Using Statistical Shape Models to Optimize TKA Implant Design
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of the SSM
2.1.1. Selection of Input Data
- Impact of pathology: arthritic versus healthy population.
- Impact of gender and ethnicity.
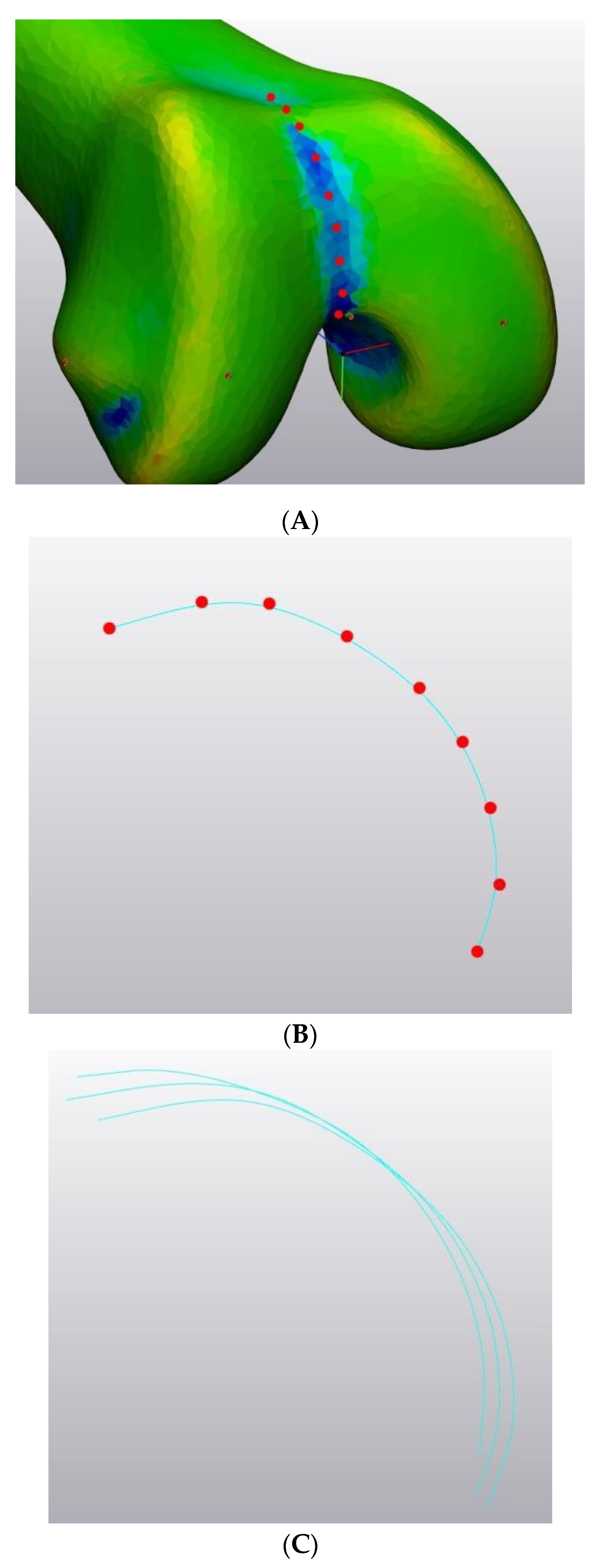
2.1.2. Generation of the SSM: Choice of the Reference System
2.2. Validation of the SSM
- The Vectorization Errors Test quantifies the error introduced by preprocessing each patient’s bone before it can be used for a SSM (all bones included in the SSM must have the same mesh).
- The Normality Test analyzes whether the population is normally distributed.
- The Compactness Test analyzes how well the modes of variation of the SSM capture the geometrical variation. If the SSM is very compact, the SSM can describe the geometrical variation observed in the input population in a few virtual modes.
- The Convergence Test analyzes the impact of adding additional patients. If convergence is achieved, additional scans no longer have an impact on the SSM. If convergence is not achieved, this test can be used to calculate the impact of adding new scans to enable the SSM to better capture the input population.
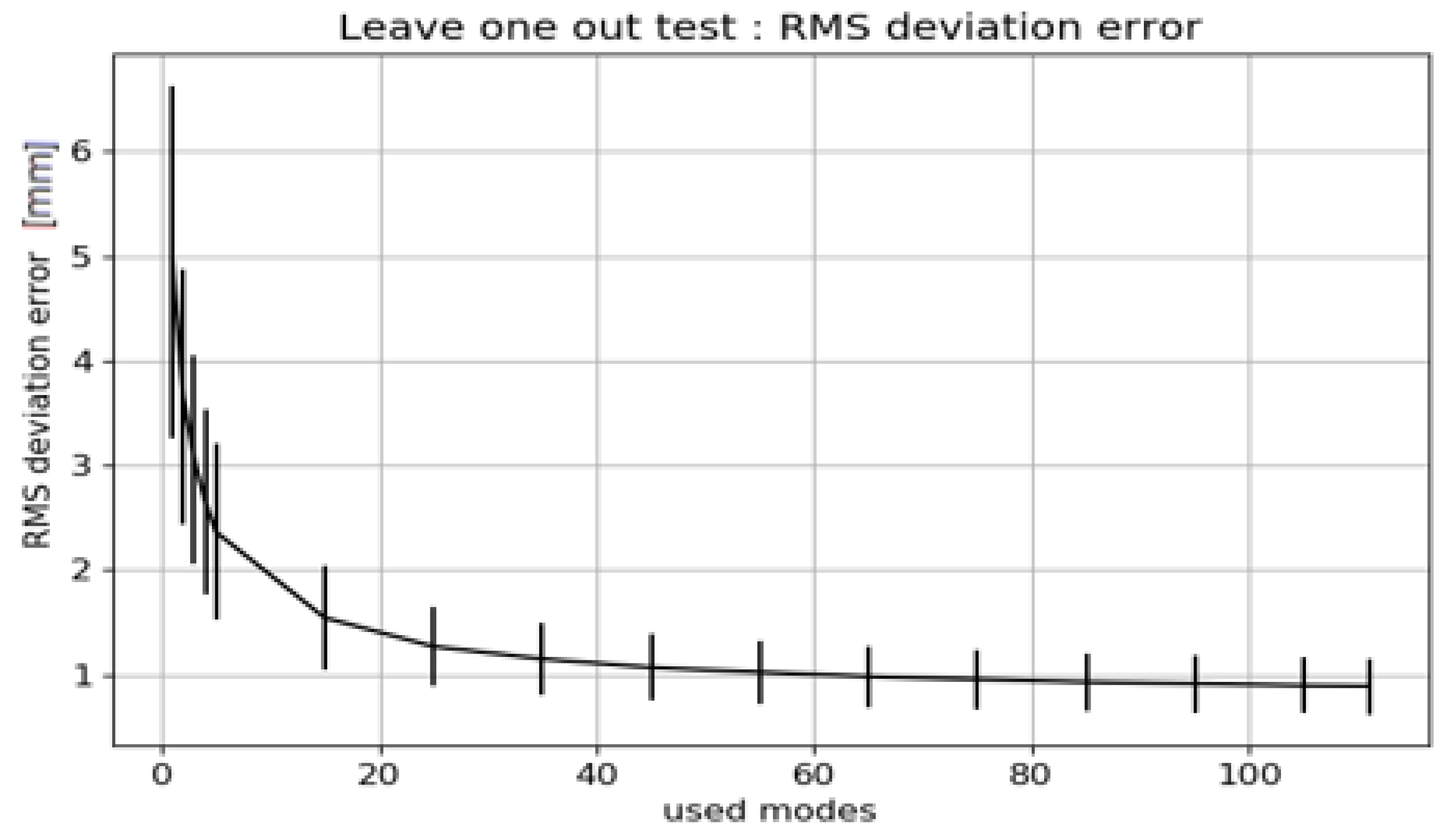
- The Leave-one-out Test analyzes how well the SSM describes patients who are not part of the input population. To perform this test multiple SSMs are generated, leaving out each input patient in turn. For example, with an input population of 112 patients, 112 different SSMs are built with 111 patients. Each of those 111 SSMs is built with an increasing number of modes. The average RMS deviation between the SSMs is computed.
2.3. Use of the SSM
2.3.1. Definition of a Representative Database of 3D Bone Models
2.3.2. Use of SSMs for Design Input
2.3.3. Use of SSMs for Design Validation
3. Results
3.1. Generation of the SSM
3.1.1. Selection of Input Data, Impact of Pathology
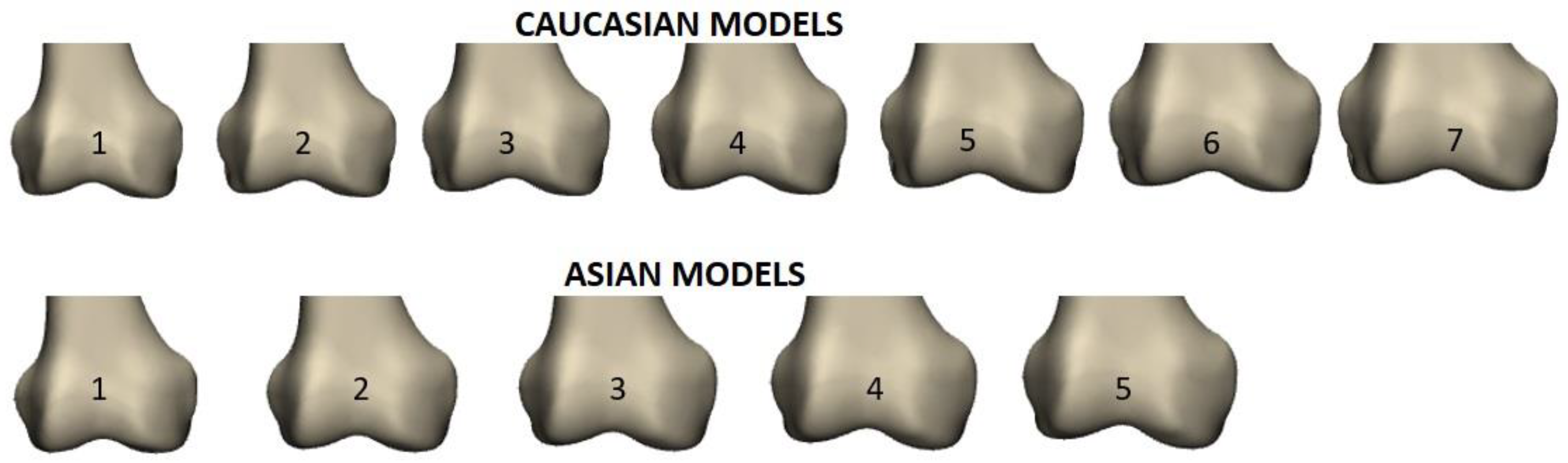
3.1.2. Selection of Input Data, Impact of Gender and Ethnicity
3.2. Generation of the SSM: Validation
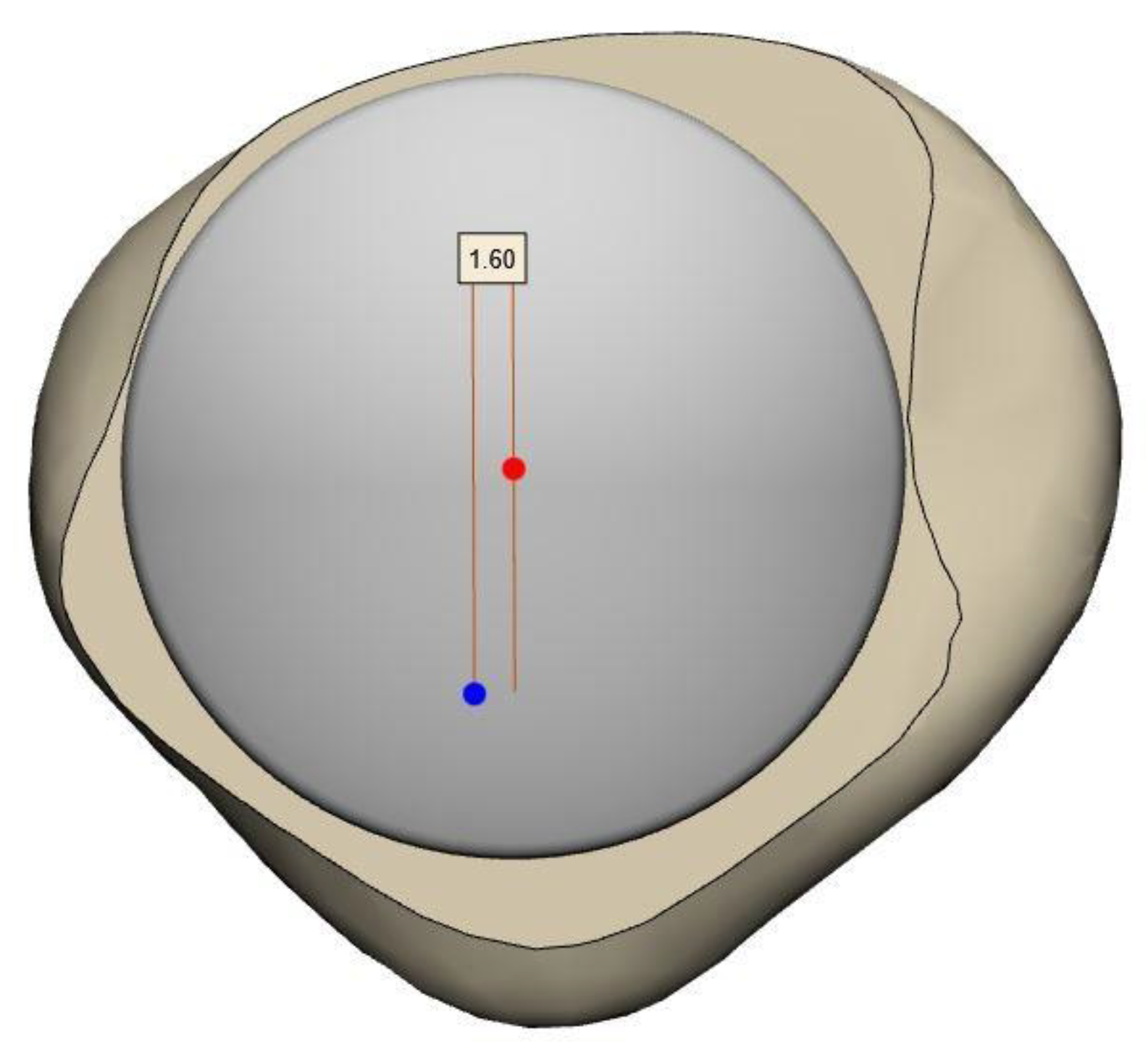
- For the SSMs used in this study 99% of the Vectorization Errors were less than 0.5 mm and the assumption of normally distributed populations was not rejected.
- Convergence Test: Results of the Convergence Test are exemplified for the Asian femur SSM. The effect of increasing the population size (to up to 112 patients) on the resulting average model is depicted Figure 3. Convergence is not achieved, as the mean RMS error does not reach zero before the populations begin to overlap (dotted line). However, with 80 patients (thus 24 overlapping scans), the error is less than 1.5 mm.
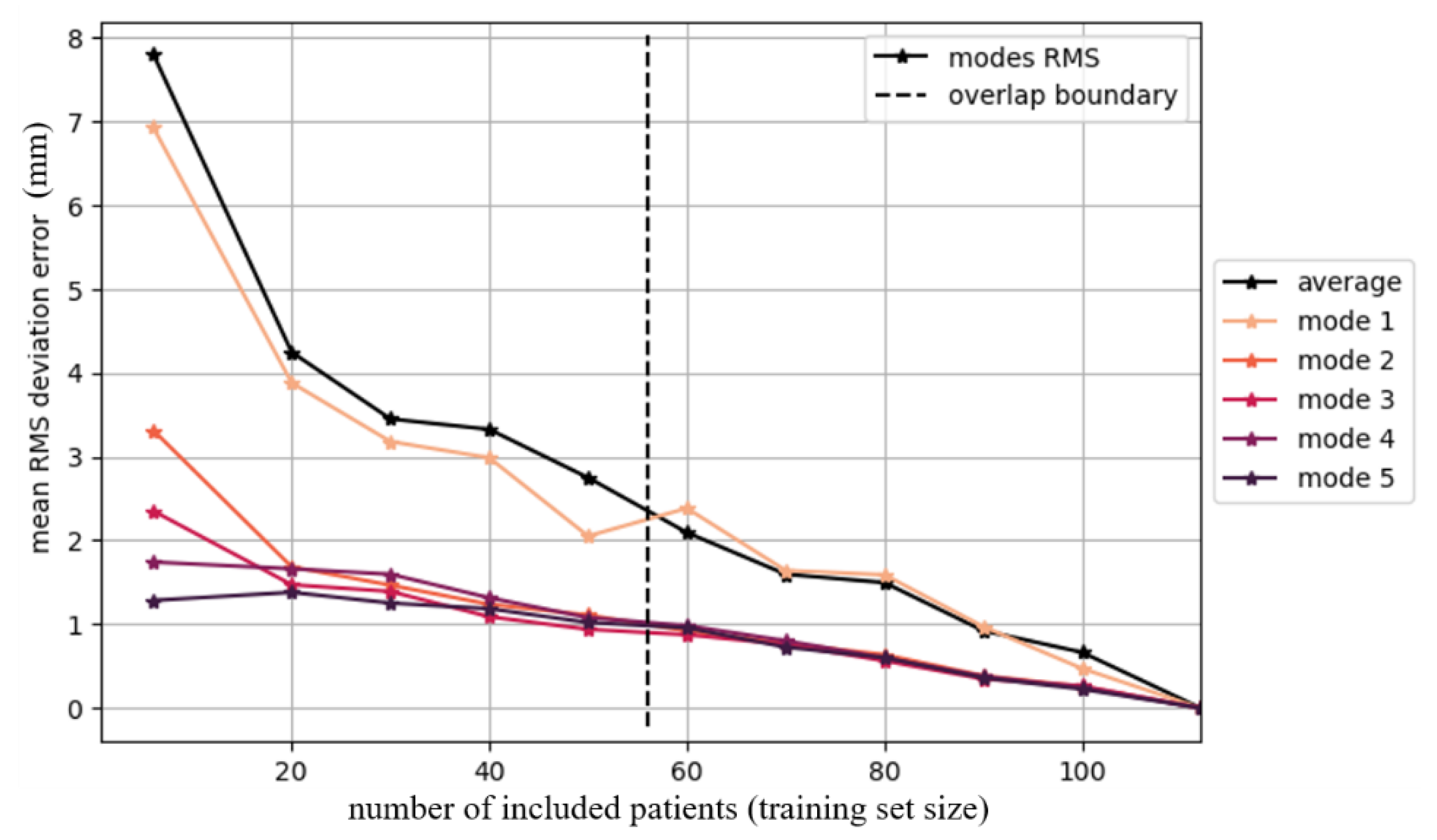
Leave-One-Out Analysis
3.3. Use of the SSM
3.3.1. Definition of a Representative Database of 3D Bone Models
3.3.2. Use of SSMs for Design Input
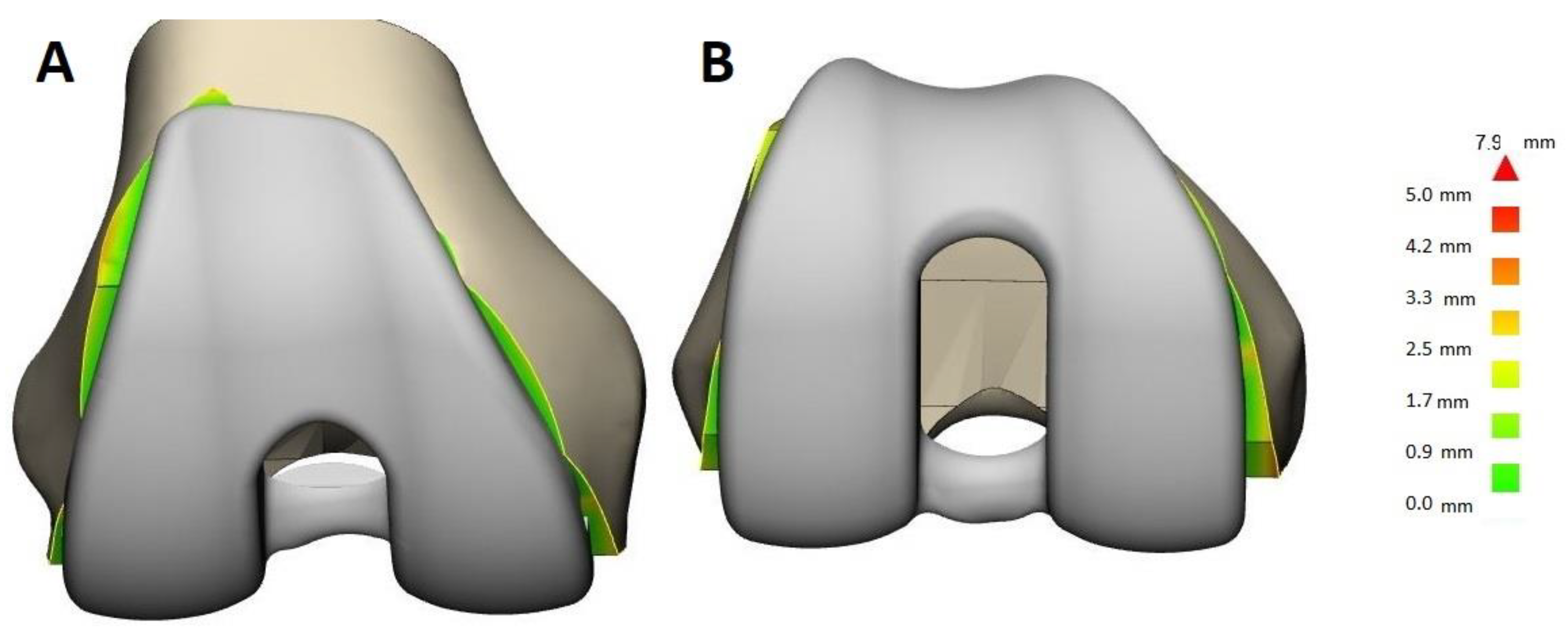
3.3.3. Use of SSMs for Design Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bonnin, M.P.; Schmidt, A.; Basiglini, L.; Bossard, N.; Dantony, E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2314–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, O.M.; Kinsey, T. Overhang of the femoral component in total knee arthroplasty: Risk factors and clinical consequences. J. Bone Jt. Surg. 2010, 92, 1115–1121. [Google Scholar] [CrossRef]
- Matz, J.; Lanting, B.A.; Howard, J.L. Understanding the patellofemoral joint in total knee arthroplasty. Can. J. Surg. 2019, 62, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.W.G.; Bloch, B.V.; James, P.J. Sagittal radius of curvature, trochlea design and ultracongruent insert in total knee arthroplasty. EFORT Open Rev. 2019, 4, 519–524. [Google Scholar] [CrossRef]
- Gu, S.; Kuriyama, S.; Nakamura, S.; Nishitani, K.; Ito, H.; Matsuda, S. Underhang of the tibial component increases tibial bone resorption after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1270–1279. [Google Scholar] [CrossRef]
- Bonnin, M.; Saffarini, M.; Shepherd, D.; Bossard, N.; Dantony, E. Oversizing the tibial component in TKAs: Incidence, consequences and risk factors. Knee Surg. Sports Traumatol. Arthrosc. 2015, 24, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Chaichankul, C.; Tanavalee, A.; Itiravivong, P. Anthropometric measurements of knee joints in Thai population: Correlation to the sizing of current knee prostheses. Knee 2011, 18, 5–10. [Google Scholar] [CrossRef]
- Cheng, F.B.; Ji, X.F.; Lai, Y.; Feng, J.C.; Zheng, W.X.; Sun, Y.F.; Fu, Y.W.; Li, Y.Q. Three dimensional morphometry of the knee to design the total knee arthroplasty for Chinese population. Knee 2009, 16, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.J.; Kang, J.Y.; Kang, Y.G.; Kim, S.J.; Kim, T.K. Clinical Implications of Femoral Anthropometrical Features for Total Knee Arthroplasty in Koreans. J. Arthroplast. 2015, 30, 1220–1227. [Google Scholar] [CrossRef]
- Yang, C.C.; Dennis, D.A.; Davenport, P.G.; Kim, R.H.; Miner, T.M.; Johnson, D.R.; Laz, P.J. Patellar component design influences size selection and coverage. Knee 2017, 24, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Morris, W.Z.; Gebhart, J.J.; Goldberg, V.M.; Wera, G.D. Implant Size Availability Affects Reproduction of Distal Femoral Anatomy. J. Knee Surg. 2016, 29, 409–413. [Google Scholar] [CrossRef]
- Dai, Y. (Ed.) Distal Femoral Morphology and Its Correlation with Two Contemporary TKA Designs. In Proceedings of the ORS 2017 Annual Meeting, San Diego, CA, USA, 19–22 March 2017. [Google Scholar]
- Ha, C.W.; Na, S.E. The correctness of fit of current total knee prostheses compared with intra-operative anthropometric measurements in Korean knees. J. Bone Jt. Surg. Br. Vol. 2012, 94, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Hitt, K.; Shurman, J.R.; Greene, K.; McCarthy, J.; Moskal, J.; Hoeman, T.; Mont, M.A. Anthropometric measurements of the human knee: Correlation to the sizing of current knee arthroplasty systems. J. Bone Jt. Surg. Am. Vol. 2003, 85 (Suppl. S4), 115–122. [Google Scholar] [CrossRef] [Green Version]
- Lonner, J.H.; Jasko, J.G.; Thomas, B.S. Anthropomorphic differences between the distal femora of men and women. Clin. Orthop. Relat. Res. 2008, 466, 2724–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clary, C.; Aram, L.; Deffenbaugh, D.; Heldreth, M. Tibial base design and patient morphology affecting tibial coverage and rotational alignment after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 3012–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Bischoff, J.E. Comprehensive assessment of tibial plateau morphology in total knee arthroplasty: Influence of shape and size on anthropometric variability. J. Orthop. Res. 2013, 31, 1643–1652. [Google Scholar] [CrossRef]
- Cootes, T.; Taylor, C.J. Statistical Models of Appearance for Computer Vision; University of Manchester: Manchester, UK, 2004. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akagi, M.; Oh, M.; Nonaka, T.; Tsujimoto, H.; Asano, T.; Hamanishi, C. An Anteroposterior Axis of the Tibia for Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2004, 420, 213–219. [Google Scholar] [CrossRef]
- Franceschini, V.; Nodzo, S.R.; Della Gonzalez Valle, A. Femoral Component Rotation in Total Knee Arthroplasty: A Comparison between Transepicondylar Axis and Posterior Condylar Line Referencing. J. Arthroplast. 2016, 31, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Akamatsu, Y.; Kumagai, K.; Kusayama, Y.; Ishigatsubo, R.; Muramatsu, S.; Saito, T. The surgical epicondylar axis is a consistent reference of the distal femur in the coronal and axial planes. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2947–2953. [Google Scholar] [CrossRef]
- Victor, J.; van Doninck, D.; Labey, L.; van Glabbeek, F.; Parizel, P.; Bellemans, J. A common reference frame for describing rotation of the distal femur: A ct-based kinematic study using cadavers. J. Bone Jt. Surg. Br. 2009, 91, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, I.; Jacobs, M.; Deckx, J.; Firmbach, F.-P.; Utz, M. Mise en Place d’un Référentiel Anatomique sur un Modèle Statistique de la Rotule; Livre des résumés des communications de la SOFCOT: Paris, France, 2019. [Google Scholar]
- Dai, Y.; Scuderi, G.R.; Penninger, C.; Bischoff, J.E.; Rosenberg, A. Increased shape and size offerings of femoral components improve fit during total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2931–2940. [Google Scholar] [CrossRef] [Green Version]
- van Dijck, C.; Wirix-Speetjens, R.; Jonkers, I.; Vander Sloten, J. Statistical shape model-based prediction of tibiofemoral cartilage. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 568–578. [Google Scholar] [CrossRef]
- Cerveri, P.; Belfatto, A.; Manzotti, A. Predicting Knee Joint Instability Using a Tibio-Femoral Statistical Shape Model. Front. Bioeng. Biotechnol. 2020, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, P.; Demol, J.; Gelaude, F.; Vander Sloten, J. Virtual anatomical reconstruction of large acetabular bone defects using a statistical shape model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 577–586. [Google Scholar] [CrossRef]
- Schierjott, R.A.; Hettich, G.; Graichen, H.; Jansson, V.; Rudert, M.; Traina, F.; Weber, P.; Grupp, T.M. Quantitative assessment of acetabular bone defects: A study of 50 computed tomography data sets. PLoS ONE 2019, 14, e0222511. [Google Scholar] [CrossRef] [Green Version]
- Hettich, G.; Schierjott, R.A.; Ramm, H.; Graichen, H.; Jansson, V.; Rudert, M.; Traina, F.; Grupp, T.M. Method for quantitative assessment of acetabular bone defects. J. Orthop. Res. 2019, 37, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.R.R.; Pereira, L.A.; Oliveira, M.J.; Wafae, G.C.; Ruiz, C.R.; Wafea, N. Morphometric analysis of the angle of the femoral trochlea. J. Morphol. Sci. 2011, 28, 250–254. [Google Scholar]
- Iranpour, F.; Merican, A.M.; Dandachli, W.; Amis, A.A.; Cobb, J.P. The geometry of the trochlear groove. Clin. Orthop. Relat. Res. 2010, 468, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monk, A.P.; Choji, K.; O’Connor, J.J.; Goodfellow, J.W.; Murray, D.W. The shape of the distal femur: A geometrical study using MRI. Bone Jt. J. 2014, 96, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, S.; Yan, M.; Yue, B.; Wang, Y. Differences between native and prosthetic knees in terms of cross-sectional morphology of the femoral trochlea: A study based on three-dimensional models and virtual total knee arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.-B.; Luo, X.; Song, C.-H.; Zhang, J.-Y.; Yang, Y.-Q.; Yu, J.-K. Comprehensive assessment of patellar morphology using computed tomography-based three-dimensional computer models. Knee 2015, 22, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Long, J.; Wright, A.; Clary, C.W. The Shape of the Resected Patella and Design Factors Affecting Patella Coverage and Restoration of Patella Anatomy. In Proceedings of the ORS 2014 Annual Meeting, New Orleans, LA, USA, 28–29 April 2014. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupraz, I.; Bollinger, A.; Deckx, J.; Schierjott, R.A.; Utz, M.; Jacobs, M. Using Statistical Shape Models to Optimize TKA Implant Design. Appl. Sci. 2022, 12, 1020. https://doi.org/10.3390/app12031020
Dupraz I, Bollinger A, Deckx J, Schierjott RA, Utz M, Jacobs M. Using Statistical Shape Models to Optimize TKA Implant Design. Applied Sciences. 2022; 12(3):1020. https://doi.org/10.3390/app12031020
Chicago/Turabian StyleDupraz, Ingrid, Arthur Bollinger, Julien Deckx, Ronja Alissa Schierjott, Michael Utz, and Marnic Jacobs. 2022. "Using Statistical Shape Models to Optimize TKA Implant Design" Applied Sciences 12, no. 3: 1020. https://doi.org/10.3390/app12031020
APA StyleDupraz, I., Bollinger, A., Deckx, J., Schierjott, R. A., Utz, M., & Jacobs, M. (2022). Using Statistical Shape Models to Optimize TKA Implant Design. Applied Sciences, 12(3), 1020. https://doi.org/10.3390/app12031020





