Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications
Abstract
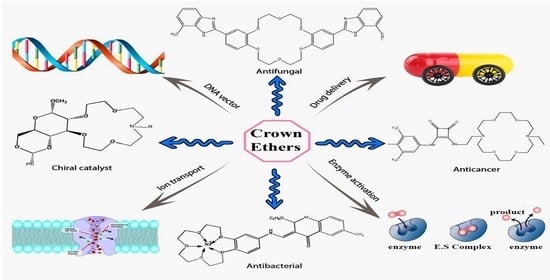
:1. Introduction
2. The History of Crown Ethers
Crown Ethers as Solvent Extraction Reagents
3. Synthesis of Crown Ethers and Their Derivatives
3.1. Synthesis of Dibenzo-18-crown-6 Ether
3.2. Synthesis of Bis-benzo-15-crown-5 Ether
3.3. Synthesis of Aza-Crown Ether-Squaramide Conjugates
3.4. Synthesis of Dibenzothiazolylodibenzo-18-crown-6 Ether
3.5. Synthesis of (γ-Arylpyridino)-dibenzoaza-14-crown-4 Ether
4. Biological and Pharmacological Analysis of Crown Ethers
4.1. Antibacterial Potential
4.2. Antifungal Potential
4.3. Anticancer Potential
4.4. Toxicology
4.5. Amyloidogenesis Inhibitory Activity
4.6. Electrochemical Sensor
4.7. Disposable Potentiometric Sensor
4.8. Chiral Catalyst
4.9. Peptide Interaction and Self-Assembly
4.10. Crown Ethers as P-Glycoprotein Inhibitors
4.11. DNA Targeting
4.12. Enzyme Activation
4.13. Crown Ethers as Vesicles
4.14. Membrane Anchors
4.15. Transfection Activity
4.16. Crown Ethers-Tyrosine Kinase Inhibitors
4.17. Fluorescent Chemosensor
4.18. Ion Transport
4.19. Computational Study
4.20. Antimicrobial Peptides
4.21. Covalent Organic Frameworks (COFs)
4.22. Quinolines Derivatives
4.23. Piperidones
4.24. Dibenzothiazolyldibenzo-18-crown-6 Ether
4.25. Benzo-15-crown-5 Substituted Coumarin
4.26. (ɤ-Arylpyridino)-dibenzoaza-14-crown-4 Ethers
4.27. Benzo-Oxo Crown Ethers and Macrocyclic Benzothio Crown Ethers
4.28. 4-((Substituted bis-indolyl)methyl)-benzo-15-crown-5 Ether
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ethers, A.; Basok, S.S.; Schepetkin, I.A.; Khlebnikov, A.I.; Lutsyuk, A.F.; Kirichenko, T.I.; Kirpotina, L.N.; Pavlovsky, V.I.; Leonov, K.A.; Vishenkova, D.A.; et al. Synthesis, Biological Evaluation, and Molecular Modeling of Aza-Crown Ethers. Molecules 2021, 26, 2225. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Crown Ethers, Cryptands and Other Compounds; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Alabdaly, B.I. Synthesis, Characterization and Antibacterial Activity of New Complexes of Some Lanthanide Ions with Benzo 18-Crown-6 and 221-Cryptand. IOSR J. Appl. Chem. 2013, 6, 32–39. [Google Scholar] [CrossRef]
- Ijeri, V.; Vocanson, F.; Martelet, C.; Jaffrezic-renault, N.; Lyon, E.C. De Capacitive Sensing of Amino Acids Using Caliraxene-Coated Silicon Transducers. Electroanalysis. 2007, 19, 510–514. [Google Scholar] [CrossRef]
- Bukhzam, A.; Bader, N. Crown Ethers: Their Complexes and Analytical Applications. J. Appl. Chem. 2014, 3, 237–244. [Google Scholar]
- Steed, J.W. First- and second-sphere coordination chemistry of alkali metal crown ether complexes. Coord. Chem. Rev. 2001, 215, 171–221. [Google Scholar] [CrossRef]
- Jedliński, Z. Novel Electron-Transfer Reactions Mediated by Alkali Metals Complexed by Macrocyclic Ligand. Acc. Chem. Res. 1998, 31, 55–61. [Google Scholar] [CrossRef]
- Ushakov, E.N.; Alfimov, M.V.; Gromov, S.P. Crown Ether Based Optical Molecular Sensors and Photocontrolled Crown Ether-Based Optical Molecular Sensors and Photocontrolled Ionophores. J. Macroheterocyc. 2010, 3, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 2, 7017–7036. [Google Scholar] [CrossRef]
- Lee, W.; Bang, E.; Yun, J.; Paik, M.; Lee, W. Enantiodiscrimination Using a Chiral Crown Ether as a Chiral Solvating Agent Using NMR Spectroscopy. J. SAGE 2019. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh Kakhki, R.; Rakhshanipour, M. Application of nanoparticle modified with crown ether in colorimetric determinations. Arab. J. Chem. 2019, 12, 3096–3107. [Google Scholar] [CrossRef] [Green Version]
- Tkachenko, N.V.; Sun, Z.; Boldyrev, A.I. Record Low Ionization Potentials of Alkali Metal Complexes with Crown Ethers and Cryptands. ChemPhysChem 2019, 20, 2060–2062. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Macrocycles (Construction, Chemistry and Nanotechnology Applications)II Front Matter; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 34–76. ISBN 9780470980200. [Google Scholar]
- Ahmed, A.; Hashmi, M.A.; Ayub, K. Permeation selectivity of alkali metal ions through crown ether based ion channels. J. Mol. Liq. 2020, 302, 112577. [Google Scholar] [CrossRef]
- Cooper, S.R. Crown Thioether Chemistry. Acc. Chem. Res. 1988, 21, 141–146. [Google Scholar] [CrossRef]
- Bond, A.H.; Dietz, M.L.; Chiarizia, R. Incorporating size selectivity into synergistic solvent extraction: A review of crown ether-containing systems. Ind. Eng. Chem. Res. 2000, 39, 3442–3464. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. The role of crown ethers in drug delivery. Supramol. Chem. 2019, 31, 221–238. [Google Scholar] [CrossRef]
- Christy, F.A.; Shrivastav, P.S. Conductometric studies on cation-crown ether complexes: A review. Crit. Rev. Anal. Chem. 2011, 41, 236–269. [Google Scholar] [CrossRef]
- De Jong, F.; Reinhoudt, D.N. Stability and Reactivity of Crown-Ether Complexes. Adv. Phys. Org. Chem. 1980, 17, 279–433. [Google Scholar] [CrossRef]
- Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. A dual-responsive supramolecular polymer gel formed by crown ether based molecular recognition. Angew. Chem.-Int. Ed. 2011, 50, 1905–1909. [Google Scholar] [CrossRef]
- Fang, A.; Kroenlein, K.; Riccardi, D.; Smolyanitsky, A. Highly mechanosensitive ion channels from graphene-embedded crown ethers. Nat. Mater. 2019, 18, 76–81. [Google Scholar] [CrossRef]
- Hendrixson, R.R.; Mack, M.P.; Palmer, R.A.; Ottolenghi, A.; Ghirardelli, R.G. Oral toxicity of the cyclic polyethers-12-crown-4, 15-crown-5, and 18-crown-6-in mice. Toxicol. Appl. Pharmacol. 1978, 44, 263–268. [Google Scholar] [CrossRef]
- Hyun, M.H. Development and application of crown ether-based HPLC chiral stationary phases. Bull. Korean Chem. Soc. 2005, 26, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Kuang, H.; Chen, W.; Yan, W.; Xu, L.; Zhu, Y.; Liu, L.; Chu, H.; Peng, C.; Wang, L.; Kotov, N.A.; et al. Crown ether assembly of gold nanoparticles: Melamine sensor. Biosens. Bioelectron. 2011, 26, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nalluri, S.K.M.; Fraser Stoddart, J. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Crown Ether-Functionalized Polybenzoxazine for Metal Ion Adsorption. Macromolecules 2020, 53, 2420–2429. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Nicoletta, F.P.; Trombino, S.; Cassano, R.; Iemma, F.; Picci, N. A new crown ether as vesicular carrier for 5-fluoruracil: Synthesis, characterization and drug delivery evaluation. Colloids Surf. B Biointerfaces 2007, 58, 197–202. [Google Scholar] [CrossRef]
- Ngo, H.L.; Lin, W. Chiral crown ether pillared lamellar lanthanide phosphonates. J. Am. Chem. Soc. 2002, 124, 14298–14299. [Google Scholar] [CrossRef]
- Reuter, K.; Rudel, S.S.; Buchner, M.R.; Kraus, F.; von Hänisch, C. Crown Ether Complexes of Alkali-Metal Chlorides from SO2. Chem.-A Eur. J. 2017, 23, 9607–9617. [Google Scholar] [CrossRef]
- Rupar, P.A.; Bandyopadhyay, R.; Cooper, B.F.T.; Stinchcombe, M.R.; Ragogna, P.J.; Macdonald, C.L.B.; Baines, K.M. Cationic Crown Ether Complexes of Germanium(II). Angew. Chemie. 2009, 121, 5257–5260. [Google Scholar] [CrossRef]
- Saddik, A.A.; Mohammed, M.; Lin, H.C. The crown ether size and stereochemistry affect the self-assembly, hydrogelation, and cellular interactions of crown ether/peptide conjugates. J. Mater. Chem. B. 2020, 8, 9961–9970. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, H.; Yang, C.; Li, Y. Synthesis and electroluminescence of novel copolymers containing crown ether spacers. J. Mater. Chem. 2003, 13, 800–806. [Google Scholar] [CrossRef]
- Tsukanov, A.V.; Dubonosov, A.D.; Bren, V.A.; Minkin, V.I. Organic chemosensors with crown-ether groups (review). Chem. Heterocycl. Compd. 2008, 44, 899–923. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and their use in drug delivery—A review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, M.; Kralj, M.; Supek, F.; Frkanec, L.; Piantanida, I.; Šmuc, T.; Tušek-Božić, L. Antitumor potential of crown ethers: Structure-activity relationships, cell cycle disturbances, and cell death studies of a series of ionophores. J. Med. Chem. 2007, 50, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Gale, P.A. Supramolecular chemistry anniversary. Chem. Soc. Rev. 2017, 46, 2376–2377. [Google Scholar] [CrossRef]
- Caridade Costa, J.M.; Rodrigues, P.M.S. Complexation Study of Alkali Metal Ions by Crown Ether Derivatives in Nonaqueous Solvents by Potentiometric Methods. Port. Electrochim. Acta. 2002, 20, 167–178. [Google Scholar] [CrossRef]
- Archive, P.L.; Razavi, R. Trace Metal role on Crown ethers Stability by DET Abstract. J. Environ. Friendly Processes 2015, 3, 10–13. [Google Scholar]
- Toke, L.; Bitter, I.; Agai, B.; Hell, Z.; Pako, P.; Fenichel, L. Synthesis and Application of Crown Ethers. Period. Polytech. Chem. Eng. 1988, 32, 121–129. [Google Scholar]
- Capel-Cuevas, S.; de Orbe-Payá, I.; Santoyo-González, F.; Capitán-Vallvey, L.F. Double-armed crown ethers for calcium optical sensors. Talanta 2009, 78, 1484–1488. [Google Scholar] [CrossRef]
- Ackman, R.G.; Brown, W.H.; Wright, G.F. The condensation of methyl ketones with furan. J. Org. Chem. 1955, 20, 1147–1158. [Google Scholar] [CrossRef]
- Dardouri, M.; Chrayet, B.; Ammari, F. Free, Grafted on Polymers and Intercaled Ethers-Crowns in Clays and Applications. J. Maroc. Heterocyl. Chem. 2021, 20, 95–107. [Google Scholar]
- Kyba, E.P.; Helgeson, R.C.; Madan, K.; Gokel, G.W.; Tarnowski, T.L.; Moore, S.S.; Cram, D.J. Host-Guest Complexation. 1. Concept and Illustration. J. Am. Chem. Soc. 1977, 99, 2564–2571. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, H.; He, T.; Li, Y.; Yin, S. Fluorescent supramolecular polymers formed by crown ether based host guest interaction. Front. Chem. 2020, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W. Supramolecular Chemistry. Supramol. Chem. 1993, 3. [Google Scholar] [CrossRef]
- Kim, H.S.; Do, K.S.; Kim, K.S.; Shim, J.H.; Cha, G.S.; Nam, H. Ammonium ion binding property of naphtho-crown ethers containing thiazole as sub-cyclic unit. Bull. Korean Chem. Soc. 2004, 25, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Kantekin, H.; Hasançebi, Ö.; Abbasoglu, R.; Gök, Y. Synthesis and characterization of a novel (E,E)-dioxime and its mono- and heterotrinuclear complexes containing a 21-membered trioxadithiadiaza macrocycle. New J. Chem. 2001, 25, 879–886. [Google Scholar] [CrossRef]
- Zhang, X.X.; Bradshaw, J.S.; Izatt, R.M. Enantiomeric recognition of amine compounds by chiral macrocyclic receptors. Chem. Rev. 1997, 97, 3313–3361. [Google Scholar] [CrossRef]
- Smith, P.W.; Still, W.C. The effect of substitution and stereochemistry on ion binding in the polyether ionophore monensin. J. Am. Chem. Soc. 1988, 110, 7917–7919. [Google Scholar] [CrossRef]
- Mcdowell, J. Crown Ethers as Solvent Extraction Reagents: Where do We Stand? Separa. Sci. Technol. 2006, 23, 1251–1268. [Google Scholar] [CrossRef]
- Mohite, B.S.; Khopkar, S.M. Solvent extraction separation of strontium as 18-crown-6 complex with picrate ion. Anal. Chem. 1987, 59, 1200–1203. [Google Scholar] [CrossRef]
- Klenc, J.; Raux, E.; Barnes, S.; Sullivan, S.; Duszynska, B.; Bojarski, A.J.; Strekowski, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino) pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265. [Google Scholar] [CrossRef]
- Hayvalı, Z.; Güler, H.; Öğütcü, H.; Sarı, N. Novel bis-crown ethers and their sodium complexes as antimicrobial agent: Synthesis and spectroscopic characterizations. Med. Chem. Res. 2014, 23, 3652–3661. [Google Scholar] [CrossRef]
- Yu, X.H.; Cai, X.J.; Hong, X.Q.; Tam, K.Y.; Zhang, K.; Chen, W.H. Synthesis and biological evaluation of aza-crown ether-squaramide conjugates as anion/cation symporters. Future Med. Chem. 2019, 11, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Lu, X.M.; Chen, Y.T.; Lu, G.Y.; Zhang, J.J.; Shao, Y.; Liu, F.; Xu, Q. Synthesis, DNA-binding, cleavage, and cytotoxic activity of new 1,7-dioxa-4,10-diazacyclododecane artificial receptors containing bisguanidinoethyl or diaminoethyl double side arms. Chem.-A Eur. J. 2007, 13, 9703–9712. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, M.; Wu, L.; Guan, Y.; Pan, Y.; Jiang, J.; Lin, C.; Wang, L. Squaramide-based tripodal receptors for selective recognition of sulfate anion. Chem. Commun. 2013, 49, 2025–2027. [Google Scholar] [CrossRef]
- Cai, X.J.; Li, Z.; Chen, W.H. Tripodal squaramide conjugates as highly effective transmembrane anion transporters. Bioorganic Med. Chem. Lett. 2017, 27, 1999–2002. [Google Scholar] [CrossRef]
- Le, T.A.; Truong, H.H.; Thi, T.P.N.; Thi, N.D.; To, H.T.; Thi, H.P.; Soldatenkov, A.T. Synthesis and biological activity of (γ-arylpyridino)-dibenzoaza-14-crown-4 ethers. Mendeleev Commun. 2015, 25, 224–225. [Google Scholar] [CrossRef]
- Thapa, P.; Karki, R.; Thapa, U.; Jahng, Y.; Jung, M.J.; Nam, J.M.; Na, Y.; Kwon, Y.; Lee, E.S. 2-Thienyl-4-furyl-6-aryl pyridine derivatives: Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study. Bioorganic Med. Chem. 2010, 18, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Thapa, P.; Kang, M.J.; Jeong, T.C.; Nam, J.M.; Kim, H.L.; Na, Y.; Cho, W.J.; Kwon, Y.; Lee, E.S. Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study of hydroxylated 2,4-diphenyl-6-aryl pyridines. Bioorganic Med. Chem. 2010, 18, 3066–3077. [Google Scholar] [CrossRef]
- Şahin Gül, D.; Ogutcu, H.; Hayvalı, Z. Investigation of photophysical behaviours and antimicrobial activity of novel benzo-15-crown-5 substituted coumarin and chromone derivatives. J. Mol. Struct. 2020, 1204, 127569. [Google Scholar] [CrossRef]
- Febles, M.; Montalvão, S.; Crespín, G.D.; Norte, M.; Padrón, J.M.; Tammela, P.; Fernández, J.J.; Daranas, A.H. Synthesis and biological evaluation of crown ether acyl derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 5591–5593. [Google Scholar] [CrossRef] [Green Version]
- Arenaza-Corona, A.; Couce-Fortúnez, M.D.; De Blas, A.; Morales-Morales, D.; Santillan, R.; Höpfl, H.; Rodríguez-Blas, T.; Barba, V. Further Approaches in the Design of Antitumor Agents with Response to Cell Resistance: Looking toward Aza Crown Ether-dtc Complexes. Inorg. Chem. 2020, 59, 15120–15134. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Rao, P.; Li, Y.; Qiu, J.; Qiu, W.; Tang, H.; Potter, M.A. Toxicological responses of the earthworm Eisenia fetida to 18-crown-6 under laboratory conditions. Bull. Environ. Contam. Toxicol. 2014, 93, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Mizuguchi, M. Crown Ethers as Transthyretin Amyloidogenesis Inhibitors. J. Med. Chem. 2019, 62, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Electrochemical Determination of Neurotransmitters at Crown Ether Modified Carbon Nanotube Composite: Application for Sub-nano-sensing of Serotonin in Human Serum. Electroanalysis 2019, 31, 1204–1214. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiao, Q.; Zhang, Q.; Liu, Z. A novel crown ether-acylhydrazone turn-on fluorescent chemosensor for Al3+ ion. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 211, 1–8. [Google Scholar] [CrossRef]
- Kumbhat, S.; Singh, U. A potassium-selective electrochemical sensor based on crown-ether functionalized self assembled monolayer. J. Electroanal. Chem. 2018, 809, 31–35. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, Y.; Dong, C.; Huang, Y.; Sun, L.; Ruan, H.; Wang, H.; Li, X.; Wu, A. Rapid colorimetric detection of potassium ions based on crown ether modified Au NPs sensor. Sens. Actuators B Chem. 2019, 281, 783–788. [Google Scholar] [CrossRef]
- Khaled, E.; Hassan, H.N.A.; Ahmed, M.A.; El-Attar, R.O. Crown Ether/Carbon Nanotubes Based Biperiden Disposable Potentiometric Sensor. Electroanalysis 2017, 29, 975–982. [Google Scholar] [CrossRef]
- Bako, P.; Keglevich, G.; Rapi, Z. Asymmetric Phase Transfer Reactions Catalyzed by Chiral Crown Ethers Derived from Monosaccharides. Lett. Org. Chem. 2010, 7, 645–656. [Google Scholar] [CrossRef]
- Paquet-Côté, P.A.; Fillion, M.; Provencher, M.È.; Otis, F.; Dionne, J.; Cardinal, S.; Collignon, B.; Bürck, J.; Lagüe, P.; Ulrich, A.S.; et al. Crown ether modified peptide interactions with model membranes. Supramol. Chem. 2019, 31, 159–171. [Google Scholar] [CrossRef]
- Guberović, I.; Marjanović, M.; Mioč, M.; Ester, K.; Martin-Kleiner, I.; Šumanovac Ramljak, T.; Mlinarić-Majerski, K.; Kralj, M. Crown ethers reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Sci. Rep. 2018, 8, 14467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löffler, P.M.G.; Hansen, A.H.; Ries, O.; Jakobsen, U.; Rabe, A.; Sørensen, K.T.; Glud, K.; Vogel, S. Lipidated Polyaza Crown Ethers as Membrane Anchors for DNA-Controlled Content Mixing between Liposomes. Sci. Rep. 2019, 9, 13856. [Google Scholar] [CrossRef] [Green Version]
- Akkemik, E.; Cicek, B.; Camadan, Y.; Calisir, U.; Onbasioglu, Z. The determination of the carbonic anhydrases activators in vitro effect of mixed donor crown ethers. J. Biochem. Mol. Toxicol. 2018, 32, e22032. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, Y.; Jiang, H.; Zou, G.; Zhang, Q. Benzo-15-crown-5 functionalized polydiacetylene-based colorimetric self-assembled vesicular receptors for lead ion recognition. J. Mater. Chem. 2011, 21, 3604–3610. [Google Scholar] [CrossRef]
- Sewbalas, A.; Islam, R.U.; Van Otterlo, W.A.L.; De Koning, C.B.; Singh, M.; Arbuthnot, P.; Ariatti, M. Enhancement of transfection activity in HEK293 cells by lipoplexes containing cholesteryl nitrogen-pivoted aza-crown ethers. Med. Chem. Res. 2013, 22, 2561–2569. [Google Scholar] [CrossRef]
- Hu, S.; Xie, G.; Zhang, D.X.; Davis, C.; Long, W.; Hu, Y.; Wang, F.; Kang, X.; Tan, F.; Ding, L.; et al. Synthesis and biological evaluation of crown ether fused quinazoline analogues as potent EGFR inhibitors. Bioorganic Med. Chem. Lett. 2012, 22, 6301–6305. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, F.; Shen, J.; Zhang, H.; Wang, T.; Ye, R.; Li, T.; Loh, T.P.; Yang, Y.Y.; Zeng, H. Buckyball-Based Spherical Display of Crown Ethers for de Novo Custom Design of Ion Transport Selectivity. J. Am. Chem. Soc. 2020, 142, 21082–21090. [Google Scholar] [CrossRef]
- Van der Ham, A.; Hansen, T.; Lodder, G.; Codée, J.D.C.; Hamlin, T.A.; Filippov, D.V. Computational and NMR Studies on the Complexation of Lithium Ion to 8-Crown-4. ChemPhysChem. 2019, 20, 2103–2109. [Google Scholar] [CrossRef] [Green Version]
- You, D.; Liu, L.; Yang, Q.; Wu, X.; Li, S.; Li, A. A far-red aza-crown ether fluorescent probe for selective G-quadruplex DNA targeting. Dye. Pigment. 2020, 176, 108222. [Google Scholar] [CrossRef]
- Gholiee, Y.; Salehzadeh, S. The solvent effect on selectivity of four well-known cryptands and crown ethers toward Na+ and K+ cations; A computational study. J. Mol. Liq. 2020, 309, 113149. [Google Scholar] [CrossRef]
- Paquet-Côté, P.A.; Paradis, J.P.; Auger, M.; Voyer, N. Crown ether modified peptides: Length and crown ring size impact on membrane interactions. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183261. [Google Scholar] [CrossRef]
- An, S.; Xu, Q.; Ni, Z.; Hu, J.; Peng, C.; Zhai, L.; Guo, Y.; Liu, H. Construction of Covalent Organic Frameworks with Crown Ether Struts. Angew. Chem.-Int. Ed. 2021, 9959–9963. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Fu, S.; Yang, K.; Hou, B.; Liu, Y.; Jiang, J.; Cui, Y. Crystalline C-C and C=C Bond-Linked Chiral Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ashram, M.; Al-Mazaideh, G.M.; Al-Zereini, W.; Al-Mustafa, A.; Mizyed, S. Synthesis, complexation and biological effects studies of new thiacrown ethers derived quinoline: Part I. J. Sulfur Chem. 2019, 40, 277–288. [Google Scholar] [CrossRef]
- Dao, T.N.; Truong, H.H.; Luu, V.B.; Soldatenkov, A.T.; Kolyadina, N.M.; Kulakova, A.N.; Khrustalev, V.N.; Wodajo, A.T.; Nguyen, H.Q.; Van Tran, T.T.; et al. Synthesis and bioactivity of novel (γ-piperidono)dibenzo-33-aza-14-crown-3 ethers. Chem. Heterocycl. Compd. 2019, 55, 654–659. [Google Scholar] [CrossRef]
- Calisir, U.; Çiçek, B. Comparison of classic and microwave-assisted synthesis of benzo-thio crown ethers, and investigation of their ion pair extractions. J. Mol. Struct. 2017, 1148, 505–511. [Google Scholar] [CrossRef]
- Bhosale, T.R.; Chandam, D.R.; Anbhule, P.V.; Deshmukh, M.B. Synthesis of Novel 4-((Substituted bis-indolyl)methyl)-benzo-15-crown-5 for the Colorimetric Detection of Hg2+ Ions in an Aqueous Medium. J. Heterocycl. Chem. 2019, 56, 477–484. [Google Scholar] [CrossRef]


























| Samples | E. coli | S. typhi H | Br. abortus | L. monocytogenes | C. albicans |
|---|---|---|---|---|---|
| 12 | 16 | 13 | 23 | 12 | 24 |
| 13 | 17 | 14 | 24 | 12 | 23 |
| 14 | - | 14 | 15 | 12 | 22 |
| K30 | 25 | 20 | - | 15 | - |
| AMP10 | 10 | 11 | - | 16 | - |
| SXT25 | 18 | 17 | - | 11 | - |
| SCF | - | - | 12 | - | - |
| NYS100 | - | - | - | - | 20 |
| Studied Compounds | Aspergillus niger | Aspergillus oryzae |
|---|---|---|
| 500 ppm | 500 ppm | |
| 16d | 24 | 28.5 |
| DMF | - | - |
| Bavistin | 33 | 22 |
| Studied Compounds | Hela Cell Line |
|---|---|
| 17b | >50 |
| 17d | >50 |
| Doxorubicin | 0.13 ± 0.01 |
| Aza-crown ether Pt complex | 6.4 ± 0.2 |
| Cisplatin | 0.53 ± 0.6 |
| Studied Compounds | Concentrations | Anti-Amyloid Genesis Activity (%) |
|---|---|---|
| Diflunisal | 0.5 µM 1.5 µM | 80 ± 9.0 35 ± 1.0 |
| 18 | 0.2 mM 0.6 mM | 99 ± 0.25 92 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.A.; Khan, M.R.; Ullah, S.; Fayyaz ur Rehman, M.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Appl. Sci. 2022, 12, 1102. https://doi.org/10.3390/app12031102
Ullah F, Khan TA, Iltaf J, Anwar S, Khan MFA, Khan MR, Ullah S, Fayyaz ur Rehman M, Mustaqeem M, Kotwica-Mojzych K, et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Applied Sciences. 2022; 12(3):1102. https://doi.org/10.3390/app12031102
Chicago/Turabian StyleUllah, Faiz, Taskin Aman Khan, Jawaria Iltaf, Saleha Anwar, Muhammad Farhan Ali Khan, Muhammad Rizwan Khan, Sami Ullah, Muhammad Fayyaz ur Rehman, Muhammad Mustaqeem, Katarzyna Kotwica-Mojzych, and et al. 2022. "Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications" Applied Sciences 12, no. 3: 1102. https://doi.org/10.3390/app12031102
APA StyleUllah, F., Khan, T. A., Iltaf, J., Anwar, S., Khan, M. F. A., Khan, M. R., Ullah, S., Fayyaz ur Rehman, M., Mustaqeem, M., Kotwica-Mojzych, K., & Mojzych, M. (2022). Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Applied Sciences, 12(3), 1102. https://doi.org/10.3390/app12031102