Lingual Lichenoid Lesion Due to Dental Amalgam Fillings: Case Report and Clinical Considerations
Abstract
1. Introduction
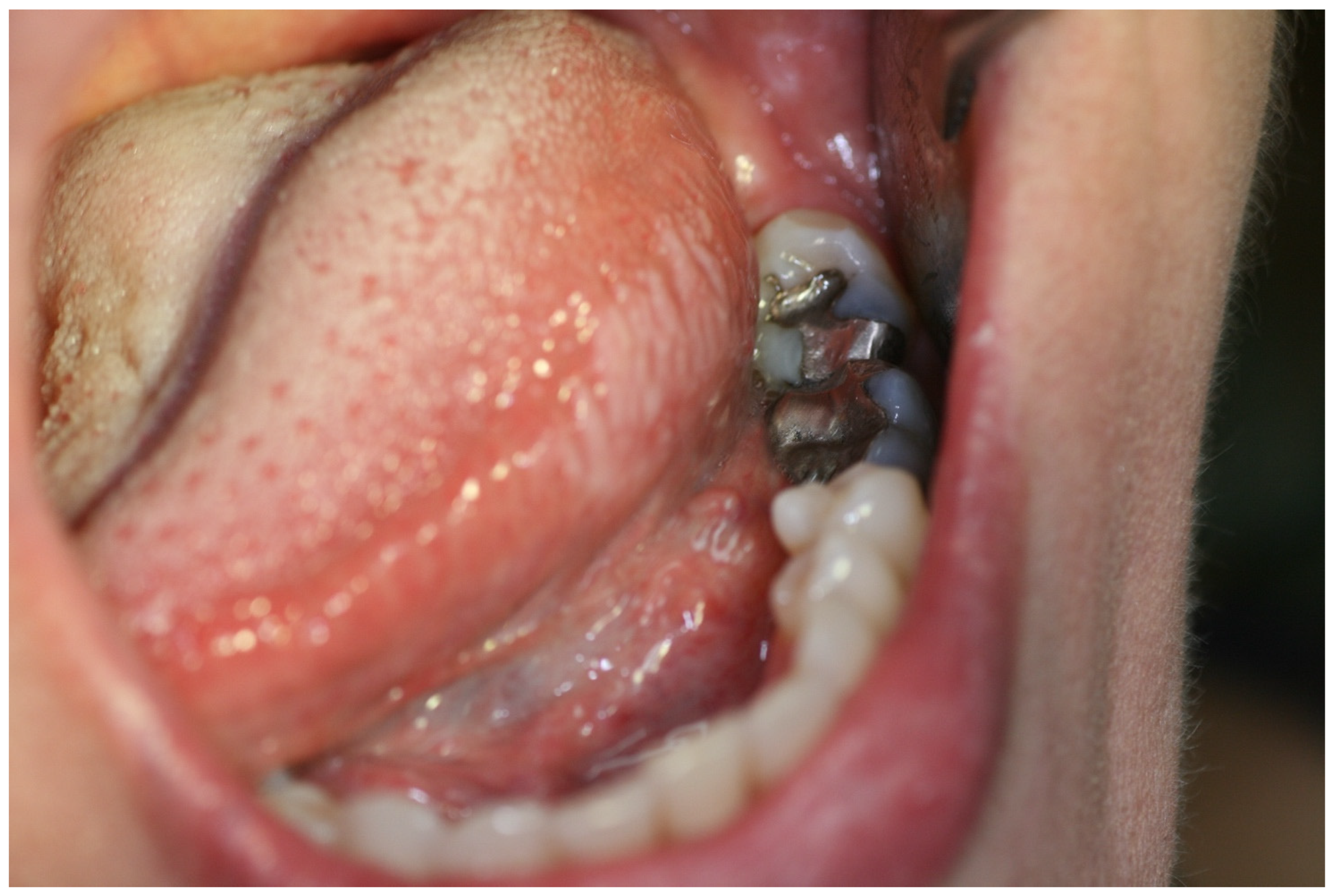
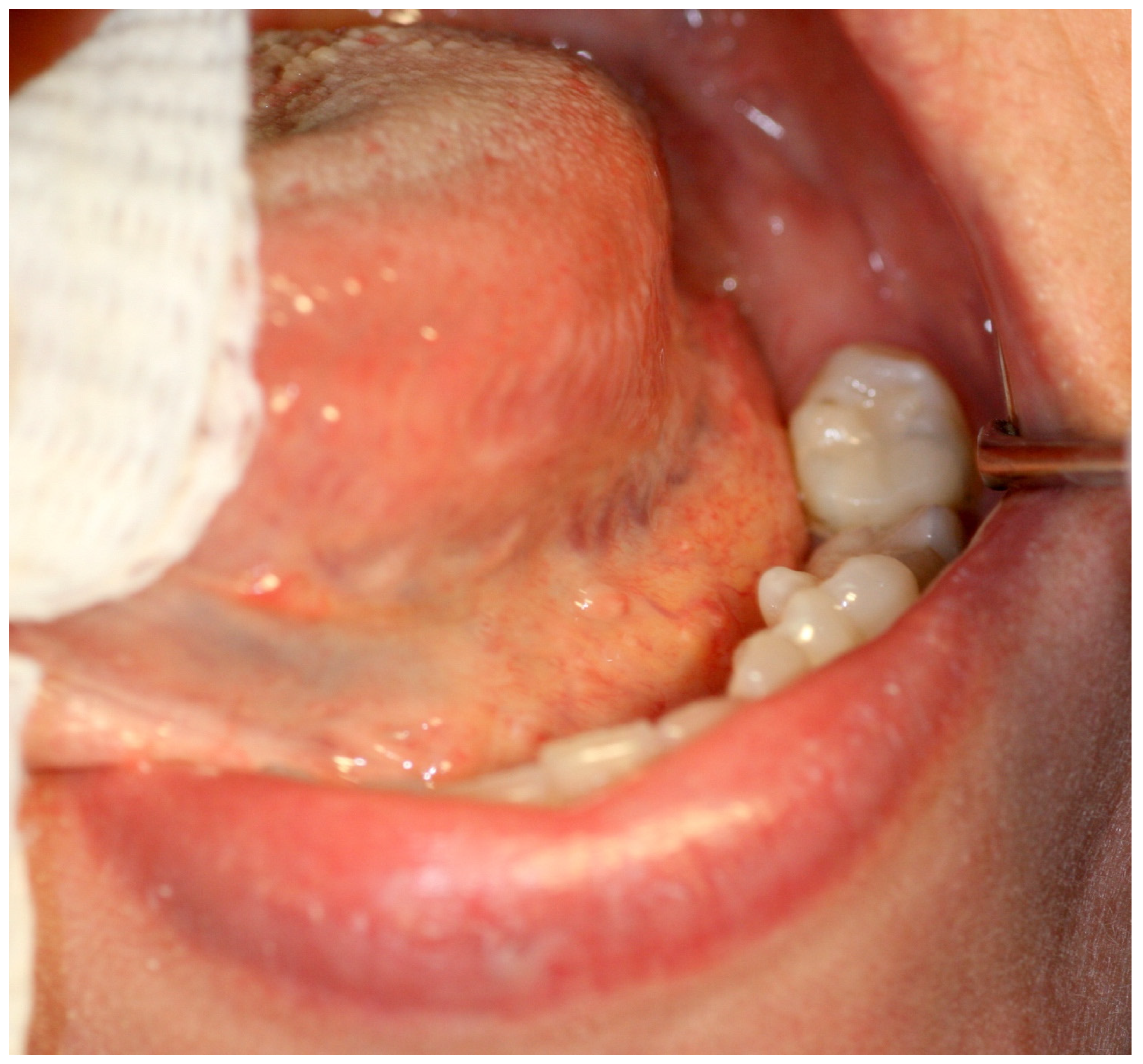
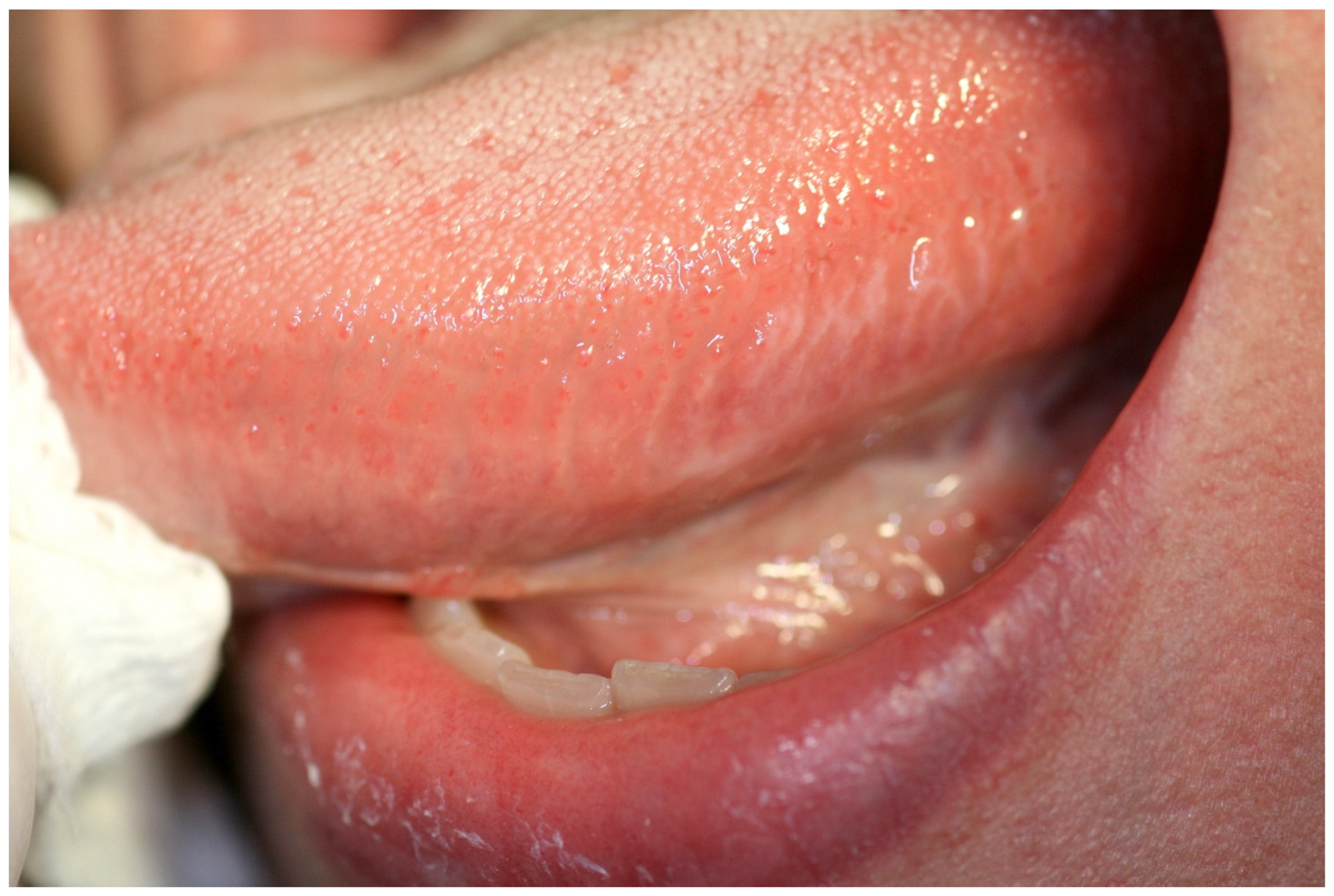
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kamath, V.V.; Setlur, K.; Yerlagudda, K. Oral lichenoid lesions—A review and update. Indian. J. Dermatol. 2015, 60, 102. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, I.; Schifter, M.; Lockhart, P.B.; Wray, D.; Brennan, M.; Migliorati, C.A.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E.; et al. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 103, S25.e1–S25.e12. [Google Scholar] [CrossRef] [PubMed]
- Baccaglini, L.; Brennan, M.T.; Lockhart, P.B.; Patton, L.L. World Workshop on Oral Medicine IV: Process and methodology for systematic review and developing management recommendations. Reference manual for management recommendations writing committees. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 103, S3.e1–S3.e19. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Warfvinge, G. The histopathology of oral mucosal lesions associated with amalgam or porcelain-fused-to-metal restorations. Oral. Dis. 1995, 1, 152–158. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Greenspan, D.; Greenspan, J.S.; Conant, M.; Petersen, V.; Silverman, S.; de Souza, Y. Oral "hairy" leucoplakia in male homosexuals: Evidence of association with both papillomavirus and a herpes-group virus. Lancet 1984, 2, 831–834. [Google Scholar] [CrossRef]
- Bravo, I.M.; Correnti, M.; Escalona, L.; Perrone, M.; Brito, A.; Tovar, V.; Rivera, H. Prevalence of oral lesions in HIV patients related to CD4 cell count and viral load in a Venezuelan population. Med. Oral. Patol. Oral. Cir. Bucal. 2006, 11, E33-9. [Google Scholar]
- Pastore, L.; De Benedittis, M.; Petruzzi, M.; Fiore, J.R.; Serpico, R. Efficacy of famciclovir in the treatment of oral hairy leukoplakia. Br. J. Dermatol. 2006, 154, 378–379. [Google Scholar] [CrossRef]
- Greenspan, J.S.; Greenspan, D.; Webster-Cyriaque, J. Hairy leukoplakia; lessons learned: 30-plus years. Oral. Dis. 2016, 22 (Suppl. 1), 120–127. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. White, red, and mixed lesions of oral mucosa: A clinicopathologic approach to diagnosis. Periodontol. 2000. 2019, 80, 89–104. [Google Scholar] [CrossRef]
- Finne, K.; Göransson, K.; Winckler, L. Oral lichen planus and contact allergy to mercury. Int. J. Oral. Surg. 1982, 11, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mallishery, S.; Bajaj, N.; Banga, K.; Mehra, A.; Desai, R. Low Prevalence of Amalgam-Associated Lichenoid Lesions in the Oral Cavity: A Prospective Study. Cureus. 2022, 14, e22696. [Google Scholar] [CrossRef] [PubMed]
- Dunsche, A.; Kästel, I.; Terheyden, H.; Springer, I.N.; Christophers, E.; Brasch, J. Oral lichenoid reactions associated with amalgam: Improvement after amalgam removal. Br J Dermatol. 2003, 148, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Bullón-Fernández, P. Lichenoid reaction associated to amalgam restoration. Med. Oral. Patol. Oral. Cir. Bucal. 2004, 9, 421–424. [Google Scholar]
- Suter, V.G.; Warnakulasuriya, S. The role of patch testing in the management of oral lichenoid reactions. J. Oral. Pathol. Med. 2016, 45, 48–57. [Google Scholar] [CrossRef]
- Rahat, S.; Kashetsky, N.; Bagit, A.; Sachdeva, M.; Lytvyn, Y.; Mufti, A.; Maibach, H.I.; Yeung, J. Can We Separate Oral Lichen Planus from Allergic Contact Dermatitis and Should We Patch Test? A Systematic Review of Chronic Oral Lichenoid Lesions. Dermatitis. 2021, 32, 144–150. [Google Scholar] [CrossRef]
- McParland, H.; Warnakulasuriya, S. Oral lichenoid contact lesions to mercury and dental amalgam—A review. J. Biomed. Biotechnol. 2012, 2012, 589569. [Google Scholar] [CrossRef]
- Lind, P.O.; Hurlen, B.; Strømme Koppang, H. Electrogalvanically-induced contact allergy of the oral mucosa. Report of a case. Int. J. Oral. Surg. 1984, 13, 339–345. [Google Scholar] [CrossRef]
- Vale, D.A.; Martins, F.M.; Silva, P.H.; Ortega, K.L. Retrospective analysis of the clinical behavior of oral hairy leukoplakia in 215 HIV-seropositive patients. Braz. Oral. Res. 2016, 30, e118. [Google Scholar] [CrossRef]
- Kanduc, D.; Serpico, R.; Lucchese, A.; Shoenfeld, Y. Correlating low-similarity peptide sequences and HIV B-cell epitopes. Autoimmun. Rev. 2008, 7, 291–296. [Google Scholar] [CrossRef]
- Alramadhan, S.A.; Bhattacharyya, I.; Cohen, D.M.; Islam, M.N. Oral Hairy Leukoplakia in Immunocompetent Patients Revisited with Literature Review. Head. Neck. Pathol. 2021, 15, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.B.; Lin, D. Morsicatio mucosae oris—A chronic oral frictional keratosis, not a leukoplakia. J Oral Maxillofac Surg. 2009, 67, 140–146. [Google Scholar] [CrossRef]
- Flores-Hidalgo, A.; Lim, S.O.; Curran, A.E.; Padilla, R.J.; Murrah, V. Considerations in the diagnosis of oral hairy leukoplakia-an institutional experience. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2018, 125, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Davari, P.; Rogers, R.S., 3rd. Contact stomatitis. Clin. Dermatol. 2017, 35, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://oralcancerfoundation.org/cdc/early-detection-diagnosis-staging/ (accessed on 4 April 2022).
- Netto, J.N.S.; Pires, F.R.; Costa, K.H.A.; Fischer, R.G. Clinical features of oral lichen planus and oral lichenoid lesions: An oral pathologist’s perspective. Braz. Dent. J. 2022, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, G.; Garola, F.; Piemonte, E.; Leonardi, N.; Panico, R.; Warnakulasuriya, S. Lichenoid proliferative leukoplakia, lichenoid lesions with evolution to proliferative leukoplakia or a continuum of the same precancerous condition? A revised hypothesis. J. Oral. Pathol. Med. 2021, 50, 129–135. [Google Scholar] [CrossRef]
- Larsson, A.; Warfvinge, G. Oral lichenoid contact reactions may occasionally transform into malignancy. Eur. J. Cancer. Prev. 2005, 14, 525–529. [Google Scholar] [CrossRef]
- Gabusi, A.; Rossi, R.; Gissi, D.B. Malignant transformation of a clinically and histologically healed lichenoid lesion after amalgam removal: A case report and literature review. J. Stomatol. Oral. Maxillofac. Surg. 2021, 122, 208–211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzi, M.; della Vella, F.; Campus, G.; Di Stasio, D.; Lauritano, D. Lingual Lichenoid Lesion Due to Dental Amalgam Fillings: Case Report and Clinical Considerations. Appl. Sci. 2022, 12, 12895. https://doi.org/10.3390/app122412895
Petruzzi M, della Vella F, Campus G, Di Stasio D, Lauritano D. Lingual Lichenoid Lesion Due to Dental Amalgam Fillings: Case Report and Clinical Considerations. Applied Sciences. 2022; 12(24):12895. https://doi.org/10.3390/app122412895
Chicago/Turabian StylePetruzzi, Massimo, Fedora della Vella, Guglielmo Campus, Dario Di Stasio, and Dorina Lauritano. 2022. "Lingual Lichenoid Lesion Due to Dental Amalgam Fillings: Case Report and Clinical Considerations" Applied Sciences 12, no. 24: 12895. https://doi.org/10.3390/app122412895
APA StylePetruzzi, M., della Vella, F., Campus, G., Di Stasio, D., & Lauritano, D. (2022). Lingual Lichenoid Lesion Due to Dental Amalgam Fillings: Case Report and Clinical Considerations. Applied Sciences, 12(24), 12895. https://doi.org/10.3390/app122412895









