Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. The Preparation of Beverages and Fermentation
2.3. Determination of Microbiological Quality and LAB Viability during Storage
2.4. Determination of pH, Titratable Acidity (TA), Total Solids Content (TSC), and Color Measurements
2.5. Preparation of Extracts
2.6. Determination of the Reducing Sugars Content (RSC), Total Free Amino Acids (TFAA) and Total Phenolic Content (TSC)
2.7. Antioxidant Activity Measurements
2.8. Rheology and Texture Measurements
2.9. Sensory evaluation
2.10. Statistical Analyses
3. Results and Discussion
3.1. The Changes in pH, TA, TSC, LAB Viability and Color
3.2. The Changes in Bioactive Compounds and Antioxidant Activity
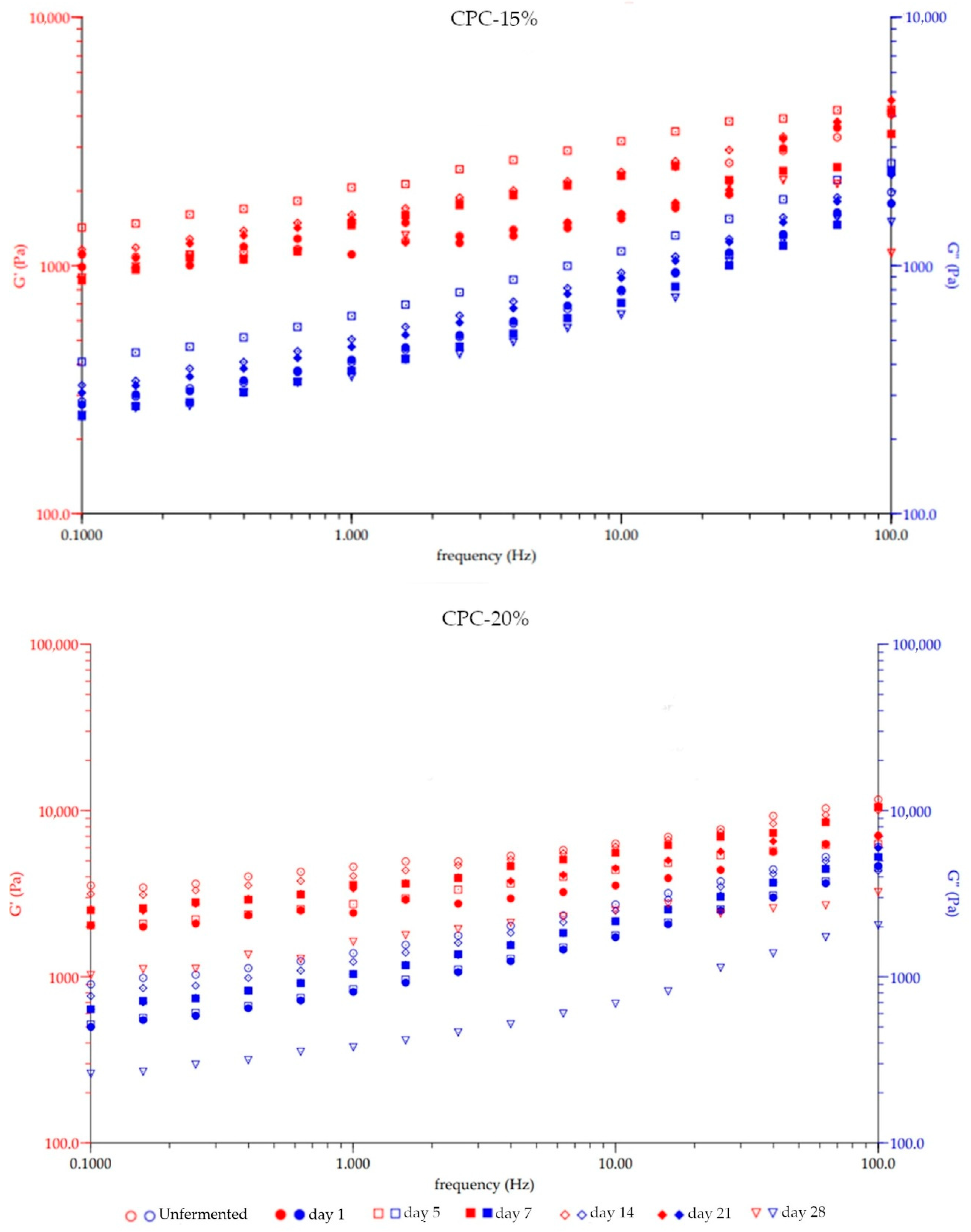
3.3. The Textural, Viscosity, and Rheological Changes
3.4. Sensory Evaluation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurasiak-Popowska, D.; Rynska, B.; Stuper-Szablewska, K. Analysis of distribution of selected bioactive compounds in Camelina sativa from seeds to pomace and oil. Agronomy 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Tavarini, S.; De Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L.G. Flaxseed and camelina meals as potential sources of health-beneficial compounds. Plants 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Kwiatkowski, P.; Bartkowiak, A. The Development of Novel Probiotic Fermented Plant Milk Alternative from Flaxseed Oil Cake Using Lactobacillus rhamnosus GG Acting as A Preservative Agent Against Pathogenic Bacteria During Short-term Refrigerated Storage. Emirates J. Food Agric. 2021, 33, 266–276. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A. Preparation and characterization of novel flaxseed oil cake yogurt-like plant milk fortified with inulin. J. Food Nutr. Res. 2020, 59, 61–70. [Google Scholar]
- Quezada, N.; Cherian, G. Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Physicochemical, microbiological and functional properties of camelina meal fermented in solid-state using food grade aspergillus fungi. Fermentation 2020, 6, 44. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Tomkowiak, A.; Człopińska, M.; Bocianowski, J.; Weigt, D.; Nawracała, J. Analysis of yield and genetic similarity of Polish and Ukrainian Camelina sativa genotypes. Ind. Crops Prod. 2018, 123, 667–675. [Google Scholar] [CrossRef]
- Terpinc, P.; Polak, T.; Makuc, D.; Ulrih, N.P.; Abramovič, H. The occurrence and characterisation of phenolic compounds in Camelina sativa seed, cake and oil. Food Chem. 2012, 131, 580–589. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, W.S.; Kumura, H.; Shimazaki, K.I. Autoaggregation and surface hydrophobicity of bifidobacteria. World J. Microbiol. Biotechnol. 2008, 24, 1593–1598. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 506/2009 of 15 June 2009 Entering a Designation in the Register of Traditional Specialities Guaranteed (Olej Rydzowy (TSG). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0506 (accessed on 21 December 2021).
- Department for Environment, Food & Rural Affairs. Protected Geographical Food and Drink Names—Olej Rydzowy Tradycyjny. Available online: https://www.gov.uk/protected-food-drink-names/olej-rydzowy-tradycyjny (accessed on 21 December 2021).
- Rahman, M.J.; Costa de Camargo, A.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Salminen, H.; Estévez, M.; Kivikari, R.; Heinonen, M. Inhibition of protein and lipid oxidation by rapeseed, camelina and soy meal in cooked pork meat patties. Eur. Food Res. Technol. 2006, 223, 461–468. [Google Scholar] [CrossRef]
- Sarv, V.; Trass, O.; Diosady, L.L. Preparation and Characterization of Camelina sativa Protein Isolates and Mucilage. J. Am. Oil Chem. Soc. 2017, 94, 1279–1285. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Gunaratne, A.; Sui, Z.Q.; Corke, H. Effects of Fermented Edible Seeds and Their Products on Human Health: Bioactive Components and Bioactivities. Compr. Rev. Food Sci. Food Saf. 2017, 16, 489–531. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kwiatkowski, P.; Bartkowiak, A.; Gefrom, A.; Sienkiewicz, M. The Effect of Fermentation with Kefir Grains on the Physicochemical and Antioxidant Properties of Beverages from Blue Lupin (Lupinus angustifolius L.) Seeds. Molecules 2020, 25, 5791. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Trakselyte-Rupsiene, K.; Navickaite, B.; Zadeike, D.; Bendoraitiene, J.; Bartkiene, E.; Lele, V.; Rueller, L.; Robert, J.; Arnoldi, A.; et al. Functionalization of soya press cake (okara) by ultrasonication for enhancement of submerged fermentation with Lactobacillus paracasei LUHS244 for wheat bread production. LWT 2021, 152, 112337. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Prebiotic potential and bioactive volatiles of hemp byproduct fermented by lactobacilli. LWT 2021, 151, 112201. [Google Scholar] [CrossRef]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT-Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Lai, K.; How, Y.; Pui, L. Microencapsulation of Lactobacillus rhamnosus GG with flaxseed mucilage using co-extrusion technique. J. Microencapsul. 2021, 38, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Bătrîna, Ș.L.; Corcionivoschi, N.; Jurcoane, S.; Linton, M.; Kelly, C.; Pinkerton, L.; Imbrea, I.M.; Sima, F.; Imbrea, F. Camelina sativa oil can inhibit Campylobacter jejuni but stimulate lactic acid bacteria growth. Rom. Biotechnol. Lett. 2021, 26, 2423–2427. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kubicka, M.M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological quality of non-sterile pharmaceutical products. Saudi Pharm. J. 2015, 23, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Froiio, F.; Cristiano, M.C.; Mancuso, A.; Iannone, M.; Paolino, D. Vegetable-milk-based yogurt-like structure: Rheological properties influenced by gluten-free carob seed flour. Appl. Sci. 2020, 10, 6963. [Google Scholar] [CrossRef]
- ISO. ISO 13299:2003—Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. Available online: https://www.iso.org/standard/37227.html (accessed on 14 January 2022).
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT-Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Bătrîna, L.S.; Jurcoane, Ș.; Popescu, I.; Marin, F.; Imbrea, I.; Crista, F.; Pop, G.; Imbrea, F. Camelina sativa: A study on amino acid content. Rom. Biotechnol. Lett. 2020, 25, 1136–1142. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Bogusławska-Wąs, E.; Drozłowska, E.; Trocer, P.; Dłubała, A.; Mazurkiewicz-Zapałowicz, K.; Bartkowiak, A. The Application of Spray-Dried and Reconstituted Flaxseed Oil Cake Extract as Encapsulating Material and Carrier for Probiotic Lacticaseibacillus rhamnosus GG. Materials 2021, 14, 5324. [Google Scholar] [CrossRef] [PubMed]
- Penna Barretto, A.L.; Converti, A.; Nogueira De Oliveira, M. Simultaneous effects of total solids content, milk base, heat treatment temperature and sample temperature on the rheological properties of plain stirred yogurt. Food Technol. Biotechnol. 2006, 44, 515. [Google Scholar]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Zhang, D.; Li, X.; Sultanbawa, Y. Phytic Acid Reduction in Canola and Camelina Meals by Fungal Fermentation for Potential Broiler Feeding. In Proceedings of the 30th Annual Australian Poultry Science Symposium, Sydney, Australia, 17–20 February 2019; p. 203. [Google Scholar]
- Ghorbani, A.; Pourahmad, R.; Fallahpour, M.; Assadi, M.M. Production of probiotic soy yogurt. Ann. Biol. Res. 2012, 3, 2750–2754. [Google Scholar]
- El-Sayed, E.M.; Abd El-Gawad, I.A.; Murad, H.A.; Salah, S.H. Utilization of laboratory-produced xanthan gum in the manufacture of yogurt and soy yogurt. Eur. Food Res. Technol. 2002, 215, 298–304. [Google Scholar] [CrossRef]
- Silva, E.G.d.S.O.; Anaya, K.; Bezerra, M.d.F.; Macêdo, C.S.; Urbano, S.A.; Borba, L.H.F.; Barbosa, I.d.M.; Ramalho, H.M.M.; Cipolat-Gotet, C.; Galdino, A.B.d.S.; et al. Physicochemical and sensory evaluation of greek style yoghurt with bovine colostrum. Food Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Lange, I.; Mleko, S.; Tomczynska-Mleko, M.; Polischuk, G.; Janas, P.; Ozimek, L. Technology and factors influencing Greek-style yogurt—A Review. Ukr. Food J. 2020, 9, 361–480. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Bertacchi, S.; Bettiga, M.; Porro, D.; Branduardi, P. Camelina sativa meal hydrolysate as sustainable biomass for the production of carotenoids by Rhodosporidium toruloides. Biotechnol. Biofuels 2020, 13, 47. [Google Scholar] [CrossRef]
- Li, N.; Qi, G.; Sun, X.S.; Wang, D. Characterization of gum isolated from Camelina seed. Ind. Crops Prod. 2016, 83, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Bienkiewicz, G.; Łopusiewicz, Ł. The influence of flaxseed oil cake extract on oxidative stability of microencapsulated flaxseed oil in spray-dried powders. Antioxidants 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, E.A.; Barimah, J.; Acheampong, R.; Addai, L.O.; Nnaji, C.O. Effect of fermentation and malting on the viscosity of maize-soyabean weaning blends. Pakistan J. Nutr. 2009, 8, 1671–1675. [Google Scholar] [CrossRef] [Green Version]
- Bortnowska, G.; Balejko, J.; Tokarczyk, G.; Romanowska-Osuch, A.; Krzemińska, N. Effects of pregelatinized waxy maize starch on the physicochemical properties and stability of model low-fat oil-in-water food emulsions. Food Hydrocoll. 2014, 36, 229–237. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I.; Mortas, M.; Dervisoglu, M. Rheological, textural, colour and sensorial properties of kefir produced with buffalo milk using kefir grains and starter culture: A comparison with cows’ milk kefir. Int. J. Dairy Technol. 2018, 71, 73–80. [Google Scholar] [CrossRef]
- Glibowski, P.; Kowalska, A. Rheological, texture and sensory properties of kefir with high performance and native inulin. J. Food Eng. 2012, 111, 299–304. [Google Scholar] [CrossRef]
- Sendra, E.; Kuri, V.; Fernández-López, J.; Sayas-Barberá, E.; Navarro, C.; Pérez-Alvarez, J.A. Viscoelastic properties of orange fiber enriched yogurt as a function of fiber dose, size and thermal treatment. LWT-Food Sci. Technol. 2010, 43, 708–714. [Google Scholar] [CrossRef]
- Gallardo-Escamilla, F.J.; Kelly, A.L.; Delahunty, C.M. Mouthfeel and flavour of fermented whey with added hydrocolloids. Int. Dairy J. 2007, 17, 308–315. [Google Scholar] [CrossRef]
- Farah, J.S.; Araujo, C.B.; Melo, L. Analysis of yoghurts’, whey-based beverages’ and fermented milks’ labels and differences on their sensory profiles and acceptance. Int. Dairy J. 2017, 68, 17–22. [Google Scholar] [CrossRef]
- Shepard, L.; Miracle, R.E.; Leksrisompong, P.; Drake, M.A. Relating sensory and chemical properties of sour cream to consumer acceptance. J. Dairy Sci. 2013, 96, 5435–5454. [Google Scholar] [CrossRef]
- Gierczynski, I.; Guichard, E.; Laboure, H. Aroma perception in dairy products: The roles of texture, aroma release and consumer physiology. A review. Flavour Fragr. J. 2011, 26, 141–152. [Google Scholar] [CrossRef]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic Acid Bacteria Convert Glucosinolates to Nitriles Efficiently Yet Differently from Enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef]
- Watanabe, H.; Usami, R.; Kishino, S.; Osada, K.; Aoki, Y.; Morisaka, H.; Takahashi, M.; Izumi, Y.; Bamba, T.; Aoki, W.; et al. Enzyme systems involved in glucosinolate metabolism in Companilactobacillus farciminis KB1089. Sci. Rep. 2021, 11, 23715. [Google Scholar] [CrossRef] [PubMed]
- Palani, K.; Harbaum-Piayda, B.; Meske, D.; Keppler, J.K.; Bockelmann, W.; Heller, K.J.; Schwarz, K. Influence of fermentation on glucosinolates and glucobrassicin degradation products in sauerkraut. Food Chem. 2016, 190, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, M.; Chang, Y. Degradation of glucosinolates in rapeseed meal by Lactobacillus delbrueckii and Bacillus subtilis. Grain Oil Sci. Technol. 2020, 3, 70–76. [Google Scholar] [CrossRef]

| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| TSC (%) | |||||||
| CPC-15% | 13.84 ± 0.76 Aa | 14.36 ± 0.04 Ba | 14.38 ± 0.05 Ba | 14.34 ± 0.08 Ba | 14.44 ± 0.08 Ba | 14.49 ± 0.07 Ba | 14.46 ± 0.06 Ba |
| CPC-20% | 17.88 ± 3.05 Ab | 19.87 ± 0.12 Bb | 19.55 ± 0.06 Bb | 19.88 ± 0.06 Bb | 19.71 ± 0.12 Bb | 19.96 ± 0.11 Bb | 19.60 ± 0.20 Bb |
| pH (-) | |||||||
| CPC-15% | 6.28 ± 0.00 Aa | 4.65 ± 0.01 Ba | 5.12 ± 0.01 Ca | 4.31 ± 0.01 Da | 4.46 ± 0.01 Ea | 4.37 ± 0.00 Fa | 4.65 ± 0.00 Ga |
| CPC-20% | 6.32 ± 0.01 Ab | 4.56 ± 0.02 Bb | 4.82 ± 0.00 Cb | 4.84 ± 0.01 Db | 4.87 ± 0.02 Eb | 4.69 ± 0.01 Fb | 4.71 ± 0.01 gb |
| TA (mg lactic acid/g) | |||||||
| CPC-15% | 0.32 ± 0.03 Aa | 0.75 ± 0.00 Ba | 0.66 ± 0.01 Ca | 0.98 ± 0.04 Da | 0.97 ± 0.01 Da | 1.25 ± 0.14 Ea | 0.94 ± 0.02 Da |
| CPC-20% | 0.37 ± 0.00 Aa | 0.89 ± 0.00 Bb | 0.89 ± 0.05 Cb | 0.83 ± 0.04 Cb | 0.88 ± 0.04 Da | 0.98 ± 0.04 Eb | 1.12 ± 0.01 Fb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| L * | |||||||
| CPC-15% | 55.11 ± 0.01 Aa | 55.65 ± 0.01 Ba | 57.15 ± 0.02 Ca | 56.51 ± 0.03 Da | 56.60 ± 0.01 EDa | 55.46 ± 0.01 Fa | 55.27 ± 0.01 Ga |
| CPC-20% | 53.32 ± 0.00 Ab | 53.26 ± 0.00 Bb | 51.07 ± 0.01 Cb | 53.51 ± 0.01 Db | 51.48 ± 0.01 Eb | 52.96 ± 0.02 Fb | 52.47 ± 0.01 Gb |
| a * | |||||||
| CPC-15% | 7.74 ± 0.01 Aa | 6.83 ± 0.02 Ba | 6.81 ± 0.01 Ba | 6.59 ± 0.01 Ca | 6.59 ± 0.01 Ca | 6.40 ± 0.01 Da | 6.68 ± 0.02 Ea |
| CPC-20% | 8.07 ± 0.01 Ab | 7.48 ± 0.01 Bb | 7.11 ± 0.01 Cb | 7.30 ± 0.01 Db | 7.17 ± 0.01 Eb | 7.08 ± 0.01 Fb | 7.49 ± 0.01 Bb |
| b * | |||||||
| CPC-15% | 25.50 ± 0.02 Aa | 25.61 ± 0.03 Ba | 27.35 ± 0.04 Ca | 24.44 ± 0.01 Da | 26.30 ± 0.03 Ea | 24.34 ± 0.04 Fa | 25.41 ± 0.02 Ga |
| CPC-20% | 24.56 ± 0.00 Ab | 24.16 ± 0.01 Bb | 23.22 ± 0.02 Cb | 24.90 ± 0.04 Db | 23.61 ± 0.03 Eb | 24.35 ± 0.01 Fb | 24.75 ± 0.03 Gb |
| ΔE | |||||||
| CPC-15% | Used as a standard | 1.00 ± 0.01 Aa | 1.78 ± 0.02 Ba | 2.08 ± 0.10 Ca | 4.55 ± 0.01 Da | 2.46 ± 0.02 Ea | 1.11 ± 0.01 Fa |
| CPC-20% | Used as a standard | 0.87 ± 0.01 Ab | 2.71 ± 0.02 Bb | 0.54 ± 0.04 Cb | 2.27 ± 0.03 Db | 1.56 ± 0.02 Eb | 0.93 ± 0.03 Fb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| RSC (mg/g) | |||||||
| CPC-15% | 51.10 ± 1.05 Aa | 38.53 ± 0.38 Ba | 35.77 ± 0.10 Ca | 37.32 ± 1.14 Da | 33.02 ± 0.57 EFa | 32.75 ± 0.76 Ea | 33.69 ± 1.52 Fa |
| CPC-20% | 41.49 ± 0.57 Ab | 33.02 ± 0.57 Bb | 29.05 ± 0.10 Cb | 26.96 ± 0.38 Db | 25.01 ± 0.10 Eb | 32.01 ± 0.10 Fa | 29.99 ± 0.10 Gb |
| TFAA (mg Gly/g) | |||||||
| CPC-15% | 1.90 ± 0.14 ABa | 2.59 ± 0.11 Da | 2.41 ± 0.01 CDa | 1.80 ± 0.06 Aa | 1.73 ± 0.01 Aa | 2.07 ± 0.06 ABCa | 2.18 ± 0.08 BCa |
| CPC-20% | 1.72 ± 0.10 Aa | 2.03 ± 0.34 Ba | 2.34 ± 0.05 Bb | 1.87 ± 0.20 Aa | 2.00 ± 0.01 Cb | 2.16 ± 0.07 Da | 2.18 ± 0.05 Da |
| TPC (mg GAE/g) | |||||||
| CPC-15% | 6.74 ± 0.26 Aa | 7.21 ± 0.14 Ba | 8.54 ± 1.37 Ba | 8.27 ± 0.10 Ba | 7.50 ± 0.06 Ca | 6.65 ± 0.47 Aa | 7.40 ± 0.05 Ca |
| CPC-20% | 6.18 ± 0.10 Aa | 6.98 ± 0.23 Ba | 8.18 ± 1.60 Ba | 6.68 ± 0.59 Ab | 6.27 ± 1.13 Aa | 6.71 ± 0.99 Aa | 7.90 ± 2.72 Ba |
| ABTS (%) | |||||||
| CPC-15% | 31.15 ± 3.42 Aa | 53.32 ± 2.66 Ba | 54.60 ± 0.28 Ba | 56.95 ± 1.52 Ba | 55.00 ± 2.75 Ba | 52.12 ± 5.13 Ba | 50.44 ± 1.23 Ba |
| CPC-20% | 40.37 ± 1.33 Ab | 55.81 ± 2.82 Ba | 54.13 ± 1.33 ABa | 59.03 ± 4.27 Bb | 50.10 ± 1.33 Aa | 47.95 ± 0.95 Ab | 54.40 ± 1.14 Aa |
| DPPH (%) | |||||||
| CPC-15% | 53.14 ± 0.25 Aa | 59.80 ± 0.90 BDa | 51.39 ± 0.16 Ca | 57.37 ± 0.25 Dea | 62.59 ± 5.33 Ba | 57.89 ± 0.33 DEa | 55.10 ± 0.49 Ea |
| CPC-20% | 56.79 ± 1.23 Ab | 57.66 ± 0.16 Ba | 58.18 ± 0.57 Db | 57.77 ± 0.00 Ca | 55.28 ± 0.25 Eb | 56.61 ± 0.33 Aa | 58.24 ± 2.79 Da |
| FRAP (mg AAE/g) | |||||||
| CPC-15% | 6.18 ± 0.41 Aa | 6.65 ± 0.23 Ba | 6.19 ± 0.24 Aa | 6.77 ± 0.40 Ba | 5.35 ± 0.44 Ca | 5.01 ± 0.36 Da | 5.72 ± 0.22 Ea |
| CPC-20% | 5.58 ± 0.52 ACb | 6.03 ± 0.29 Bb | 6.13 ± 0.47 Bb | 5.47 ± 0.45 Ada | 5.38 ± 0.32 Da | 6.02 ± 0.47 Bb | 5.75 ± 0.46 Ca |
| RP (-) | |||||||
| CPC-15% | 1.220 ± 0.014 Aa | 1.250 ± 0.011 Ba | 1.154 ± 0.006 Ca | 1.278 ± 0.008 Da | 1.070 ± 0.021 Ea | 1.037 ± 0.003 Fa | 1.049 ± 0.004 Ga |
| CPC-20% | 1.095 ± 0.003 Ab | 1.167 ± 0.009 Bb | 1.172 ± 0.011 Cb | 1.093 ± 0.003 Db | 1.165 ± 0.011 Eb | 1.254 ± 0.006 Fb | 1.087 ± 0.004 Gb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Unfermented | 1 | 5 | 7 | 14 | 21 | 28 | |
| Springiness (N) | |||||||
| CPC-15% | 0.99 ± 0.07 Aa | 0.84 ± 0.19 Ba | 0.81 ± 0.17 Ca | 1.01 ± 0.08 Da | 1.06 ± 0.15 Ea | 0.92 ± 0.17 Fa | 0.79 ± 0.15 Ga |
| CPC-20% | 0.83 ± 0.14 Ab | 0.60 ± 0.33 Bb | 1.03 ± 0.08 Cb | 1.28 ± 0.17 Db | 0.97 ± 0.12 Eb | 0.89 ± 0.19 Fb | 1.09 ± 0.15 Gb |
| Gumminess (N) | |||||||
| CPC-15% | 0.02 ± 0.01 Aa | 0.07 ± 0.01 Ba | 0.16 ± 0.02 CDa | 0.18 ± 0.02 Da | 0.18 ± 0.02 Da | 0.20 ± 0.01 Ea | 0.15 ± 0.02 Fa |
| CPC-20% | 0.04 ± 0.03 Ab | 0.10 ± 0.01 Bb | 0.34 ± 0.03 Cb | 0.37 ± 0.04 Db | 0.42 ± 0.03 Eb | 0.47 ± 0.06 Fb | 0.44 ± 0.10 Gb |
| Chewiness (N) | |||||||
| CPC-15% | 0.02 ± 0.01 Aa | 0.08 ± 0.01 Ba | 0.13 ± 0.04 Ca | 0.18 ± 0.02 Da | 0.19 ± 0.03 Ea | 0.19 ± 0.03 Ea | 0.11 ± 0.03 Fa |
| CPC-20% | 0.04 ± 0.02 Ab | 0.10 ± 0.00 Bb | 0.36 ± 0.02 Cb | 0.47 ± 0.07 Db | 0.41 ± 0.07 Eb | 0.42 ± 0.11 Fb | 0.48 ± 0.09 Gb |
| Cohesiveness (N) | |||||||
| CPC-15% | 0.93 ± 0.35 Aa | 0.82 ± 0.65 Ba | 0.71 ± 0.09 Ca | 0.75 ± 0.17 Da | 0.66 ± 0.05 Ea | 0.72 ± 0.05 Fa | 0.51 ± 0.11 Ga |
| CPC-20% | 0.93 ± 0.28 Ab | 0.33 ± 0.49 Bb | 0.65 ± 0.06 Cb | 0.61 ± 0.05 Db | 0.65 ± 0.06 Eb | 0.68 ± 0.03 Fb | 0.68 ± 0.22 Gb |
| Hardness (N) | |||||||
| CPC-15% | 0.02 ± 0.00 Aa | 0.21 ± 0.01 Ba | 0.23 ± 0.03 Ca | 0.24 ± 0.03 Da | 0.27 ± 0.02 Ea | 0.28 ± 0.01 Ea | 0.27 ± 0.02 Ea |
| CPC-20% | 0.02 ± 0.00 Aa | 0.01 ± 0.00 Ba | 0.51 ± 0.03 Cb | 0.58 ± 0.07 Db | 0.63 ± 0.04 Eb | 0.67 ± 0.08 Fb | 0.62 ± 0.08 Gb |
| Viscosity (Pa s) | |||||||
| CPC-15% | 1.78 ± 0.01 Aa | 2.84 ± 0.05 Ba | 2.05 ± 0.03 Ca | 3.20 ± 0.01 Da | 3.50 ± 0.00 Ea | 3.16 ± 0.00 Fa | 3.26 ± 0.02 Ga |
| CPC-20% | 0.39 ± 0.01 Ab | 0.24 ± 0.03 Bb | 0.37 ± 0.02 Cb | 0.36 ± 0.05 Db | 0.29 ± 0.00 Eb | 0.23 ± 0.03 Fb | 1.56 ± 0.02 Gb |
| Sample * | Time of Storage (Days) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 7 | 14 | 21 | 28 | |
| Color | |||||||
| CPC-15% | 4.0 ± 0.00 Aa | 4.2 ± 0.10 Ba | 4.3 ± 0.00 Ca | 4.2 ± 0.10 Ba | 4.2 ± 0.05 Da | 4.3 ± 0.27 Ea | 4.4 ± 0.10 Ba |
| CPC-20% | 4.0 ± 0.00 Aa | 4.0 ± 0.55 Bb | 4.2 ± 0.10 Ca | 4.4 ± 0.11 Ba | 4.4 ± 0.15 Db | 4.5 ± 0.09 Eb | 4.5 ± 0.10 Eb |
| Smell | |||||||
| CPC-15% | 3.8 ± 0.05 Aa | 4.5 ± 0.10 Ba | 4.6 ± 0.10 Ca | 4.8 ± 0.10 Da | 4.5 ± 0.05 Ba | 4.5 ± 0.10 Ba | 4.4 ± 0.10 Ba |
| CPC-20% | 3.5 ± 0.10 Ab | 4.5 ± 0.00 Bb | 4.7 ± 0.00 Cb | 4.6 ± 0.00 Db | 4.6 ± 0.10 Eb | 4.5 ± 0.15 Fb | 4.5 ± 0.10 Gb |
| Consistency | |||||||
| CPC-15% | 3.5 ± 0.19 Aa | 4.2 ± 0.12 Ba | 4.5 ± 0.10 Ca | 4.4 ± 0.05 CDa | 4.5 ± 0.10 Ca | 4.3 ± 0.31 CDa | 4.1 ± 0.05 Ea |
| CPC-20% | 3.5 ± 0.19 Aa | 4.4 ± 0.05 Bb | 4.5 ± 0.21 BCb | 4.5 ± 0.27 Cb | 4.6 ± 0.12 Da | 4.2 ± 0.00 Eb | 4.2 ± 0.24 Fb |
| Mouth feel | |||||||
| CPC-15% | 3.0 ± 0.23 Aa | 4.0 ± 0.10 Ba | 4.0 ± 0.12 Ba | 4.1 ± 0.25 Ca | 4.4 ± 0.40 Da | 4.4 ± 0.10 Ea | 4.3 ± 0.00 Fa |
| CPC-20% | 2.8 ± 0.15 Ab | 4.0 ± 0.00 Bb | 4.1 ± 0.20 Cb | 4.2 ± 0.00 Db | 4.6 ± 0.12 Eb | 4.4 ± 0.10 Fb | 4.3 ± 0.10 Gb |
| Taste | |||||||
| CPC-15% | 2.5 ± 0.45 Aa | 3.0 ± 0.55 Ba | 3.5 ± 0.70 Ca | 4.2 ± 0.20 Da | 4.2 ± 0.10 Ea | 4.5 ± 0.00 Fa | 4.6 ± 0.20 Ga |
| CPC-20% | 2.1 ± 0.23 Ab | 3.1 ± 0.20 Bb | 3.5 ± 0.10 Cb | 3.9 ± 0.16 Db | 4.1 ± 0.15 Eb | 4.5 ± 0.10 Fb | 4.5 ± 0.15 Gb |
| Overall acceptability | |||||||
| CPC-15% | 3.1 ± 0.19 Aa | 4.0 ± 0.01 Ba | 4.4 ± 0.19 Ca | 4.4 ± 0.45 Da | 4.5 ± 0.23 Ea | 4.4 ± 0.11 Fa | 4.3 ± 0.45 Ga |
| CPC-20% | 2.6 ± 0.10 Aa | 3.8 ± 0.20 Ba | 4.4 ± 0.31 Cb | 4.4 ± 0.55 Db | 4.5 ± 0.15 Eb | 4.5 ± 0.23 Fb | 4.3 ± 0.20 Gb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E. Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake. Appl. Sci. 2022, 12, 1085. https://doi.org/10.3390/app12031085
Łopusiewicz Ł, Kwiatkowski P, Drozłowska E. Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake. Applied Sciences. 2022; 12(3):1085. https://doi.org/10.3390/app12031085
Chicago/Turabian StyleŁopusiewicz, Łukasz, Paweł Kwiatkowski, and Emilia Drozłowska. 2022. "Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake" Applied Sciences 12, no. 3: 1085. https://doi.org/10.3390/app12031085








