conditions. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2011.
- ASTM D7081-05; Standard Specification for Non-Floating Biodegradable Plastics in the Marine Environment. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2005.
- DIN EN 13432:2000-12; Packaging Requirements for Packaging Recoverable through Composting and Biodegradation Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. Deutsches Institut für Normung (DIN): Berlin, Germany, 2010.
- ISO 14855-2:2018; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions. International Organisation for Standarisation (ISO): Geneva, Switzerland, 2018.
- Neogi, P. Diffusion in Polymers; Marcel Dekker: New York, NY, USA, 1996; Volume 32, ISBN 082479530X. [Google Scholar]
- Le Marec, P.E.; Ferry, L.; Quantin, J.-C.; Bénézet, J.-C.; Bonfils, F.; Guilbert, S.; Bergeret, A. Influence of Melt Processing Conditions on Poly(Lactic Acid) Degradation: Molar Mass Distribution and Crystallization. Polym. Degrad. Stab. 2014, 110, 353–363. [Google Scholar] [CrossRef]
- Dragostin, O.; Profire, L. Molecular Weight of Polymers Used in Biomedical Applications, 1st ed.; Tanzi, S.F.M.C., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007372. [Google Scholar]
- Arrieta, M.P.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M.M. PLA-PHB/Cellulose Based Films: Mechanical, Barrier and Disintegration Properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Arrieta, M.P.; Gaspar, G.; de la Orden, M.U.; Urreaga, J.M. Effect of Iignocellulosic Nanoparticles Extracted from Yerba Mate (Ilex Paraguariensis) on the Structural, Thermal, Optical and Barrier Properties of Mechanically Recycled Poly(Lactic Acid). Polymers 2020, 12, 1690. [Google Scholar] [CrossRef]
- Way, C.; Dean, K.; Wu, D.Y.; Palombo, E. Biodegradation of Sequentially Surface Treated Lignocellulose Reinforced Polylactic Acid Composites: Carbon Dioxide Evolution and Morphology. Polym. Degrad. Stab. 2012, 97, 430–438. [Google Scholar] [CrossRef]
- Arias, V.; Höglund, A.; Odelius, K.; Albertsson, A.C. Polylactides with “Green” Plasticizers: Influence of Isomer Composition. J. Appl. Polym. Sci. 2013, 130, 2962–2970. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of Linear Poly(Lactic Acid)/Polyethylene Glycol Blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Dobircau, L.; Delpouve, N.; Herbinet, R.; Domenek, S.; Le Pluart, L.; Delbreilh, L.; Ducruet, V.; Dargent, E. Molecular Mobility and Physical Ageing of Plasticized Poly(Lactide). Polym. Eng. Sci. 2015, 55, 858–865. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; MacNaughtan, B.; Hill, S.; Mitchell, J.; Enrione, J. Reduction of Enthalpy Relaxation in Gelatine Films by Addition of Polyols. Int. J. Biol. Macromol. 2018, 109, 634–638. [Google Scholar] [CrossRef]
- Habibi, Y.; Dufresne, A. Highly Filled Bionanocomposites from Functionalized Polysaccharide Nanocrystals. Biomacromolecules 2008, 9, 1974–1980. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel Nanocomposites Based on Fatty Acid Modified Cellulose Nanofibers/Poly(Lactic Acid): Morphological and Physical Properties. Food. Packag. Shelf. Life. 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Fujisawa, S.; Saito, T.; Kimura, S.; Iwata, T.; Isogai, A. Surface Engineering of Ultrafine Cellulose Nanofibrils toward Polymer Nanocomposite Materials. Biomacromolecules 2013, 14, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Flame-Retardancy Properties of Intumescent Ammonium Poly(Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Badia, J.D.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A.; Martínez-Felipe, A.; Ribes-Greus, A. Reprocessed Polylactide: Studies of Thermo-Oxidative Decomposition. Bioresour. Technol. 2012, 114, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.K.; Mohanty, S.; Nayak, S.K. Effect of PEG on PLA/PEG Blend and Its Nanocomposites: A Study of Thermo-Mechanical and Morphological Characterization. Polym. Compos. 2014, 35, 283–293. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Ismail, H.; Chow, W.S. Thermal and Morphological Properties of Poly (Lactic Acid)/Nanocellulose Nanocomposites. Procedia. Chem. 2016, 19, 788–794. [Google Scholar] [CrossRef] [Green Version]
- CQE. Academy Design Verification & Validation for Quality Engineers. Available online: http://www.cqeacademy.com/cqe-body-of-knowledge/product-process-design/ (accessed on 1 July 2022).
- Park, S.H.; Lee, S.G.; Kim, S.H. Isothermal Crystallization Behavior and Mechanical Properties of Polylactide/Carbon Nanotube Nanocomposites. Compos. Part. A Appl. Sci. Manuf. 2013, 46, 11–18. [Google Scholar] [CrossRef]
- Qu, P.; Gao, Y.; Wu, G.F.; Zhang, L.P. Nanocomposites of Poly(Lactic Acid) Reinforced with Cellulose Nanofibrils. Bioresources 2010, 5, 1811–1823. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M.; Ismail, H. Tensile and Morphological Properties of Nanocrystalline Cellulose and Nanofibrillated Cellulose Reinforced PLA Bionanocomposites: A Review. Polym. Eng. Sci. 2021, 61, 22–38. [Google Scholar] [CrossRef]
- Berthé, V.; Ferry, L.; Bénézet, J.C.; Bergeret, A. Ageing of Different Biodegradable Polyesters Blends Mechanical and Hygrothermal Behavior. Polym. Degrad. Stab. 2010, 95, 262–269. [Google Scholar] [CrossRef]
- Badia, J.D.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A. Hygrothermal Ageing of Reprocessed Polylactide. Polym. Degrad. Stab. 2012, 97, 1881–1890. [Google Scholar] [CrossRef] [Green Version]
- Gil-Castell, O.; Badia, J.D.; Kittikorn, T.; Strömberg, E.; Ek, M.; Karlsson, S.; Ribes-Greus, A.; Strömberg, E.; Ek, M.; Karlsson, S.; et al. Impact of Hydrothermal Ageing on the Thermal Stability, Morphology and Viscoelastic Performance of PLA/Sisal Biocomposites. Polym. Degrad. Stab. 2016, 132, 87–96. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S. Characterization of Hydrolytic Degradation of Polylactic Acid/Rice Hulls Composites in Water at Different Temperatures. Express. Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Le Duigou, A.; Davies, P.; Baley, C. Seawater Ageing of Flax/Poly(Lactic Acid) Biocomposites. Polym. Degrad. Stab. 2009, 94, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Yew, G.H.; Mohd Yusof, A.M.; Mohd Ishak, Z.A.; Ishiaku, U.S. Water Absorption and Enzymatic Degradation of Poly(Lactic Acid)/Rice Starch Composites. Polym. Degrad. Stab. 2005, 90, 488–500. [Google Scholar] [CrossRef]
- Hrib, J.; Sirc, J.; Hobzova, R.; Hampejsova, Z.; Bosakova, Z.; Munzarova, M.; Michalek, J. Nanofibers for Drug Delivery—Incorporation and Release of Model Molecules, Influence of Molecular Weight and Polymer Structure. Beilstein. J. Nanotechnol. 2015, 6, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The Effect of Molecular Weight and Content of PEG on In Vitro Drug Release of Electrospun Curcumin Loaded PLA/PEG Nanofibers. J. Drug. Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wang, C.; Abdalkarim, S.Y.H. Cellulose Nanocrystals/Polyethylene Glycol as Bifunctional Reinforcing/Compatibilizing Agents in Poly(Lactic Acid) Nanofibers for Controlling Long-Term in Vitro Drug Release. Cellulose 2017, 24, 4461–4477. [Google Scholar] [CrossRef]
- Leu, Y.Y.; Chow, W.S. Kinetics of Water Absorption and Thermal Properties of Poly(Lactic Acid)/Organomontmorillonite/Poly(Ethylene Glycol) Nanocomposites. J. Vinyl. Addit. Technol. 2011, 17, 40–47. [Google Scholar] [CrossRef]
- Norazlina, H.; Hadi, A.A.; Qurni, A.U.; Amri, M.; Mashelmie, S.; Kamal, Y. Effects of Multi-Walled Carbon Nanotubes (MWCNTs) on the Degradation Behavior of Plasticized PLA Nanocomposites. Polym. Bull. 2019, 76, 1453–1469. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chen, D.Z.; Yue, T.M.; Chan, K.C.; Tsui, C.P.; Yu, P.H.F. Water Absorption and Solubility of PHBHV/HA Nanocomposites. Compos. Sci. Technol. 2008, 68, 1927–1934. [Google Scholar] [CrossRef]
- Joseph, P.V.; Rabello, M.S.; Mattoso, L.H.C.; Joseph, K.; Thomas, S. Environmental Effects on the Degradation Behaviour of Sisal Fibre Reinforced Polypropylene Composites. Compos. Sci. Technol. 2002, 62, 1357–1372. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A Review on the Degradability of Polymeric Composites Based on Natural Fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of Water Absorption on the Mechanical Properties of Hemp Fibre Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal Ageing of Polylactide/Sisal Biocomposites. Studies of Water Absorption Behaviour and Physico-Chemical Performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]









| Designation | PLA (wt%) | PEG-400 (wt%) | PEG-1500 (wt%) | NFC (wt%) |
|---|---|---|---|---|
| PLA | 100.00 | - | - | - |
| PLA-400 | 92.50 | 7.50 | - | - |
| PLA-400-NFC1 | 91.59 | 7.42 | - | 0.99 |
| PLA-400-NFC3 | 89.81 | 7.28 | - | 2.91 |
| PLA-400-NFC5 | 88.10 | 7.14 | - | 4.76 |
| PLA-1500 | 92.50 | - | 7.50 | - |
| PLA-1500-NFC1 | 91.59 | - | 7.42 | 0.99 |
| PLA-1500-NFC3 | 89.81 | - | 7.28 | 2.91 |
| PLA-1500-NFC5 | 88.10 | - | 7.14 | 4.76 |
| To (°C) | Tp (°C) | To (°C) | Tp (°C) | ||||
|---|---|---|---|---|---|---|---|
| PLA | P | 332.1 (±0.6) | 355.6 (±1.7) | ||||
| D | 333.0 (±2.1) | 354.3 (±0.3) | |||||
| F | 332.4 (±4.2) | 355.9 (±1.8) | |||||
| PLA-400 | C | 330.5 (±1.1) | 357.0 (±2.7) | PLA-1500 | C | 333.0 (±1.3) | 358.4 (±1.8) |
| D | 333.1 (±1.4) | 355.0 (±2.4) | D | 324.7 (±0.1) | 353.5 (±2.6) | ||
| F | 326.3 (±2.0) | 356.2 (±0.5) | F | 326.3 (±0.2) | 351.0 (±1.7) | ||
| PLA-400-NFC1 | C | 334.8 (±2.7) | 359.9 (±0.6) | PLA-1500-NFC1 | C | 333.9 (±3.4) | 359.6 (±1.1) |
| D | 334.9 (±1.5) | 359.5 (±1.3) | D | 331.2 (±1.2) | 356.2 (±0.3) | ||
| F | 323.5 (±0.7) | 355.5 (±0.3) | F | 327.4 (±2.0) | 356.0 (±1.5) | ||
| PLA-400-NFC3 | C | 337.6 (±1.2) | 359.4 (±1.4) | PLA-1500-NFC3 | C | 334.6 (±1.5) | 358.5 (±0.5) |
| D | 330.8 (±2.6) | 357.2 (±0.8) | D | 330.0 (±2.2) | 352.2 (±3.0) | ||
| F | 317.9 (±1.9) | 349.6 (±2.9) | F | 326.3 (±1.9) | 351.9 (±2.7) | ||
| PLA-400-NFC5 | C | 332.8 (±0.4) | 357.9 (±0.4) | PLA-1500-NFC5 | C | 331.3 (±0.3) | 356.2 (±0.2) |
| D | 329.7 (±1.7) | 354.5 (±1.1) | D | 329.9 (±0.6) | 352.2 (±1.3) | ||
| F | 319.3 (±1.6) | 350.1 (±1.3) | F | 330.2 (±1.2) | 351.2 (±0.8) |
| Xc0 (%) | Xc frac (%) | |
|---|---|---|
| PLA | 15.3 (±2.6) | 15.0 (±4.0) |
| PLA-400 | 18.5 (±0.8) | 42.0 (±2.2) |
| PLA-400-NFC1 | 19.1 (±0.1) | 39.8 (±0.2) |
| PLA-400-NFC3 | 15.9 (±0.5) | 37.0 (±4.9) |
| PLA-400-NFC5 | 16.6 (±1.1) | 32.6 (±3.8) |
| PLA-1500 | 20.8 (±1.4) | 40.8 (±0.6) |
| PLA-1500-NFC1 | 25.7 (±2.6) | 42.6 (±0.8) |
| PLA-1500-NFC3 | 20.5 (±0.2) | 36.4 (±3.5) |
| PLA-1500-NFC5 | 20.6 (±0.3) | 34.2 (±3.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Castell, O.; Wolf, M.H.; Cea, J.; Carrasco, J.C.; Giacinti Baschetti, M.; Ribes-Greus, A. Structure–Properties Relationship of Reprocessed Bionanocomposites of Plasticized Polylactide Reinforced with Nanofibrillated Cellulose. Appl. Sci. 2022, 12, 12821. https://doi.org/10.3390/app122412821
Gil-Castell O, Wolf MH, Cea J, Carrasco JC, Giacinti Baschetti M, Ribes-Greus A. Structure–Properties Relationship of Reprocessed Bionanocomposites of Plasticized Polylactide Reinforced with Nanofibrillated Cellulose. Applied Sciences. 2022; 12(24):12821. https://doi.org/10.3390/app122412821
Chicago/Turabian StyleGil-Castell, O., M. H. Wolf, J. Cea, J. C. Carrasco, M. Giacinti Baschetti, and A. Ribes-Greus. 2022. "Structure–Properties Relationship of Reprocessed Bionanocomposites of Plasticized Polylactide Reinforced with Nanofibrillated Cellulose" Applied Sciences 12, no. 24: 12821. https://doi.org/10.3390/app122412821
APA StyleGil-Castell, O., Wolf, M. H., Cea, J., Carrasco, J. C., Giacinti Baschetti, M., & Ribes-Greus, A. (2022). Structure–Properties Relationship of Reprocessed Bionanocomposites of Plasticized Polylactide Reinforced with Nanofibrillated Cellulose. Applied Sciences, 12(24), 12821. https://doi.org/10.3390/app122412821








