A Simple Analysis Method of Specific Anammox Activity Using a Respirometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anammox Granules
2.2. Batch Test
2.3. Analysis
3. Results and Discussion
3.1. Changes in N2 Gas Production and rSAA According to the Reaction Time
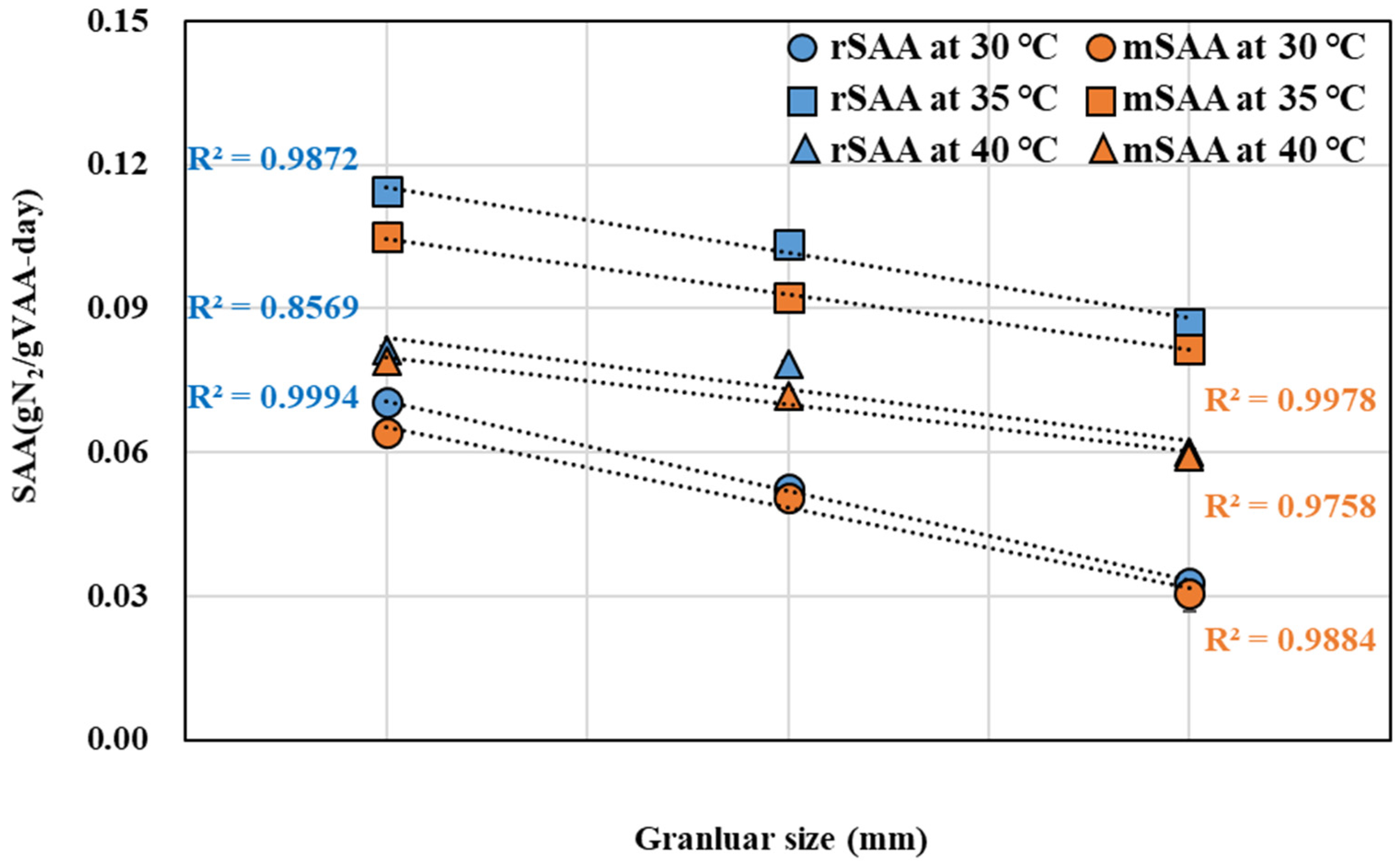
3.2. Comparison of rSAA and mSAA According to Temperature and Granule Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fenu, A.; Smolders, S.; De Gussem, K.; Weemaes, M. Conflicting carbon footprint and energy saving in a side-stream Anammox Process. Biochem. Eng. J. 2019, 151, 107336. [Google Scholar] [CrossRef]
- Kosgey, K.; Chandran, K.; Gokal, J.; Kiambi, S.L.; Bux, F.; Kumari, S. Critical Analysis of Biomass Retention Strategies in Mainstream and Sidestream ANAMMOX-Mediated Nitrogen Removal Systems. Environ. Sci. Technol. 2021, 55, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.; Yu, J.; Zheng, P. The inhibition of the Anammox process: A review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.; Kuenen, J.G.; Jetten, M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Daverey, A.; Chei, P.C.; Dutta, K.; Lin, J. Statistical analysis to evaluate the effects of temperature and pH on anammox activity. Int. Biodeterior. Biodegrad. 2015, 102, 89–93. [Google Scholar] [CrossRef]
- Puyol, D.; Carvajal-Arroyo, J.M.; Garcia, B.; Sierra-Alvarez, R.; Field, J.A. Kinetic characterization of Brocadia spp.-dominated anammox cultures. Bioresour. Technol. 2013, 139, 94–100. [Google Scholar] [CrossRef]
- Dsane, V.F.; An, S.; Oh, T.; Hwang, J.; Choi, Y.; Choi, Y. Saline conditions effect on the performance and stress index of anaerobic ammonium oxidizing (anammox) bacteria. Chemosphere 2021, 267, 129227. [Google Scholar] [CrossRef]
- Yoong, E.T.; Lant, P.A.; Greenfield, P.F. In situ respirometry in an SBR treating wastewater with high phenol concentrations. Water Res. 2000, 34, 239–245. [Google Scholar] [CrossRef]
- Sampson, M.; Blake, R., II. The cell attachment and oxygen consumption of two strains of Thiobacillus ferrooxidans. Miner. Eng. 1999, 12, 671–686. [Google Scholar] [CrossRef]
- Harahuc, L.; Lizama, H.M.; Suzuki, I. Selective Inhibition of the Oxidation of Ferrous Iron or Sulfur in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 2000, 66, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Buvignier, A.; Peyre-Lavigne, M.; Robin, O.; Bounouba, M.; Patapy, C.; Bertron, A.; Paul, E. Influence of Dissolved-Aluminum Concentration on Sulfur-Oxidizing Bacterial Activity in the Biodeterioration of Concrete. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- du Plessis, C.A.; Barnard, P.; Naldrett, K.; de Kock, S.H. Development of respirometry methods to assess the microbial activity of thermophilic bioleaching archaea. J. Microbiol. Methods 2001, 47, 189–198. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Puente, F.; Schmid, T.; Pelayo, M.; Rodríguez-Rastrero, M.; Herraiz, M.S.; O’Neill, T.; López-Martínez, J. Abiotic factors influencing soil microbial activity in the northern Antarctic Peninsula region. Sci. Total Environ. 2021, 750, 141602. [Google Scholar] [CrossRef] [PubMed]
- Rauglas, E.; Martin, S.; Bailey, K.; Magnuson, M.; Phillips, R.; Harper, W.F., Jr. The effect of malathion on the activity, performance, and microbial ecology of activated sludge. J. Environ. Manag. 2016, 183, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Raper, E.; Stephenson, T.; Fisher, R.; Anderson, D.R.; Soares, A. Characterisation of thiocyanate degradation in a mixed culture activated sludge process treating coke wastewater. Bioresour. Technol. 2019, 288, 121524. [Google Scholar] [CrossRef] [PubMed]
- Amha, Y.M.; Sinha, P.; Lagman, J.; Gregori, M.; Smith, A.L. Elucidating microbial community adaptation to anaerobic co-digestion of fats, oils, and grease and food waste. Water Res. 2017, 123, 277–289. [Google Scholar] [CrossRef]
- Echavarri-Bravo, V.; Paterson, L.; Aspray, T.J.; Porter, J.S.; Winson, M.K.; Hartl, M.G. Natural marine bacteria as model organisms for the hazard-assessment of consumer products containing silver nanoparticles. Mar. Environ. Res. 2017, 130, 293–302. [Google Scholar] [CrossRef]
- Bonaglia, S.; Broman, E.; Brindefalk, B.; Hedlund, E.; Hjorth, T.; Rolff, C.; Nascimento, F.J.; Udekwu, K.; Gunnarsson, J.S. Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 2020, 248, 126023. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, C.; Wei, L.; Wei, D.; Zhao, M.; Zhao, Z.; Zhang, J.; Chang, C. Activated sludge and other aerobic suspended culture processes. Water Environ. Res. 2020, 92, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- R.C. Team. R: A Language and Environment for Statistical Computing; RC Team: Vienna, Austria, 2013. [Google Scholar]
- Tomaszewski, M.; Cema, G.; Ziembińska-Buczyńska, A. Influence of temperature and pH on the anammox process: A review and meta-analysis. Chemosphere 2017, 182, 203–214. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Xu, X.; Yang, F.; Li, Z. Comparison of the characteristics of anammox granules of different sizes. Biotechnol. Bioprocess Eng. 2013, 18, 446–454. [Google Scholar] [CrossRef]
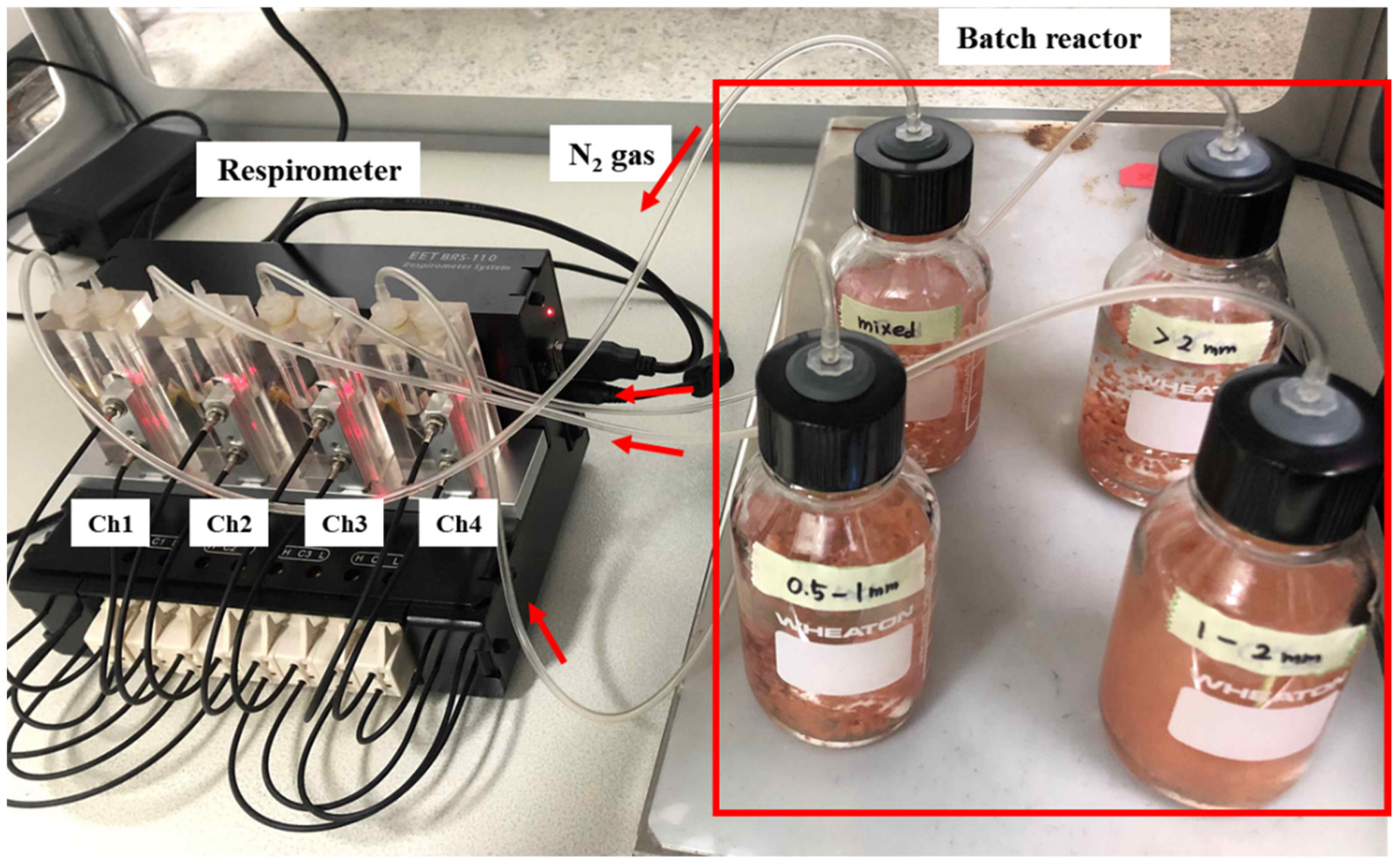
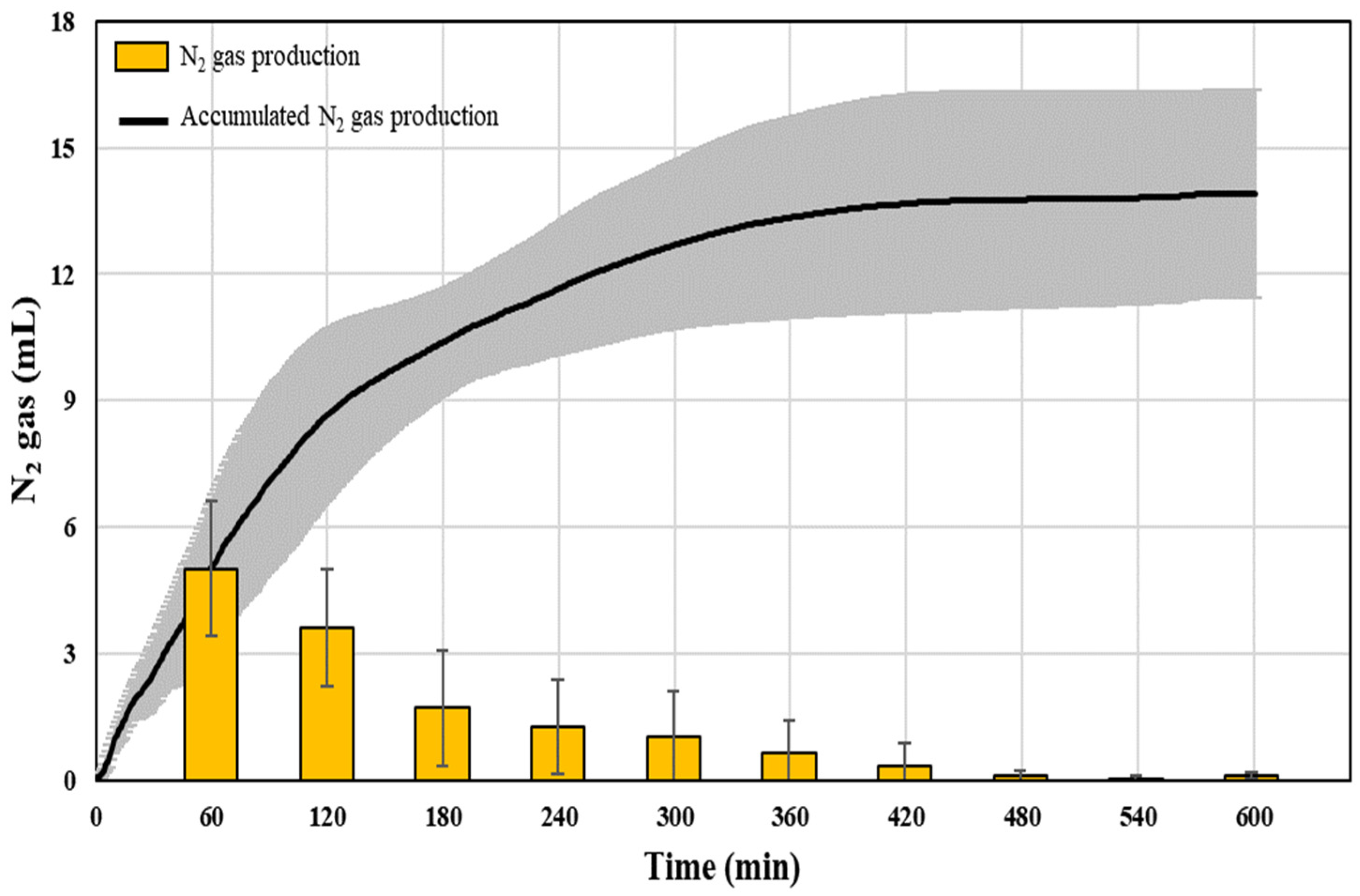
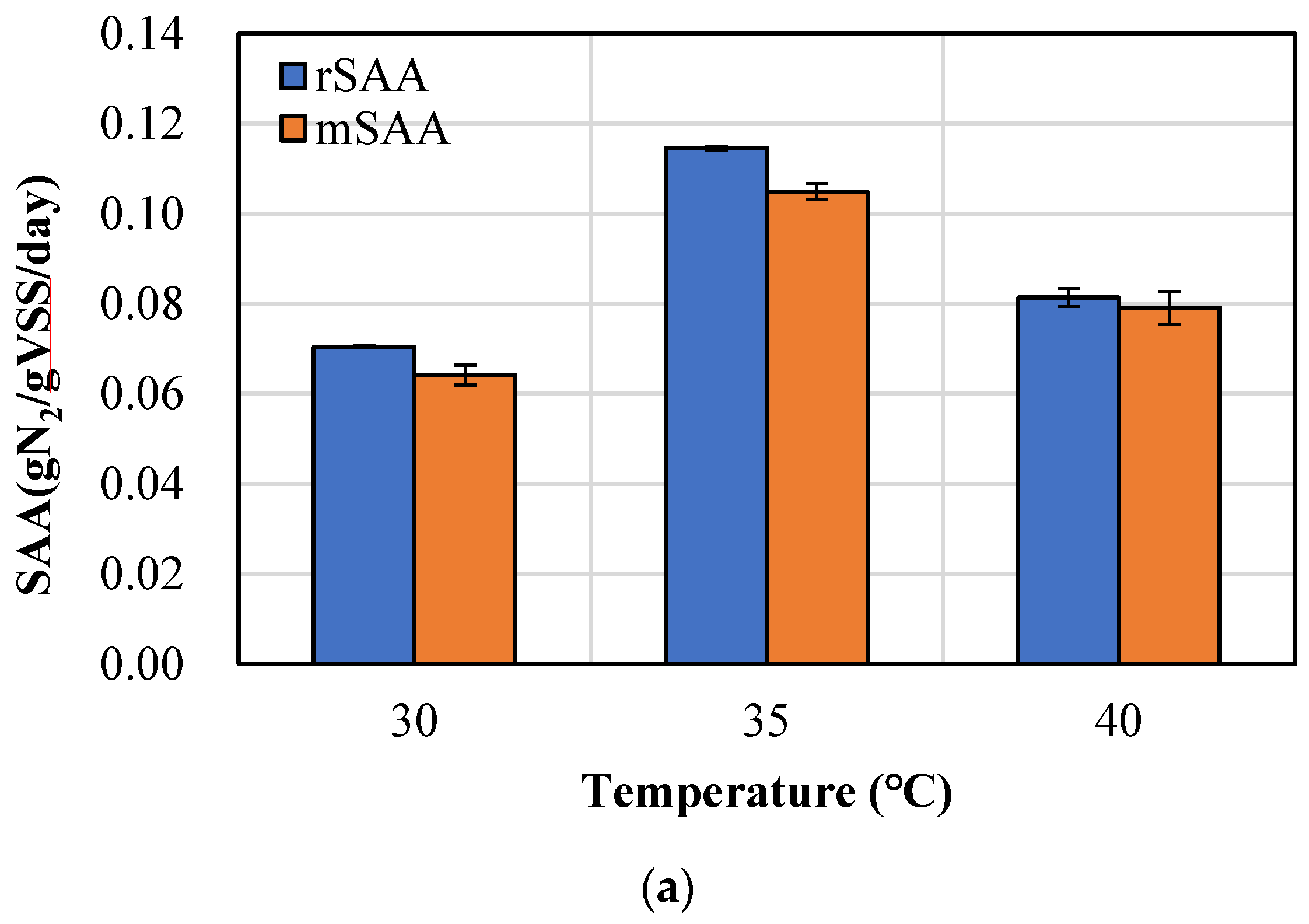
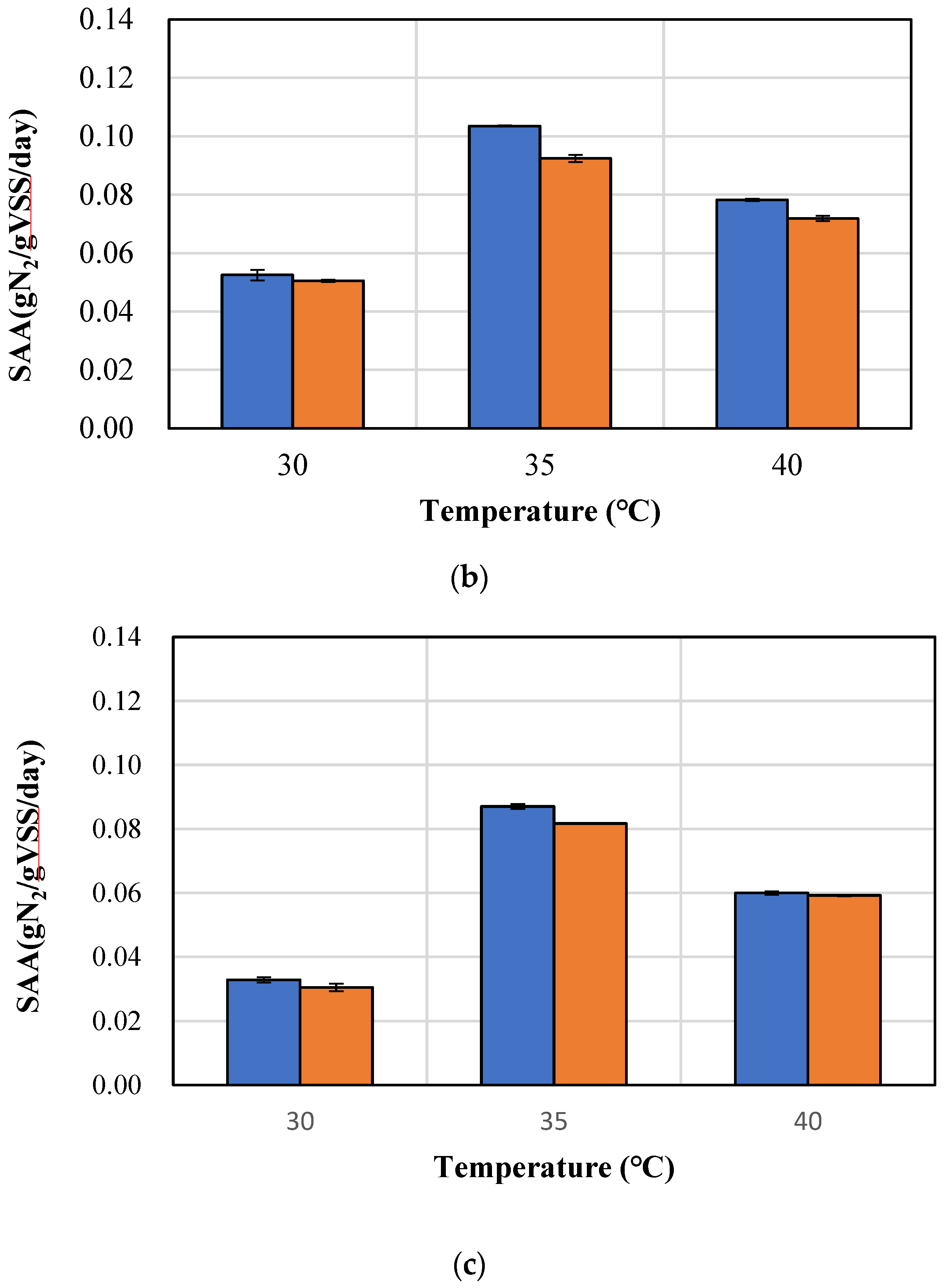
| Time (min) | SAA (gN2/gVSS/Day) |
|---|---|
| 60 | 0.449 ± 0.133 |
| 120 | 0.384 ± 0.083 |
| 360 | 0.197 ± 0.033 |
| 600 | 0.111 ± 0.022 |
| Item | rSAA30°C | mSAA30°C | rSAA35°C | mSAA35°C | rSAA40°C | mSAA40°C |
| No. 1 | 6 | 6 | 6 | 6 | 6 | 6 |
| N test 2 | 0.276 | 0.402 | 0.232 | 0.430 | 0.051 | 0.487 |
| T test | 0.711 | 0.223 | 0.591 | 0.394 | ||
| W test 3 | 0.485 | 0.240 | ||||
| Item | rSAA<1 mm | mSAA<1 mm | rSAA1–2 mm | mSAA1–2 mm | rSAA>2 mm | mSAA>2 mm |
| No. | 6 | 6 | 6 | 6 | 6 | 6 |
| N test | 0.066 | 0.401 | 0.267 | 0.337 | 0.249 | 0.189 |
| T test | 0.604 | 0.326 | 0.601 | 1.000 | ||
| W test | 0.485 | 0.589 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Kim, Y.; Kim, J.; Jeong, S.; Park, S.; Lee, T. A Simple Analysis Method of Specific Anammox Activity Using a Respirometer. Appl. Sci. 2022, 12, 1121. https://doi.org/10.3390/app12031121
Yu J, Kim Y, Kim J, Jeong S, Park S, Lee T. A Simple Analysis Method of Specific Anammox Activity Using a Respirometer. Applied Sciences. 2022; 12(3):1121. https://doi.org/10.3390/app12031121
Chicago/Turabian StyleYu, Jaecheul, Yeonju Kim, Jeongmi Kim, Soyeon Jeong, Seongjae Park, and Taeho Lee. 2022. "A Simple Analysis Method of Specific Anammox Activity Using a Respirometer" Applied Sciences 12, no. 3: 1121. https://doi.org/10.3390/app12031121






