The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
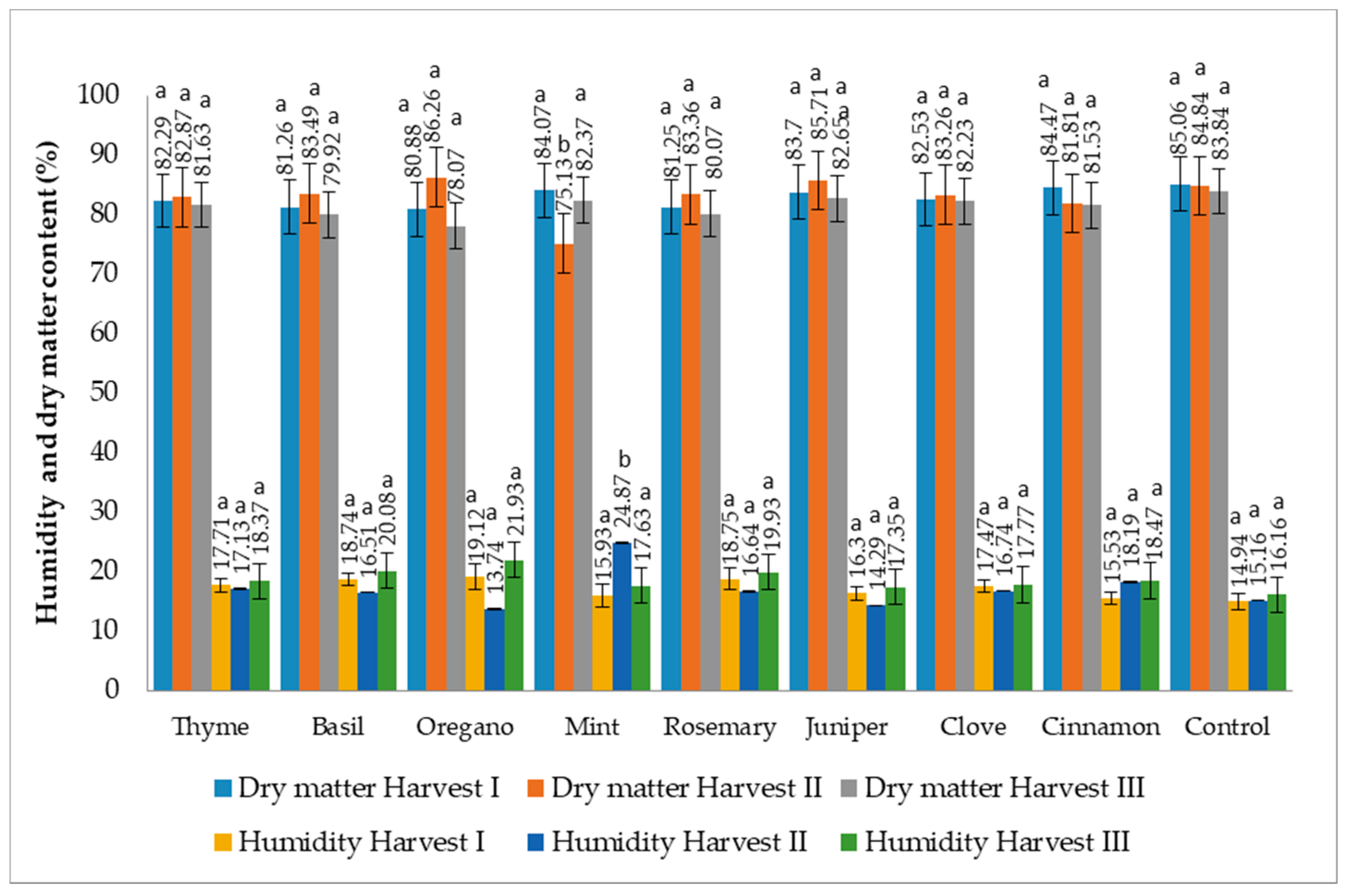
2.2. Determination of Humidity by Drying Method
- G1—the weight of petri dish and sample before drying (g);
- G2—the weight of petri dish and sample after drying (g);
- G3—the weight of Petri dish (g).
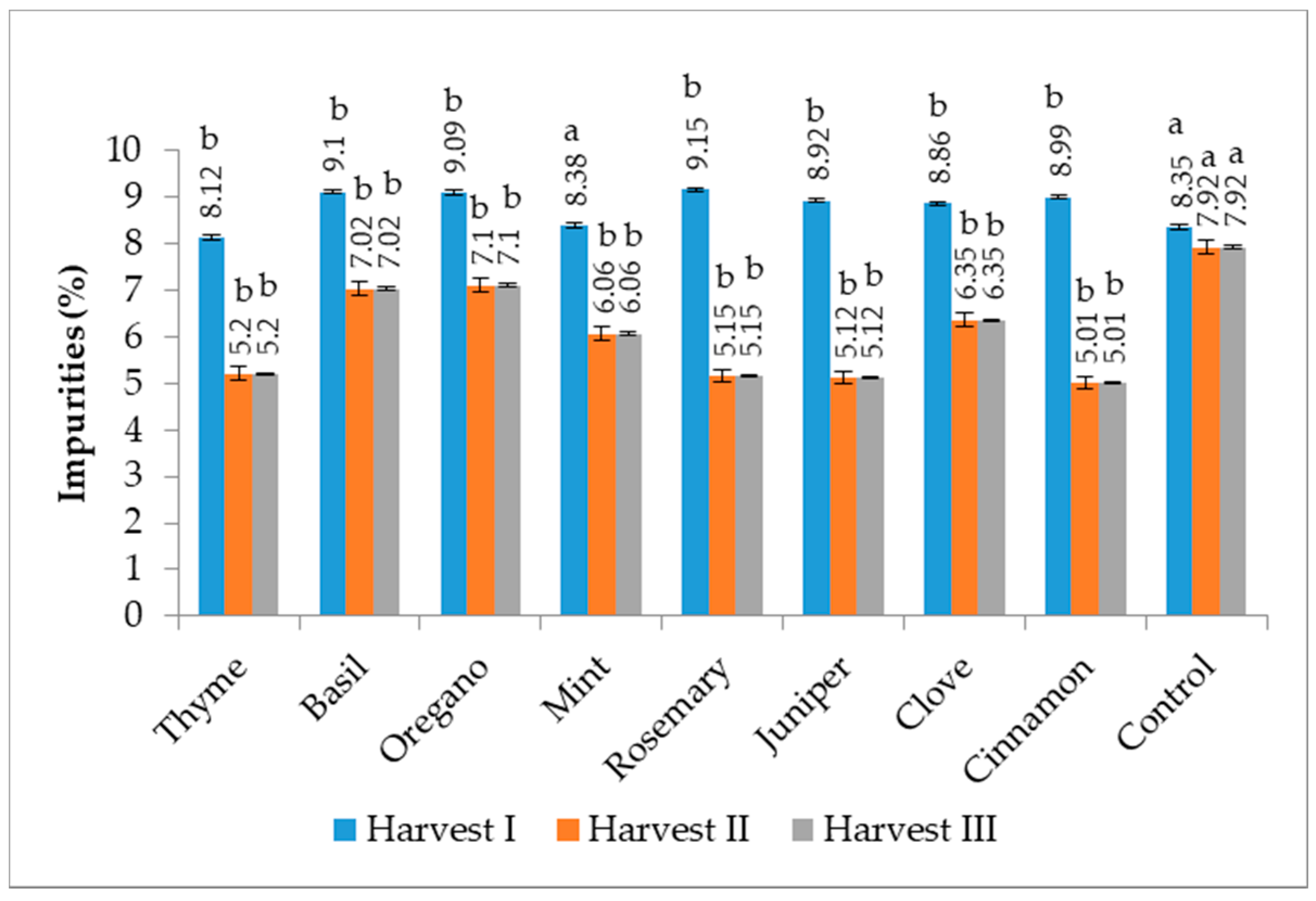
2.3. Determination of Impurities
- I—represents the quantity of impurities (%);
- m1—represents the mass of the sample taken for analysis (g);
- m2—represents the mass of residue left on the filter paper after drying (g).
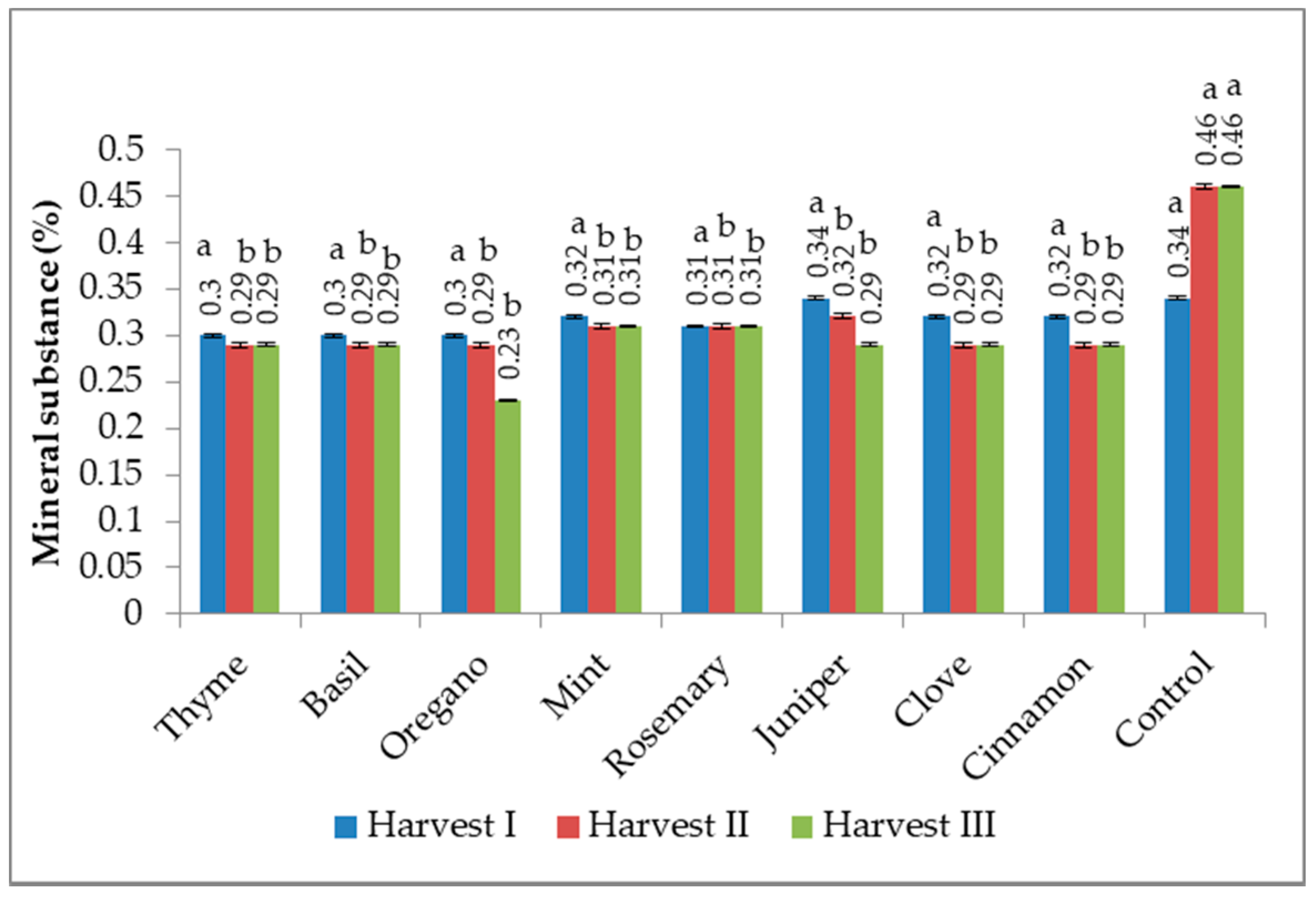
2.4. Mineral Substance Content (Ash) Determination
- m—represents the mass of the melting pot with the ash obtained after calcination (g);
- m1—represents the mass of the empty melting pot (g);
- m2—represents the mass of the melting pot with honey (g).
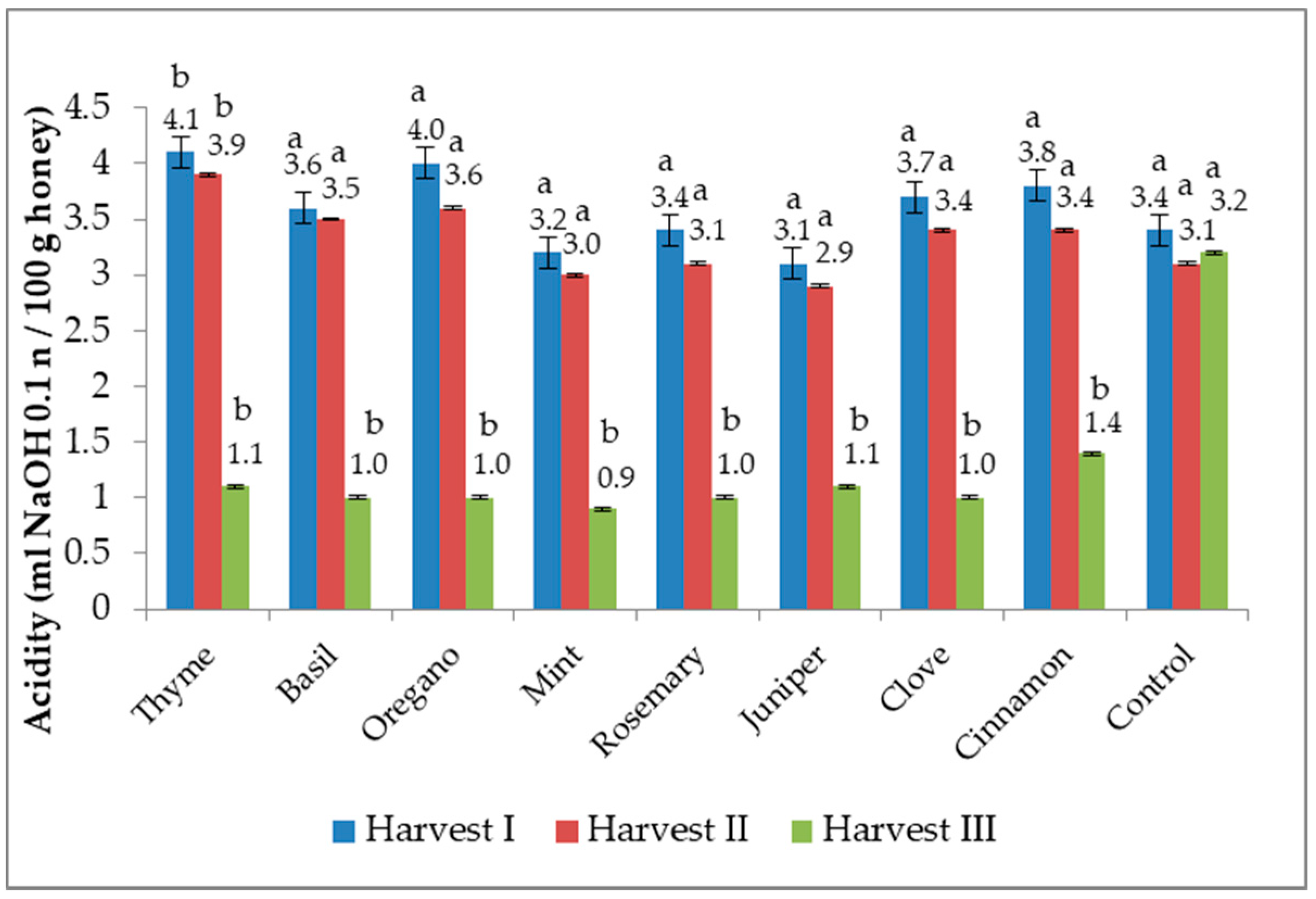
2.5. Determination of Acidity
- V—represents the volume of sodium hydroxide solution used in the titration (mL);
- 0.1—represents the normality of sodium hydroxide solution used for titration.
2.6. Determination of pH
2.7. Determination of Total Phenolic Content (TPC)
2.8. Determination of Reducing Sugar
- m—represents the amount of invert sugar (mg);
- m1—represents the amount of honey analyzed (g);
- 10—represents the ratio between the volume of the solution in the 200-mL volumetric container and the volume of the solution taken for dilution;
- 5—represents the ratio between the volume of the solution in the 100-mL volumetric container and the volume of the diluted solution taken for analysis.
2.9. Determination of Flavonoid Content (FC)
2.10. Determination of Antioxidant Activity (AA) by DPPH
- AA—represents the antioxidant activity of the analyzed sample;
- ABSsample—represents the absorbance of the sample measured at a wavelength of 518 nm;
- ABScontrol—represents the absorbance of the DPPH sample measured at a wavelength of 518 nm;
- ABSblank—represents the absorbance of the alcohol sample measured at a wavelength of 518 nm [44].
2.11. Antimicrobial Activity
Bacterial Culture
3. Results
3.1. Humidity (H) and Dry Matter (D.M.) Content
3.2. Impurities Content
3.3. Mineral Substance (Ash) Content
3.4. Acidity Profile
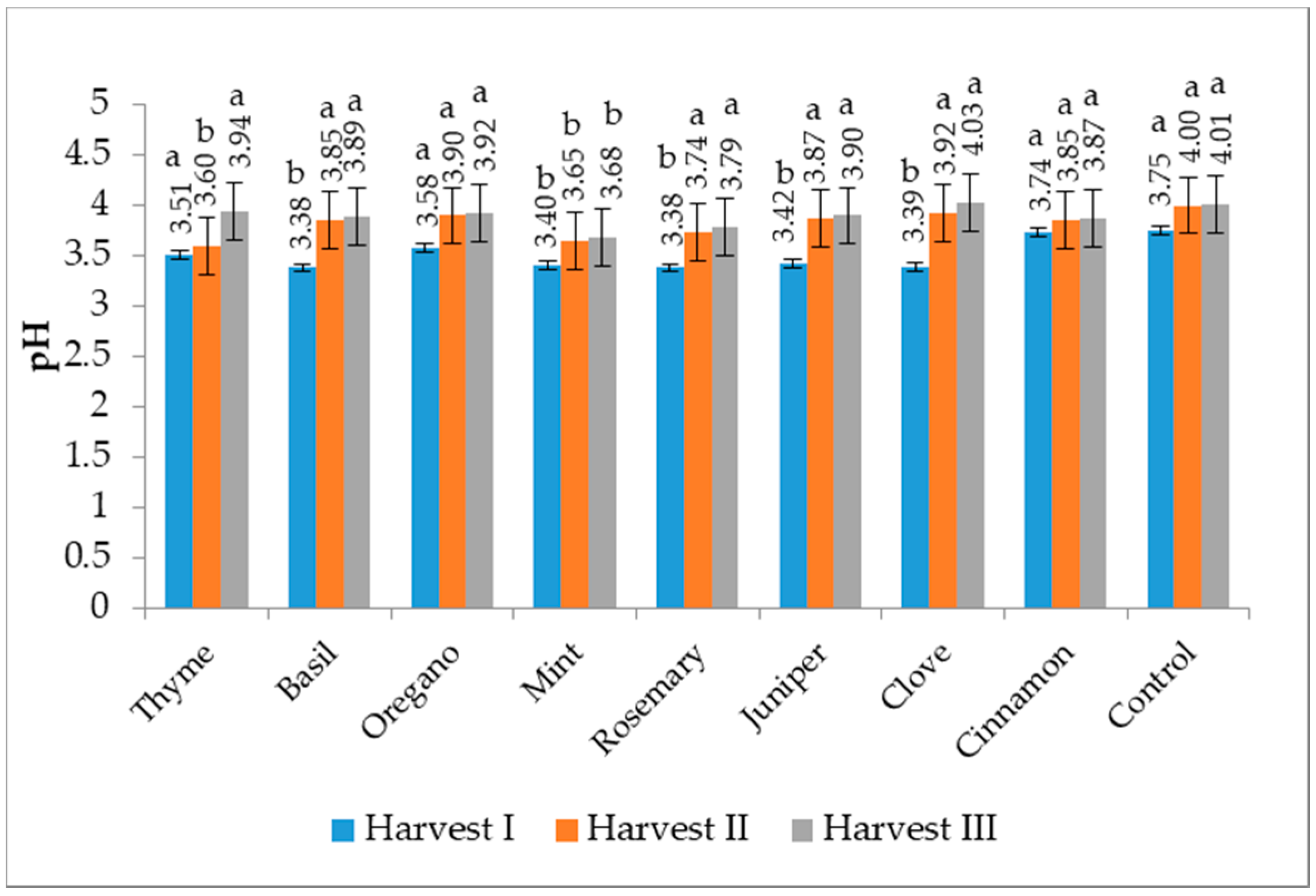
3.5. pH Value
3.6. Total Phenolic Content (TPC)
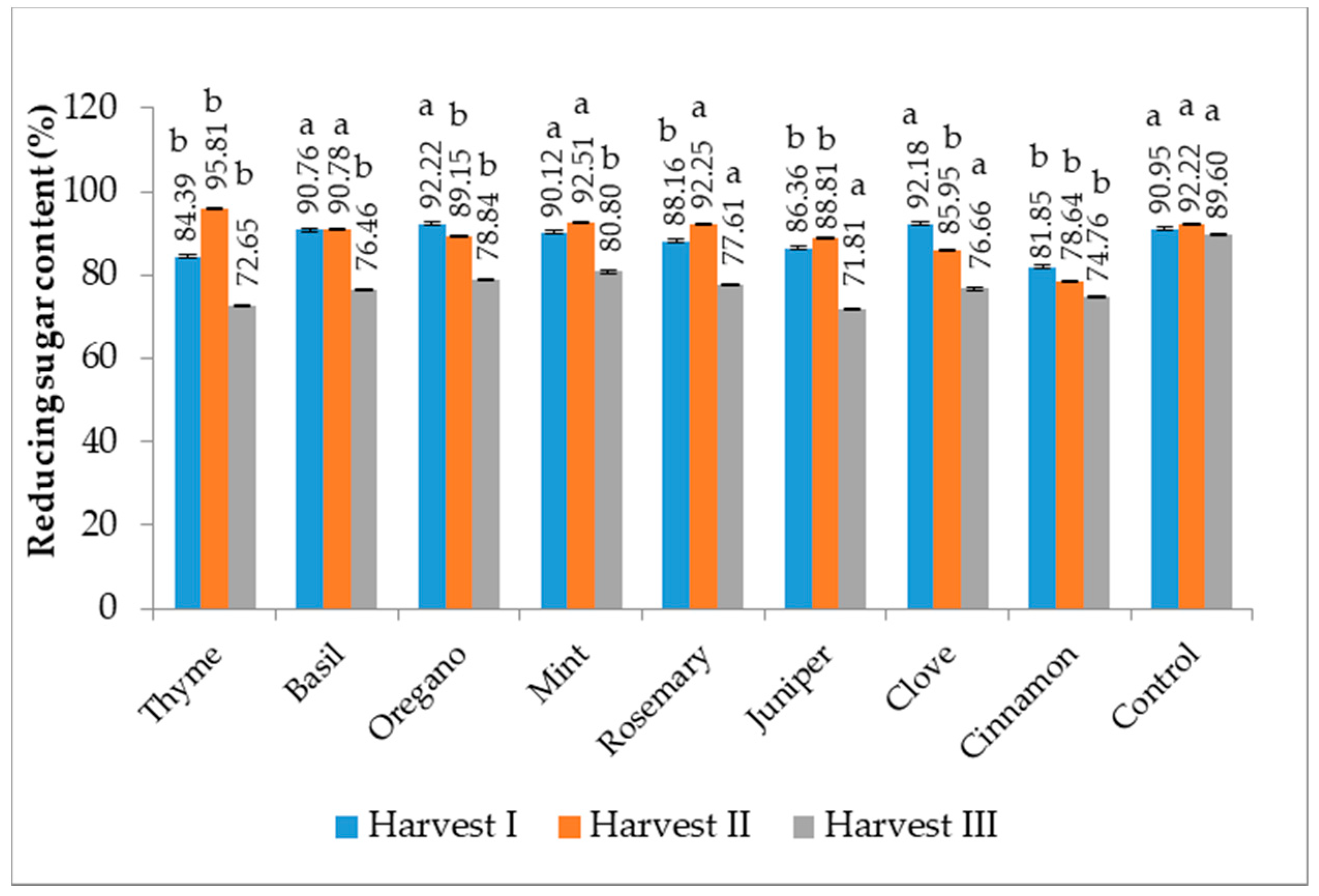
3.7. Reducing Sugar Content
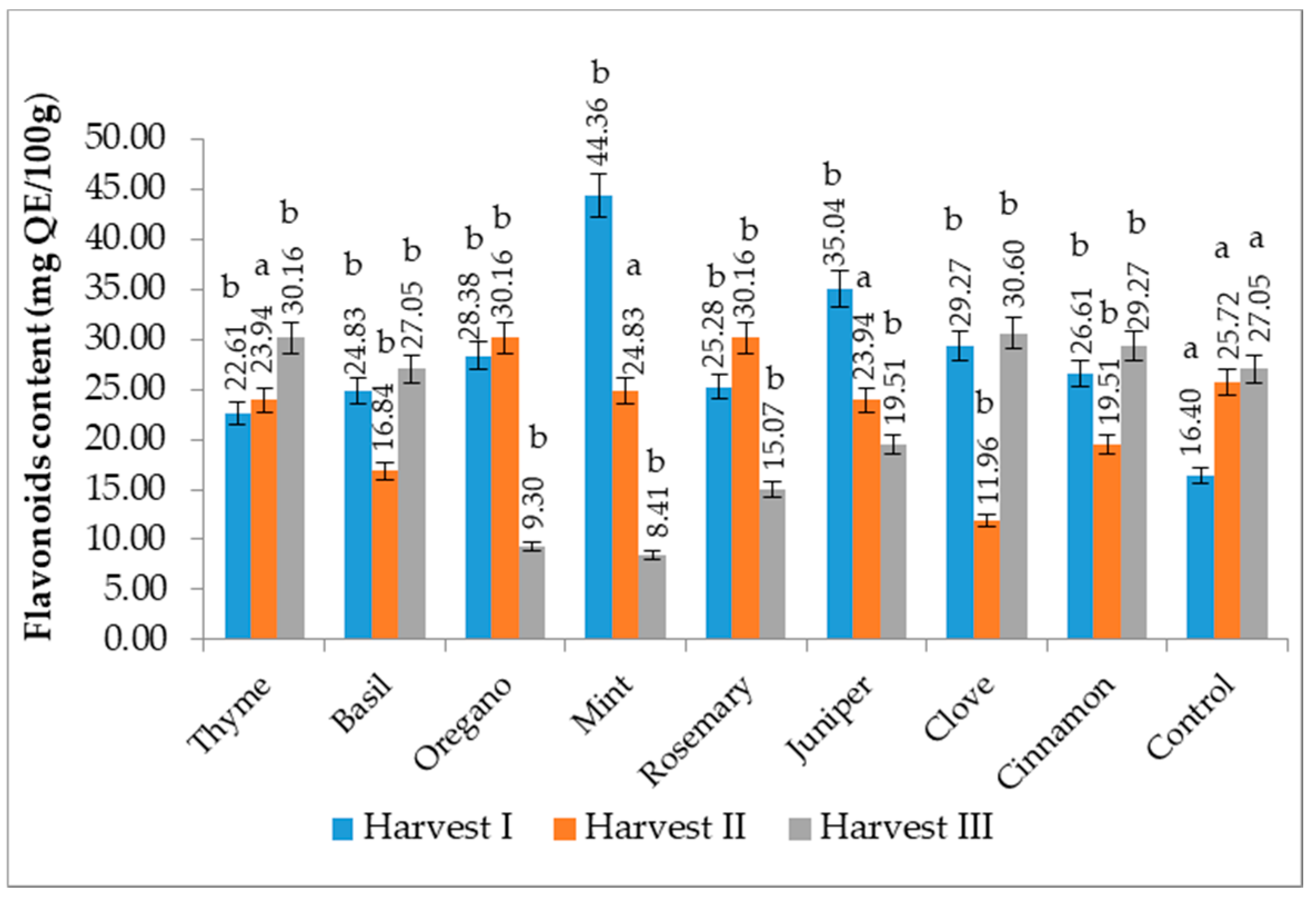
3.8. Flavonoid Content (FC)
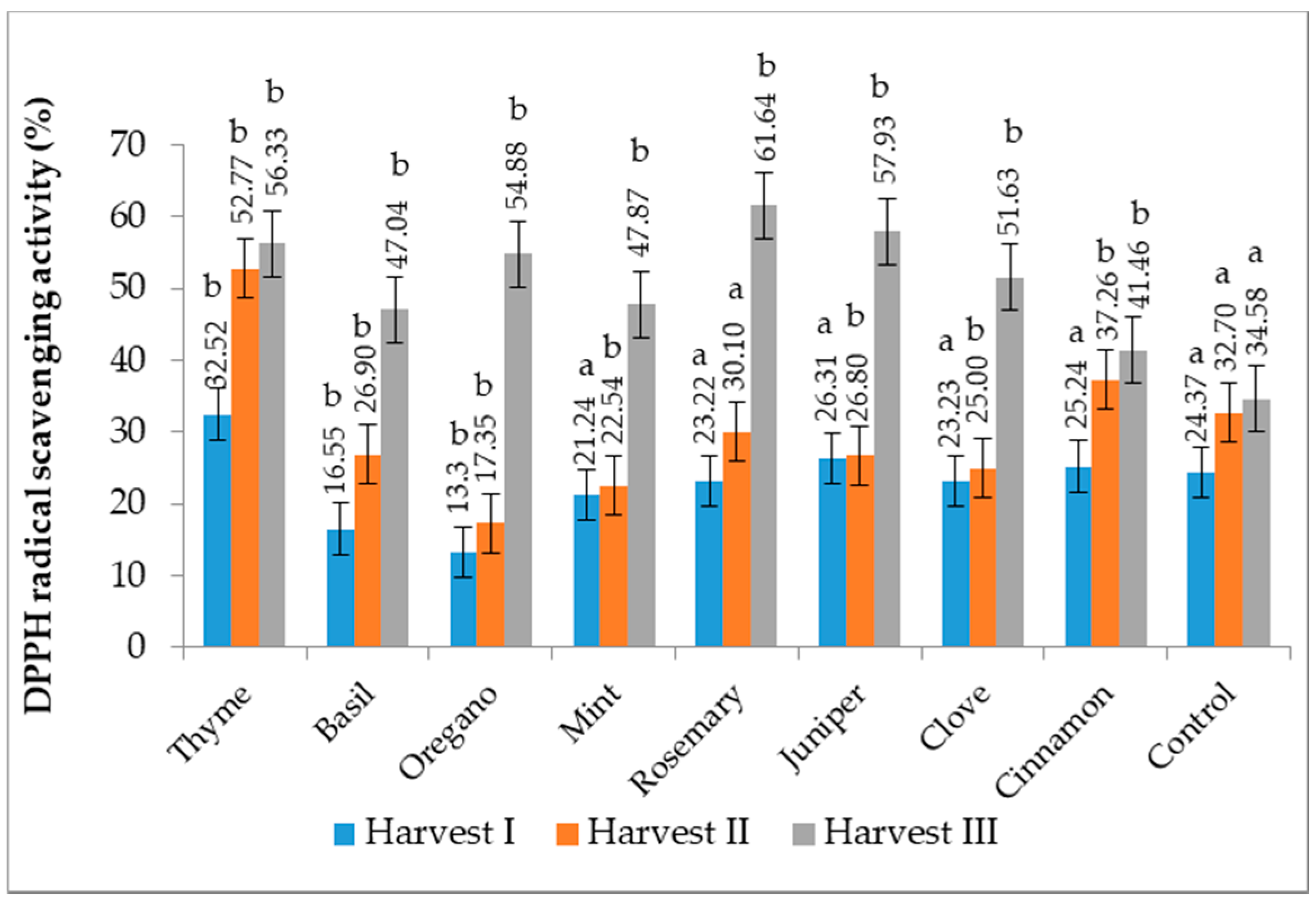
3.9. Antioxidant Activity by DPPH
3.10. Antimicrobial Activity
4. Discussion
4.1. Chemical Composition
4.2. Antimicrobial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and microbial infections: A review supporting the use of honey for microbial control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef]
- Abdellah, F.; Boukraâ, L.; Mohamed, H.S.; Alzahrani, H.A.; Bakhotmah, B. Synergistic effect of Honey and Thymus ciliatus against pathogenic bacteria. Open Nutraceuticals J. 2012, 5, 174–178. [Google Scholar] [CrossRef][Green Version]
- Cooper, R.A.; Molan, P.C.; Harding, K.G. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med. 1999, 92, 283–285. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G. Physicochemical characterization and antioxidant activity of commercial portuguese honeys. J. Food Sci. 2013, 78, 1159–1165. [Google Scholar] [CrossRef]
- Zainol, M.I.; Mohd Yusoff, K.; Mohd Yusof, M.Y. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013, 13, 129. Available online: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/1472-6882-13-129 (accessed on 20 December 2021).
- Wahdan, H.A. Causes of the antimicrobial activity of honey. Infection 1998, 26, 26–31. [Google Scholar] [CrossRef]
- Adeleke, O.E.; Olaitan, J.O.; Okpekpe, E.I. Comparative antibacterial activity of honey and gentamicina gainst Escherichiacoliand Pseudomonas aeruginosa. Ann. Burn. Fire Disasters 2006, 19, 201–204. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3188119/ (accessed on 20 December 2021).
- Khoo, Y.T.; Halim, A.S.; Singh, K.K.B.; Mohamad, N.A. Wound contraction effects and antibacterial properties of Tualang honey on full-thickness burn wounds in rats in comparison to hydrofibre. BMC Complement. Altern. Med. 2010, 10, 48. Available online: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/1472-6882-10-48 (accessed on 20 December 2021). [CrossRef]
- Montenegro, G.; Mejías, E. Biological applications of honeys produced by Apis mellifera. Biol. Res. 2013, 46, 341–345. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/112647/WHO_HSE_PED_AIP_?sequence=1 (accessed on 20 December 2021).
- Nishio, E.K.; Ribeiro, J.M.; Oliveira, A.G.; Andrade, G.C.T.J.; Proni, E.A.; Kobayashi, R.K.T.; Nakazato, G. Antibacterial synergic effect of honey from two stingless bees: Scaptotrigona bipunctata Lepeletier, 1836, and S. postica Latreille. 1807. Sci. Rep. 2016, 6, 21641. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Global Health Guardian: Climate Change, Air Pollution and Antimicrobial Resistance; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Coates, A.R.M.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef]
- Valgas, C.; Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Kim, S.I.; Roh, J.Y.; Kim, D.H.; Lee, H.S.; Ahn, Y.J. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 2003, 39, 293–303. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. Available online: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/1472-6882-6-39 (accessed on 20 December 2021). [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, R.N.; Pătruică, S. Use of Essential Oils in Bees. Sci. Pap. Anim. Sci. Biotechnol. 2020, 53, 74–79. Available online: http://conference.spasb.ro/index.php/issbar/2020/paper/view/1249 (accessed on 20 December 2021).
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey antibacterial effect boosting using Origanum vulgare L. essential oil. Evid.-Based Complement. Altern. Med. 2018, 2018, 7842583. [Google Scholar] [CrossRef]
- Boukra, L.; Alzahrani, H.A.; Abdelah, F.; Bakhotmah, B.; Hammoudi, S.M. Synergistic Effect of Monofloral Honeys and Essential Oils against Pseudomonas aeruginosa. Br. Microbiol. Res. J. 2013, 3, 564–573. [Google Scholar] [CrossRef]
- Lazăr, R.N.; Moț, D.; Simiz, E.; Pătruică, S. Research on the use of essential oils on the health of bee families. Sci. Pap. Anim. Sci. Biotechnol. 2020, 53, 161–164. Available online: https://www.spasb.ro/index.php/spasb (accessed on 20 December 2021).
- Lazăr, R.N.; Moț, D.; Alexa, E.; Boldea, M.; Ștef, L.; Pătruică, S. Influence of essential oils on bioproductive indices and health of bee families. In Scientific Papers; Series D; Animal Science: Bucharest, Romania, 2021; Volume LXIV, pp. 247–253. [Google Scholar]
- Medhat, M.A.; Mohsen, M.S.A. Chemical composition, antioxidant and antimicrobial activity of the essential oil of the thyme and rosemary. Int. J. Acad. Res. Part A 2013, 5, 186–195. [Google Scholar] [CrossRef]
- Imelouane, B.; Amhamdi, H.; Wathelet, J.P.; Ankit, M.; Khedid, K.; El Bachiri, A. Chemical composition of the essential oil of thyme (Thymus vulgaris) from Eastern Morocco. Int. J. Agric. Biol. 2009, 11, 205–208. [Google Scholar]
- Schulz, H.; Schrader, B.; Quilitzsch, R.; Pfeffer, S.; Kruger, H. Rapid classification of basil chemotypes by variousvibrational spectroscopy methods. J. Agric. Food Chem. 2003, 51, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical Composition of Essential Oils, Chemistry, Safety and Applications, 1st ed.; Hashemi, S.M.B., Khaneghah, A.M., de Souza Sant’ Ana, A., Eds.; IFT Press, Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 119–122. ISBN 978-1-119-14934-7. [Google Scholar]
- Kokkini, S.; Karousou, R.; Hanlidou, E. Herbs of Labiatae. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3082–3090. [Google Scholar] [CrossRef]
- Muntean, D.; Licker, M.; Alexa, E.; Popescu, I.; Jianu, C.; Buda, V.; Dehelean, C.A.; Ghiulai, R.; Horhat, F.; Horhat, D.; et al. Evaluation of essential oil obtained from Mentha piperita L. against multidrug-resistant strains. Infect Drug Resist. 2019, 12, 2905–2914. [Google Scholar] [CrossRef]
- Alexa, V.T.; Galuscan, A.; Popescu, I.; Tirziu, E.; Obistioiu, D.; Floare, A.D.; Perdiou, A.; Jumanca, D. Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity. Molecules 2019, 24, 4043. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Burt, S.; Vlielander, R.; Haagsman, H.; Veldhuizen, E. Increase in activity of essential oil components carvacroland thymol against Escherichia coli O157:H7 by addition offood stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraivab, J.A.; Nunesa, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Javanivić, S.; Vukojević, J.; Marin, P.D. The Chemical Composition of some Lauraceae Essential Oils and Their Antifungal Activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef]
- Adams, R.P. Investigation of Juniperus species of the United States for new sources of cedarwood oil. Econ. Bot. 1987, 41, 48–54. Available online: https://www.jstor.org/stable/4254934 (accessed on 20 December 2021). [CrossRef]
- Pătruică, S.; Moț, D. The effect of using prebiotic and probiotic products on intestinal micro-flora of the honeybee (Apis mellifera carpatica). Bull. Entomol. Res. 2012, 102, 619–623. [Google Scholar] [CrossRef]
- Pătruică, S.; Huțu, I. Economic benefits of using prebiotic and probiotic products as supplements in stimulation feeds administered to bee colonies. Turk. J. Vet. Anim. Sci. 2013, 37, 259–263. [Google Scholar] [CrossRef]
- Pătruică, S.; Peț, I.; Simiz, E. Beekeeping in the context of climate change. In Scientific Papers; Series D; Animal Science: Bucharest, Romania, 2021; Volume LXIV, ISSN Online 2393-2260. [Google Scholar]
- Bobiș, R. Researches on the Nutritional and Biological Value of Bee Pollen. Doctoral Thesis, the Faculty of Animal Husbandry and Biothnology, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Cluj-Napoca, Romania, 2014. Available online: http://www.usamvcluj.ro/files/teze/2014/margaoan.pdf (accessed on 20 December 2021).
- International Honey Commision. World Network of Honey Science. HarmonisedMethods of the International Honey Commission. 2009. Available online: http://ihc-platform.net/ihcmethods2009.pdf (accessed on 20 December 2021).
- Gergen, I. Agri-Food Products Analysis; EUROSTAMPA Publishing House: Timișoara, Romania, 2004; 316p, ISBN 973-687-271-8. [Google Scholar]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Liu, W.; Xiong, S.-H.; Tang, J.; Lou, Z.-H.; Xie, M.-X.; Xia, B.-H.; Lin, L.-M.; Liao, D.-F. Determination of Total Flavonoids Contents and Antioxidant Activity of Ginkgo biloba Leaf by Near-Infrared Reflectance Method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Alexa, V.T.; Szuhanek, C.; Cozma, A.; Galuscan, A.; Borcan, F.; Obistioiu, D.; Dehelean, C.A.; Jumanca, D. Natural Preparations Based on Orange, Bergamot and Clove Essential Oils and Their Chemical Compounds as Antimicrobial Agents. Molecules 2020, 25, 5502. [Google Scholar] [CrossRef]
- Gary, N.E. Activities and Behaviour of Honey Bees; Graham, J.M., Ed.; The Hive and the Honey Bee; Bookmasters Inc.: Mansfield, UK, 1992; Volume 8, pp. 269–361. [Google Scholar]
- European Union Directive (EU). European Union Directive 2001/110/EC, 32001L0110 Relating to Honey. Available online: www.eumonitor.eu/9353000/1/ (accessed on 20 December 2021).
- Turkish Food Codex. Number 26026. 2005. Available online: https://www.tariff-tr.com/legislation/item/6840.aspx (accessed on 20 December 2021).
- Bakirdere, S.; Yaroglu, T.; Tirik, N.; Demirez, M.; Karaca, A. Analysis of some physical, chemical and microbiological aspects of honey samples produced and consumed in Turkey. Rev. Fac. Cienc. Agrar. UNCuyo 2016, 50, 263–271. [Google Scholar]
- White, J.W. Composición y Propriedades De La Miel; McGregor, S.E., Ed.; La Apicultura en los Estados Unidos; Limusa: Mexico City, Mexico, 1979; pp. 57–66. [Google Scholar]
- Akharaiyi, F.C. Physicochemical Analysis and Mineral Contents of Honey from Farmers in Western States of Nigeria. J. Nat. Sci. Res. 2016, 6, 19. [Google Scholar]
- AL-Khalifa, A.S.; AL-Arify, I.A. Physicochemical characteristics and pollen spectrum of some Saudi honeys. Food Chem. 1999, 67, 21–25. [Google Scholar] [CrossRef]
- Molan, P.C. Authenticity of honey. In Food Authentication; Springer: Boston, MA, USA, 2006; pp. 259–303. [Google Scholar]
- Tafere Dessie Ashagrie, Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [CrossRef]
- Sohaimya, S.A.E.; Masryb, S.H.D.; Shehataa, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Duca, A. Contributions to the Experimental Characterization of Indigenous Propolis Varieties. Ph.D. Thesis, “Victor Babeş” University of Medicine and Pharmacy, Timișoara, Romania, 2018. [Google Scholar]
- Duca, A.; Sturza, A.; Moacă, E.A.; Negrea, M.; Lalescu, V.D.; Lungescu, D.; Dehelean, C.A.; Muntean, D.M.; Alexa, E. Identification of resveratrol as bioactive compound of propolis from western Romania and characterization of phenolic profile and antioxidant activity of ethanolic extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Benedetti, S.; Cosio, M.S. Evaluation of the antioxidant power of honey, propolis and royal jelly by amperometric flow injection analysis. Talanta 2007, 71, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Tenore, G.; Ritieni, A.; Campiglia, P. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula). Food Chem. Toxicol. 2012, 50, 1955–1961. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V.; Bermejo, J.O. Characterization of avocado honey (Persea americana Mill.) produced in Southern Spain. Food Chem. 2019, 287, 214–221. [Google Scholar] [CrossRef]
- Pauliuc, D.; Oroian, M. Sugars content and physicochemical parameters of romanian rape honey. In Scientific Bulletin; Series F. Biotechnologies; University of Agronomic Sciences and Veterinary Medicine of Bucharest Faculty of Biotechnology: Bucharest, Romania, 2020; Volume XXIV, pp. 69–75, ISSN 2285-1364, CD-ROM ISSN 2285-5521, ISSN Online 2285-1372, ISSN-L 2285-1364. [Google Scholar]
- Gul, A.; Pelhivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Ibrahimi, H.; Hajdari, A. Phenolic and flavonoid content, and antioxidant activity of honey from Kosovo. J. Apic. Res. 2020, 59, 452–457. [Google Scholar] [CrossRef]
- Marghitas, L.A.; Dezmirean, D.S.; Pocol, C.B.; Ilea, M.; Bobis, O.; Gergen, I. The Development of a Biochemical Profile of Acacia Honey by Identifying Biochemical Determinants of its Quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 84–90. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Luís, G.; Pereira, D.E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, E.O.; Ogunsanya, T.; Aiwerioba, O.I.R. Conventional use of honey as antibacterial agent. Ann. Afr. Med. 2006, 5, 79–81. Available online: https://www.ajol.info/index.php/aam/article/view/8379 (accessed on 20 December 2021).
- Mundo, M.A.; Padilla-Zakour, O.I.; Worobo, R.W. Growth inhibition of food borne pathogens and foods spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taormina, P.J.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef]

| EOs | Chemical Composition | References |
|---|---|---|
| Thyme (Thymus Vulgaris L.) | Borneol 17.15% Alfa terpineol 6.05% Camphene 14.41% | Lazar et al. [22] |
| Thymol 39.44 p-Cymene 23.6 y-Terpinene 12.51 Ledol 2.24 | Medhat et al. [23] | |
| Camphor 38.54% Borneol 4.91% Camphene 17.19% | Imelouane et al. [24] | |
| Basil (Ocimum basilicum) | Estragol 55.73% Linalool 38.64% | Lazar et al. [22] |
| Estragol 80% Linalool 35–50% | Schultz et al. [25] | |
| Linalool 28.6% Estragole 21.7% | Politeo et al. [26] | |
| Rosemary (Rosmarinus officinalis) | Eucalyptol 52.82% Alfa pinene 17.56% Camphor 10.01% | Lazar et al. [22] |
| Alfa pinene 7.4% Beta pinene 5.0% 1.8 Cineole 43.6% Camphor 12.3% | Eslahi et al. [27] | |
| Borneol 24.13 Alfa pinene 9.72 Camphor 5.01 | Medhat and Mouhsen [23] | |
| Mint (Mentha piperita) | Menthone 30.73% Neomenthol 17.37% Limonene 9.52% | Lazar et al. [22] |
| Menthol 27–51% Limonene 0.5–6% Methyl acetate 2–4% | Kokkini et al. [28] | |
| Menthone 0.461% Beta Myrcene 1.659% p-Mentha-1.8-diene 29.576% | Muntean et al. [29] | |
| Clove (Syzygium aromaticum) | Eugenol 85.17% Carryophillene 8.15% Eugenol acetate 5.44% | Lazar et al. [22] |
| Eugenol 80.11% Eugenol acetate 13.54% | Alexa et al. [30] | |
| Eugenol 76.8% Limonene 0.1% Beta Caryophyllene 17.4% | Jirovetz et al. [31] | |
| Oregano (Origanum vulgare) | Carvacrol 33.42% O-cymene 22.98% Gamma terpinene 17.44% | Lazar et al. [22] |
| Carvacrol 80% Thymol 64% | Burt et al. [32] | |
| Carvacrol 14.5% Thymol 12.6% Beta fenchyl alcohol 12.8% | Teixeira et al. [33] | |
| Cinnamon (Cinnamomum verum) | Cinnamaldehyde (E) 69.28% Cinnamaldehyde-o-methoxy 14.30% Cinnamil acetate 6.55% | Lazar et al. [22] |
| (E)-cinnamaldehyde 77.1% Eugenol 7.2% p-cymene 6.1% | Eslahi et al. [27] | |
| Trans-Cinamaldehyde 62.79% Limonene 8.31% Alfa-pinene 1.83% | Simić et al. [34] | |
| Juniper (Juniperus communis L.) | Alfa-pinene 49.75% Beta-pinene 16.70% Beta-myrcene 9.27% | Lazar et al. [22] |
| Alfa pinene 33.7% Sabinene 27.6% Myrcene 5.5% | Eslahi et al. [27] | |
| Limonene 27.8% Sabinene 38.0% Myrcene 22.6% | Adams, 1987 [35] |
| Experimental Variant | Extra Feeding | Quantity of Honey Harvested (g) | ||||
|---|---|---|---|---|---|---|
| Sugar Syrup (l) | EOs (µL/L) | The Period of Feeding | At 7 Days after the EOs Administration (Harvest I) (g) | At 20 Days after the EOs Administration(Harvest II) (g) | After the Final Harvest of Production (Rapeseed) (Harvest III) (g) | |
| Thyme | 1 l | 20 | 30.03 07.04 14.04 | 105.3 | 116.8 | 115.2 |
| Basil | 1 l | 20 | 102.6 | 106.1 | 107.6 | |
| Rosemary | 1 l | 20 | 102.9 | 103.2 | 103.5 | |
| Juniper | 1 l | 20 | 104.8 | 105.5 | 105.9 | |
| Mint | 1 l | 20 | 110.9 | 110.9 | 111.0 | |
| Oregano | 1 l | 20 | 109.5 | 111.3 | 112.5 | |
| Cloves | 1 l | 20 | 106.8 | 110.1 | 110.6 | |
| Cinnamon | 1 l | 20 | 103.8 | 103.2 | 109.7 | |
| Control | 1 l | - | 115.1 | 110.5 | 109.5 | |
| Harvest | Sample | S. pyogenes | S. aureus | S. flexneri | P. aeruginosa | E. coli | S. typhimurium | H. influenzae Type B | C. parapsilopsis | C. albicans |
|---|---|---|---|---|---|---|---|---|---|---|
| II | C 12.5% | 0.734 | 0.452 | 0.936 | 1.139 | 1.212 | 0.826 | 0.675 | 0.491 | 0.239 |
| C 25% | 0.740 | 0.605 | 0.933 | 1.198 | 0.921 | 0.898 | 1.054 | 0.801 | 0.547 | |
| C 37.5% | 0.748 | 0.784 | 0.923 | 1.199 | 0.859 | 0.905 | 1.147 | 0.870 | 0.874 | |
| C 50% | 0.811 | 0.796 | 0.878 | 1.204 | 0.777 | 1.527 | 1.219 | 1.149 | 0.959 | |
| B 12.5% | 0.825 | 0.613 | 1.014 | 1.223 | 0.938 | 1.121 | 1.075 | 0.623 | 0.686 | |
| B 25% | 0.842 | 0.755 | 0.922 | 1.201 | 0.814 | 0.875 | 1.091 | 0.785 | 0.854 | |
| B 37.5% | 0.877 | 0.988 | 0.91 | 1.138 | 0.696 | 0.837 | 1.186 | 0.843 | 1.025 | |
| B 50% | 0.899 | 0.999 | 0.882 | 1.117 | 0.632 | 0.812 | 1.261 | 0.940 | 1.175 | |
| T 12.5% | 0.875 | 0.710 | 0.795 | 1.222 | 1.28 | 1.204 | 1.115 | 1.190 | 0.473 | |
| T 25% | 1.183 | 0.843 | 0.918 | 1.287 | 0.931 | 1.147 | 1.148 | 0.836 | 0.765 | |
| T 37.5% | 1.163 | 1.163 | 1.014 | 1.47 | 0.882 | 1.027 | 1.271 | 0.735 | 0.843 | |
| T 50% | 1.177 | 1.183 | 1.047 | 1.557 | 0.769 | 1.004 | 1.436 | 0.694 | 0.892 | |
| R 12.5% | 1.135 | 0.725 | 0.849 | 1.281 | 0.826 | 0.895 | 1.127 | 0.597 | 1.042 | |
| R 25% | 1.037 | 0.721 | 0.838 | 1.103 | 0.936 | 1.27 | 1.441 | 1.224 | 1.318 | |
| R 37.5% | 0.747 | 0.67 | 0.777 | 0.927 | 1.138 | 1.638 | 1.551 | 1.430 | 1.431 | |
| R 50% | 0.719 | 0.534 | 0.705 | 0.946 | 1.207 | 1.696 | 1.569 | 1.635 | 1.522 | |
| O 12.5% | 0.787 | 0.662 | 0.711 | 1.289 | 1.223 | 1.201 | 1.089 | 1.079 | 0.325 | |
| O 25% | 0.943 | 0.581 | 0.830 | 1.304 | 1.139 | 0.898 | 1.141 | 0.886 | 0.606 | |
| O 37.5% | 1.079 | 0.535 | 1.025 | 1.336 | 1.039 | 0.732 | 1.287 | 0.818 | 0.758 | |
| O 50% | 1.098 | 0.513 | 1.057 | 1.407 | 0.995 | 0.683 | 1.433 | 0.795 | 0.825 | |
| J 12.5% | 0.982 | 0.722 | 0.830 | 1.125 | 0.977 | 0.995 | 1.197 | 0.850 | 0.985 | |
| J 25% | 1.089 | 0.7 | 0.968 | 1.15 | 1.094 | 0.991 | 1.236 | 1.010 | 1.471 | |
| J 37.5% | 1.286 | 0.675 | 1.049 | 1.159 | 1.370 | 1.141 | 1.411 | 1.192 | 1.461 | |
| J 50% | 1.294 | 0.536 | 1.066 | 1.271 | 1.464 | 1.245 | 1.528 | 1.243 | 1.499 | |
| CL 12.5% | 0.780 | 0.625 | 0.789 | 1.031 | 0.595 | 1.099 | 1.220 | 1.010 | 0.224 | |
| CL 25% | 0.915 | 0.671 | 0.740 | 1.203 | 0.679 | 1.167 | 1.038 | 0.764 | 0.464 | |
| CL 37.5% | 1.022 | 0.721 | 0.703 | 1.22 | 0.731 | 1.227 | 0.993 | 0.639 | 0.685 | |
| CL 50% | 1.034 | 0.775 | 0.646 | 1.308 | 0.782 | 1.264 | 0.902 | 0.538 | 0.859 | |
| CI 12.5% | 1.268 | 0.545 | 0.753 | 1.417 | 0.838 | 0.937 | 1.319 | 0.766 | 0.609 | |
| CI 25% | 1.171 | 0.616 | 0.734 | 1.348 | 0.794 | 1.156 | 1.267 | 0.730 | 0.732 | |
| CI 37.5% | 1.029 | 1.008 | 0.722 | 1.219 | 0.777 | 1.198 | 1.233 | 0.868 | 0.768 | |
| CI 50% | 0.863 | 1.068 | 0.714 | 1.196 | 0.705 | 1.285 | 1.103 | 0.949 | 0.805 | |
| M 12.5% | 0.704 | 0.885 | 0.784 | 0.927 | 1.282 | 1.077 | 1.102 | 1.025 | 0.849 | |
| M 25% | 0.902 | 0.943 | 0.973 | 0.932 | 0.932 | 0.975 | 1.127 | 0.995 | 0.941 | |
| M 37.5% | 0.995 | 0.996 | 0.984 | 0.977 | 0.93 | 0.907 | 1.144 | 0.886 | 0.997 | |
| M 50% | 1.008 | 1.007 | 1.142 | 0.998 | 0.833 | 0.881 | 1.179 | 0.797 | 1.005 | |
| BHI | 0.796 | 0.621 | 0.956 | 1.043 | 1.353 | 1.043 | 1.234 | 0.914 | 1.029 |
| Harvest | Sample | S. pyogenes | S. aureus | S. flexneri | P. aeruginosa | E. coli | S. typhimurium | H. influenzae Type B | C. parapsilopsis | C. albicans |
|---|---|---|---|---|---|---|---|---|---|---|
| III | C 12.5% | 0.952 | 0.534 | 0.72 | 1.199 | 0.974 | 0.976 | 1.273 | 0.806 | 0.502 |
| C 25% | 0.943 | 0.735 | 0.736 | 1.129 | 0.906 | 0.933 | 1.575 | 0.762 | 0.633 | |
| C 37.5% | 0.865 | 0.738 | 0.78 | 1.082 | 0.881 | 0.913 | 1.13 | 0.695 | 0.733 | |
| C 50% | 0.701 | 0.823 | 0.95 | 1.03 | 0.825 | 0.887 | 0.151 | 0.659 | 0.902 | |
| B 12.5% | 0.782 | 0.552 | 0.881 | 1.018 | 1.223 | 0.985 | 0.834 | 0.540 | 0.686 | |
| B 25% | 0.723 | 0.464 | 0.733 | 1.13 | 0.964 | 0.928 | 1.183 | 0.734 | 0.746 | |
| B 37.5% | 0.710 | 0.423 | 0.722 | 1.247 | 0.941 | 0.852 | 1.244 | 0.980 | 0.834 | |
| B 50% | 0.714 | 0.406 | 0.682 | 1.38 | 0.927 | 0.796 | 1.251 | 0.996 | 0.906 | |
| T 12.5% | 0.831 | 0.670 | 0.731 | 1.321 | 0.955 | 1.242 | 1.321 | 0.991 | 0.730 | |
| T 25% | 0.939 | 0.644 | 0.885 | 1.209 | 0.936 | 1.199 | 1.335 | 0.950 | 0.859 | |
| T 37.5% | 1.079 | 0.625 | 1.056 | 0.931 | 0.872 | 1.144 | 1.459 | 0.885 | 0.956 | |
| T 50% | 1.099 | 0.61 | 1.089 | 0.918 | 0.884 | 0.977 | 1.461 | 0.775 | 1.060 | |
| R 12.5% | 0.855 | 0.564 | 0.716 | 1.271 | 0.972 | 1.018 | 1.241 | 0.739 | 0.575 | |
| R 25% | 0.804 | 0.602 | 0.682 | 1.189 | 0.87 | 0.983 | 1.166 | 0.681 | 0.612 | |
| R 37.5% | 0.761 | 0.624 | 0.637 | 1.184 | 0.75 | 0.903 | 1.134 | 0.616 | 0.736 | |
| R 50% | 0.722 | 0.621 | 0.628 | 0.997 | 0.704 | 0.880 | 0.682 | 0.587 | 0.779 | |
| O 12.5% | 0.812 | 0.686 | 0.756 | 1.301 | 1.193 | 1.166 | 1.122 | 0.958 | 0.451 | |
| O 25% | 0.889 | 0.601 | 0.799 | 1.321 | 1.124 | 0.958 | 1.197 | 0.853 | 0.583 | |
| O 37.5% | 1.004 | 0.521 | 0.977 | 1.345 | 1.014 | 0.843 | 1.234 | 0.796 | 0.678 | |
| O 50% | 1.113 | 0.497 | 1.038 | 1.397 | 0.982 | 0.705 | 1.362 | 0.708 | 0.789 | |
| J 12.5% | 1.041 | 0.699 | 0.809 | 1.125 | 0.876 | 0.904 | 1.174 | 0.799 | 0.921 | |
| J 25% | 1.102 | 0.658 | 0.924 | 1.18 | 0.947 | 0.925 | 1.205 | 0.882 | 1.246 | |
| J 37.5% | 1.158 | 0.576 | 1.008 | 1.199 | 1.178 | 1.054 | 1.351 | 0.943 | 1.308 | |
| J 50% | 1.198 | 0.502 | 1.034 | 1.205 | 1.264 | 1.154 | 1.429 | 1.087 | 1.412 | |
| CL 12.5% | 0.668 | 0.589 | 0.687 | 0.947 | 0.548 | 0.987 | 1.156 | 0.970 | 0.274 | |
| CL 25% | 0.847 | 0.628 | 0.657 | 1.084 | 0.597 | 1.027 | 1.005 | 0.789 | 0.387 | |
| CL 37.5% | 0.987 | 0.697 | 0.602 | 1.124 | 0.731 | 1.121 | 0.984 | 0.618 | 0.452 | |
| CL 50% | 1.008 | 0.724 | 0.581 | 1.189 | 0.772 | 1.184 | 0.859 | 0.555 | 0.548 | |
| CI 12.5% | 1.028 | 0.541 | 0.689 | 1.258 | 0.785 | 0.887 | 1.114 | 0.687 | 0.609 | |
| CI 25% | 0.987 | 0.589 | 0.620 | 1.208 | 0.702 | 0.954 | 1.007 | 0.741 | 0.678 | |
| CI 37.5% | 0.905 | 0.741 | 0.587 | 1.115 | 0.644 | 1.089 | 0.941 | 0.799 | 0.724 | |
| CI 50% | 0.824 | 0.789 | 0.524 | 1.041 | 0.601 | 1.117 | 0.874 | 0.852 | 0.799 | |
| M 12.5% | 0.885 | 0.458 | 0.942 | 1.353 | 1.286 | 1.040 | 1.197 | 1.182 | 0.561 | |
| M 25% | 0.729 | 0.623 | 0.872 | 1.154 | 1.101 | 1.183 | 1.098 | 0.858 | 0.622 | |
| M 37.5% | 0.716 | 1.378 | 0.875 | 1.018 | 1.094 | 1.416 | 1.21 | 0.746 | 0.721 | |
| M 50% | 0.705 | 1.442 | 0.829 | 0.934 | 1.049 | 1.521 | 1.068 | 0.724 | 0.886 | |
| BHI | 0.796 | 0.621 | 0.956 | 1.043 | 1.353 | 1.043 | 1.234 | 0.914 | 1.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, R.N.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Pătruică, S. The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity. Appl. Sci. 2022, 12, 1094. https://doi.org/10.3390/app12031094
Lazăr RN, Alexa E, Obiștioiu D, Cocan I, Pătruică S. The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity. Applied Sciences. 2022; 12(3):1094. https://doi.org/10.3390/app12031094
Chicago/Turabian StyleLazăr, Roxana Nicoleta, Ersilia Alexa, Diana Obiștioiu, Ileana Cocan, and Silvia Pătruică. 2022. "The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity" Applied Sciences 12, no. 3: 1094. https://doi.org/10.3390/app12031094
APA StyleLazăr, R. N., Alexa, E., Obiștioiu, D., Cocan, I., & Pătruică, S. (2022). The Effect of the Use of Essential Oils in the Feed of Bee Families on Honey Chemical Composition and Antimicrobial Activity. Applied Sciences, 12(3), 1094. https://doi.org/10.3390/app12031094







