Mechanism of Removal of Hexavalent Chromium from Aqueous Solution by Fe-Modified Biochar and Its Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Fe-Modified Biochar
2.3. Materials Characterization
2.4. Batch Adsorption Experiments
2.5. Adsorption Isotherm
2.6. Adsorption Kinetic
2.7. Statistical Analysis
3. Results and Discussion
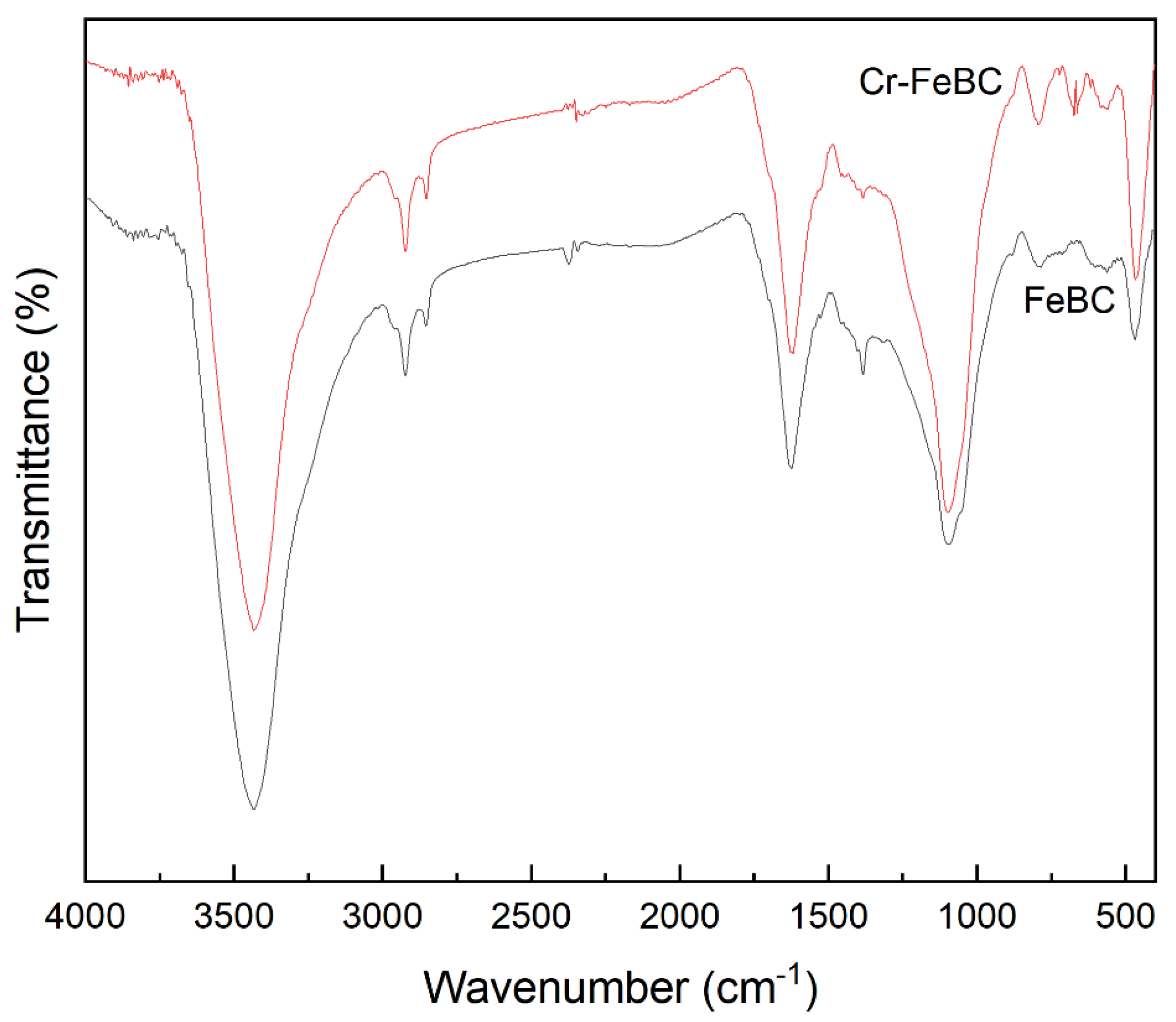
3.1. Characterization of BC and FeBC
3.2. Effect of Modifier Mass Ratio and Dosage of FeBC on Cr(VI) Removal
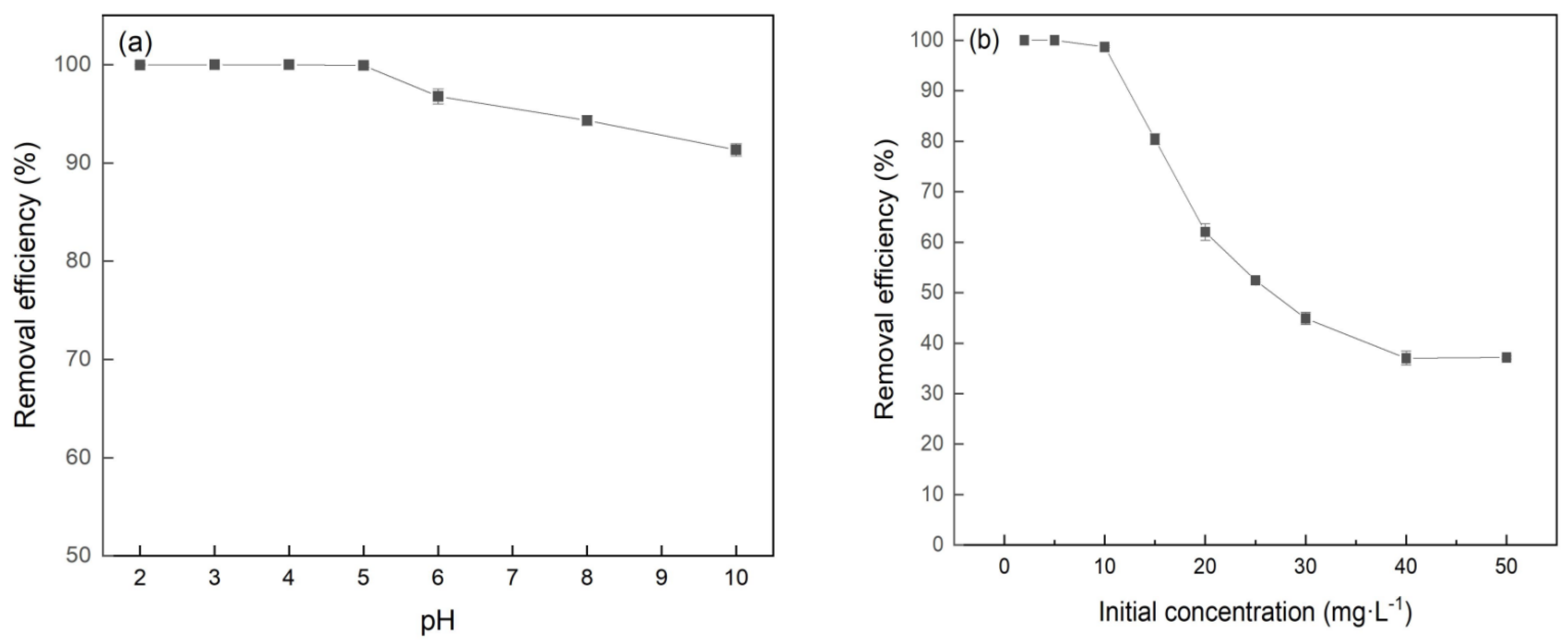
3.3. Effect of pH and Initial Concentration on Cr(VI) Removal by FeBC
3.4. Effect of Coexisting Ions in Solution
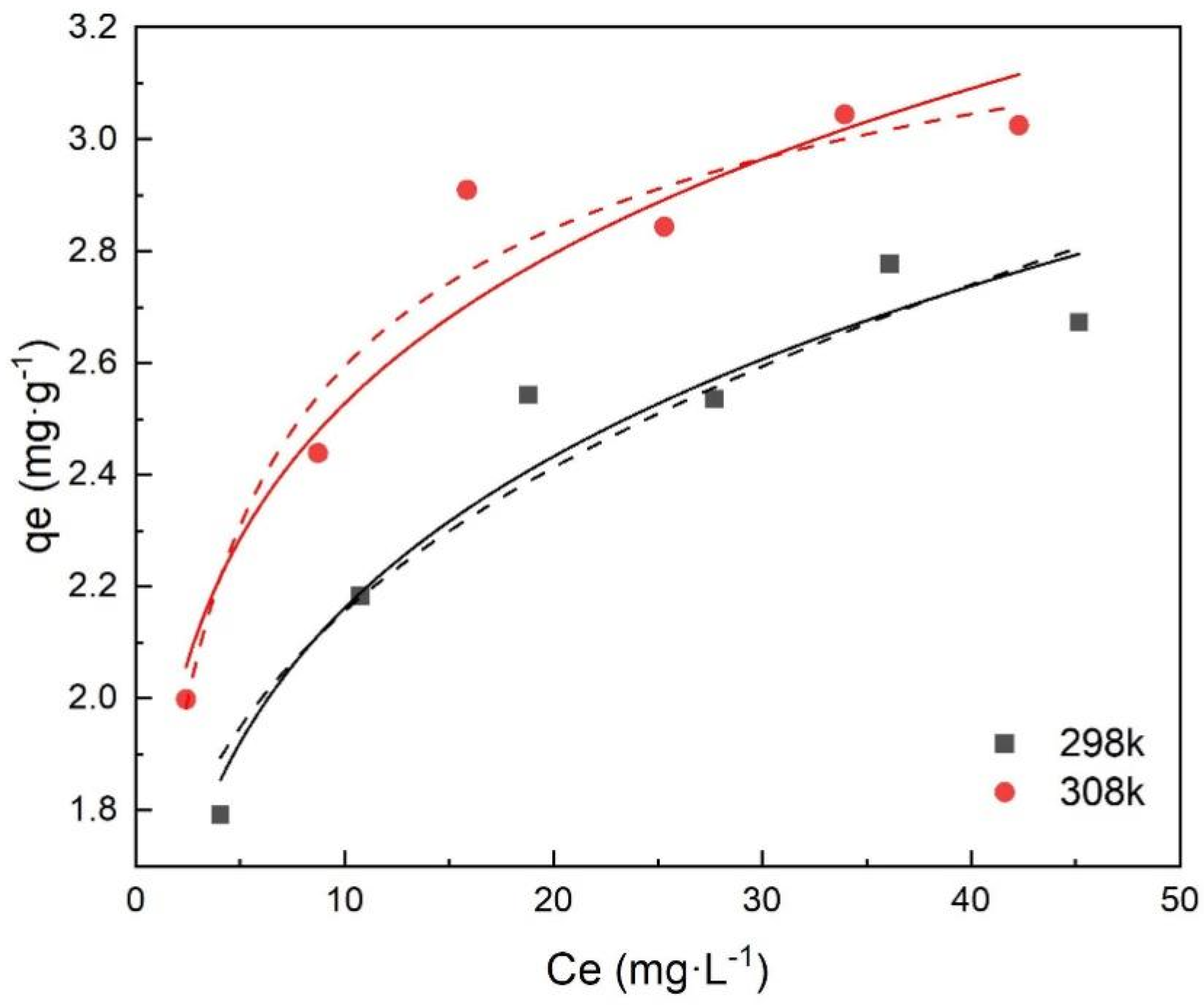
3.5. Adsorption Isotherms
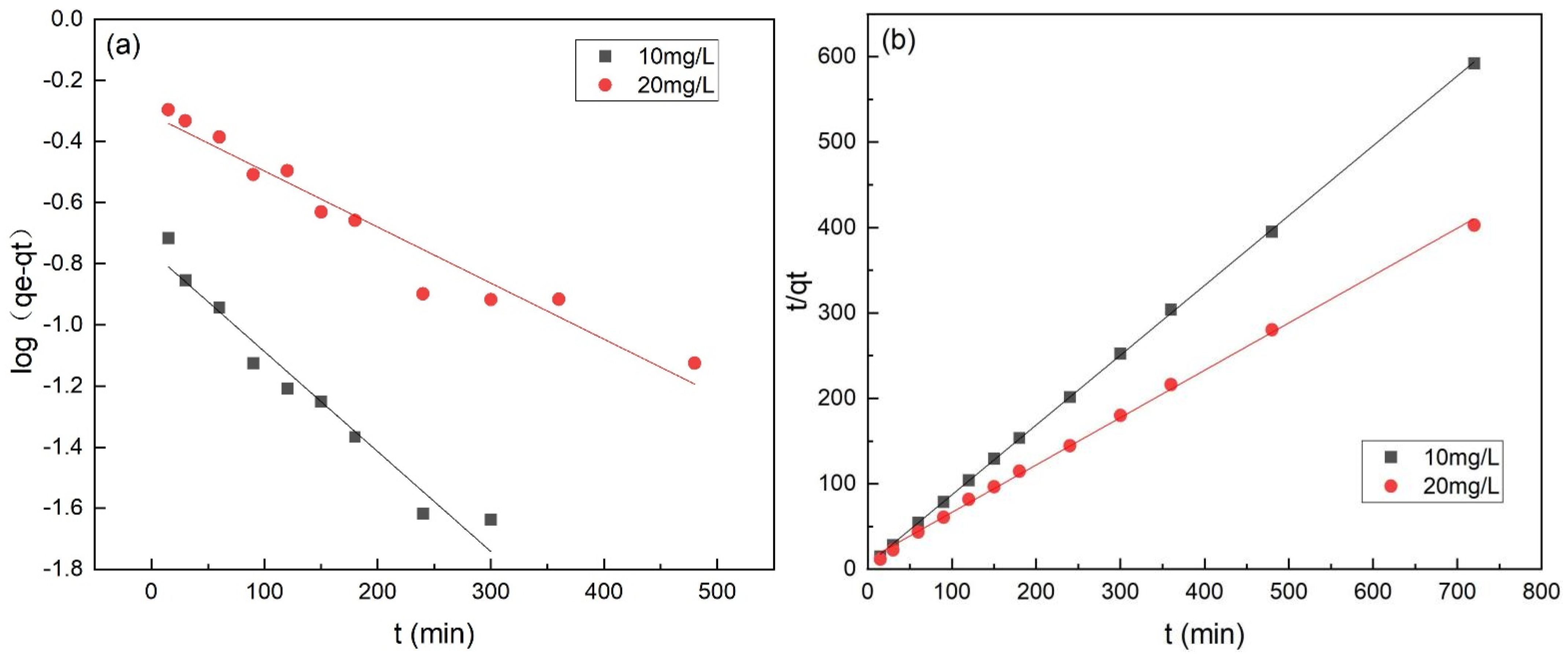
3.6. Adsorption Kinetics
3.7. Proposed Adsorption Mechanism of Cr(VI) Removal
3.8. Removal Capability of FeBC in Actual Industrial Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Oliveira, H.; Spano, M.; Guevara, M.A.; Santos, T.M.; Santos, C.; Pereira, M.D. Evaluation of in vivo reproductive toxicity of potassium chromate in male mice. Exp. Toxicol. Pathol. 2010, 62, 391–404. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. Adsorption of chromium (Cr) from tannery wastewater using low-cost spent tea leaves adsorbent. Appl. Water Sci. 2018, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Yan, X.; Peacock, C.L.; Zhang, S.; Li, W.; Zhang, J.; Feng, X.; Liu, F.; Yin, H. Adsorption of Cr(VI) on Al-substituted hematites and its reduction and retention in the presence of Fe2+ under conditions similar to subsurface soil environments. J. Hazard. Mater. 2020, 390, 122014. [Google Scholar] [CrossRef]
- Cely, P.; Gasco, G.; Paz-Ferreiro, J.; Mendez, A. Agronomic properties of biochars from different manure wastes. J. Anal. Appl. Pyrol. 2015, 111, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Iamsaard, K.; Weng, C.-H.; Yen, L.-T.; Tzeng, J.-H.; Poonpakdee, C.; Lin, Y.-T. Adsorption of metal on pineapple leaf biochar: Key affecting factors, mechanism identification, and regeneration evaluation. Bioresour. Technol. 2021, 344, 126131. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, Z.; Jeyakumar, P.; Bolan, N.; Wang, H. Characteristics and applications of biochar for remediating Cr(VI)-contaminated soils and wastewater. Environ. Geochem. Health 2020, 42, 1543–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Wang, J.J.; Gaston, L.A.; Zhou, B.Y.; Li, M.L.; Xiao, R.; Wang, Q.; Zhang, Z.Q.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Yu, Y.; An, Q.; Jin, L.; Luo, N.; Li, Z.; Jiang, J. Unraveling sorption of Cr (VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: Implicit mechanism and application. Bioresour. Technol. 2020, 297, 122466. [Google Scholar] [CrossRef]
- Lyu, H.H.; Tang, J.C.; Huang, Y.; Gai, L.S.; Zeng, E.Y.; Liber, K.; Gong, Y.Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Zhang, G.; Meng, F.; Li, L.; Wu, S. Removal of hexavalent chromium from aqueous solution by different surface-modified biochars: Acid washing, nanoscale zero-valent iron and ferric iron loading. Bioresour. Technol. 2018, 261, 142–150. [Google Scholar] [CrossRef]
- Pan, J.-j.; Jiang, J.; Xu, R.-K. Removal of Cr(VI) from aqueous solutions by Na2SO3/FeSO4 combined with peanut straw biochar. Chemosphere 2014, 101, 71–76. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Wang, G.; Yang, Z.; Xian, J.; Yang, Y.; Li, T.; Pu, Y.; Jia, Y.; Li, Y. Adsorption and reduction of Cr (VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105407. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. 2018, 6, 2335–2343. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Ma, J.; Ning, P. An efficient Egeria najas-derived biochar supported nZVI composite for Cr (VI) removal: Characterization and mechanism investigation based on visual MINTEQ model. Environ. Res. 2020, 189, 109912. [Google Scholar] [CrossRef]
- Cho, D.-W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of magnetic biochar as a treatment medium for As (V) via pyrolysis of FeCl3-pretreated spent coffee ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.J.; Jiang, J.; Xu, R.K. Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J. Environ. Sci. 2013, 25, 1957–1965. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, W.; Wu, J.G.; Huang, Z.Q.; Dai, J.Y. Mechanism of Cu(II) adsorption inhibition on biochar by its aging process. J. Environ. Sci. 2014, 26, 2123–2130. [Google Scholar] [CrossRef]
- Yuan, Y.; Bolan, N.; Prevoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H.L. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Nakarmi, A.; Bourdo, S.E.; Ruhl, L.; Kanel, S.; Nadagouda, M.; Alla, P.K.; Pavel, I.; Viswanathan, T. Benign zinc oxide betaine-modified biochar nanocomposites for phosphate removal from aqueous solutions. J. Environ. Manag. 2020, 272, 111048. [Google Scholar] [CrossRef]
- Huang, Z.; Dai, X.; Huang, Z.; Wang, T.; Cui, L.; Ye, J.; Wu, P. Simultaneous and efficient photocatalytic reduction of Cr(VI) and oxidation of trace sulfamethoxazole under LED light by rGO@Cu2O/BiVO4 p-n heterojunction composite. Chemosphere 2019, 221, 824–833. [Google Scholar] [CrossRef]
- Dong, H.R.; Deng, J.M.; Xie, Y.K.; Zhang, C.; Jiang, Z.; Cheng, Y.J.; Hou, K.J.; Zeng, G.M. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D.; Singh, A.; Gupta, T. Removal of hexavalent chromium upon interaction with biochar under acidic conditions: Mechanistic insights and application. Environ. Sci. Pollut. Res. 2017, 24, 16786–16797. [Google Scholar] [CrossRef]
- Sass, B.M.; Rai, D. Solubility of amorphous chromium(III)-iron(III) hydroxide solid-solutions. Inorg. Chem. 1987, 26, 2228–2232. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Shao, Y.; Huang, W.; Cheng, X. Different adsorption behaviors and mechanisms of a novel amino-functionalized hydrothermal biochar for hexavalent chromium and pentavalent antimony. Bioresour. Technol. 2020, 310, 123438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Chen, S.J.; Zhang, H.W.; Wang, X.K. Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars. Bioresour. Technol. 2017, 238, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Lyonga, F.N.; Hong, S.H.; Cho, E.J.; Kang, J.K.; Lee, C.G.; Park, S.J. As(III) adsorption onto Fe-impregnated food waste biochar: Experimental investigation, modeling, and optimization using response surface methodology. Environ. Geochem. Health 2021, 43, 3303–3321. [Google Scholar] [CrossRef]
- Gan, C.; Liu, Y.G.; Tan, X.F.; Wang, S.F.; Zeng, G.M.; Zheng, B.H.; Li, T.T.; Jiang, Z.J.; Liu, W. Effect of porous zinc-biochar nanocomposites on Cr(VI) adsorption from aqueous solution. RSC Adv. 2015, 5, 35107–35115. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Bioresour. Technol. 2013, 134, 94–100. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, Y.G.; Peng, Q.Q.; Hu, X.J.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, H.Y.; Li, H.H.; He, X.Y.; Shi, Y.J.; Kruse, A. Microwave digestion-assisted HFO/biochar adsorption to recover phosphorus from swine manure. Sci. Total Environ. 2018, 621, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Lee, D.; Yoon, M. Bioinspired 2D-Carbon Flakes and Fe3O4 Nanoparticles Composite for Arsenite Removal. Acs Appl. Mater. Inter. 2016, 8, 23876–23885. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, Y.T.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.Y.; Wang, Q.Y. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef]
- Lyu, H.H.; Zhao, H.; Tang, J.C.; Gong, Y.Y.; Huang, Y.; Wu, Q.H.; Gao, B. Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 2018, 194, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) removal by magnetic greigite/biochar composites. Sci. Total Environ. 2020, 700, 134414. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- CHINA. Integrated Wastewater Discharge Standard GB/T 8978. 1996. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/199801/t19980101_66568.htm (accessed on 1 October 2021).
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
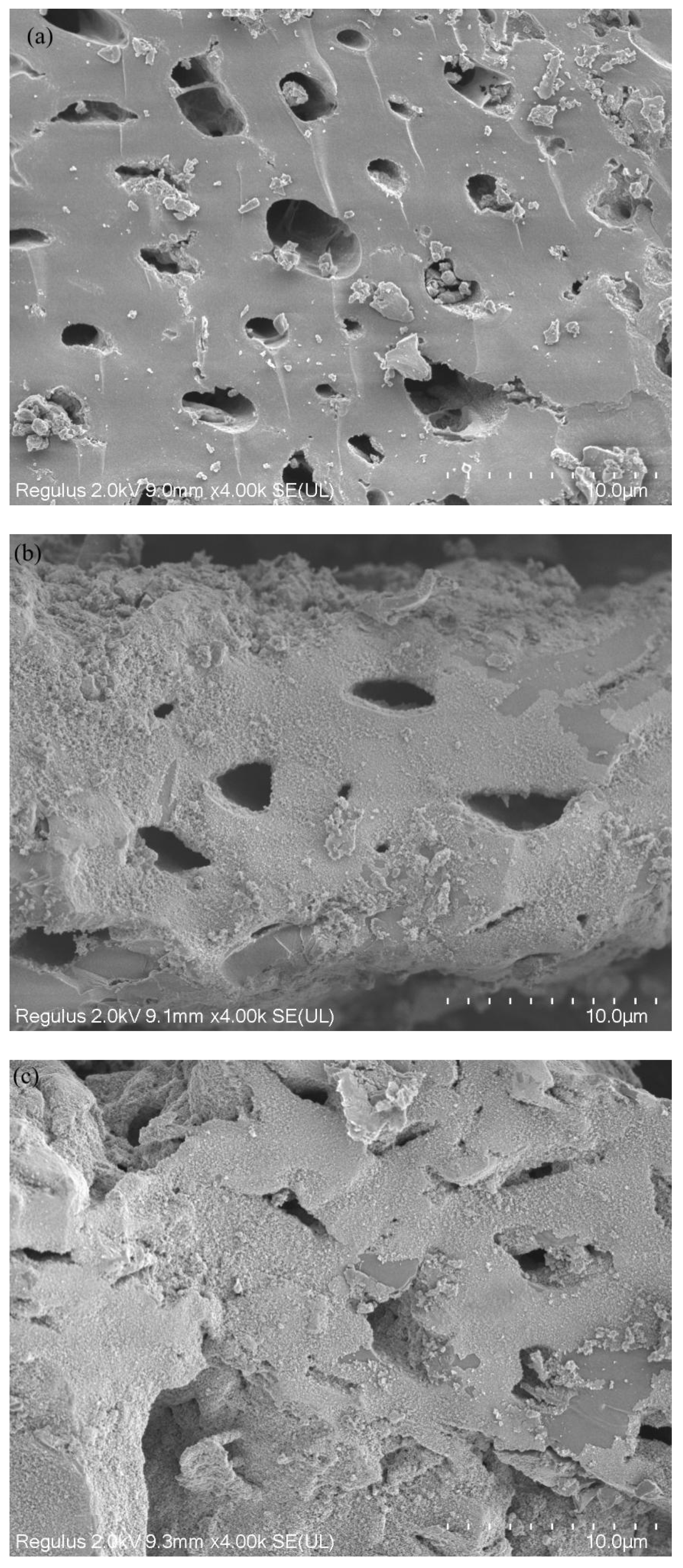
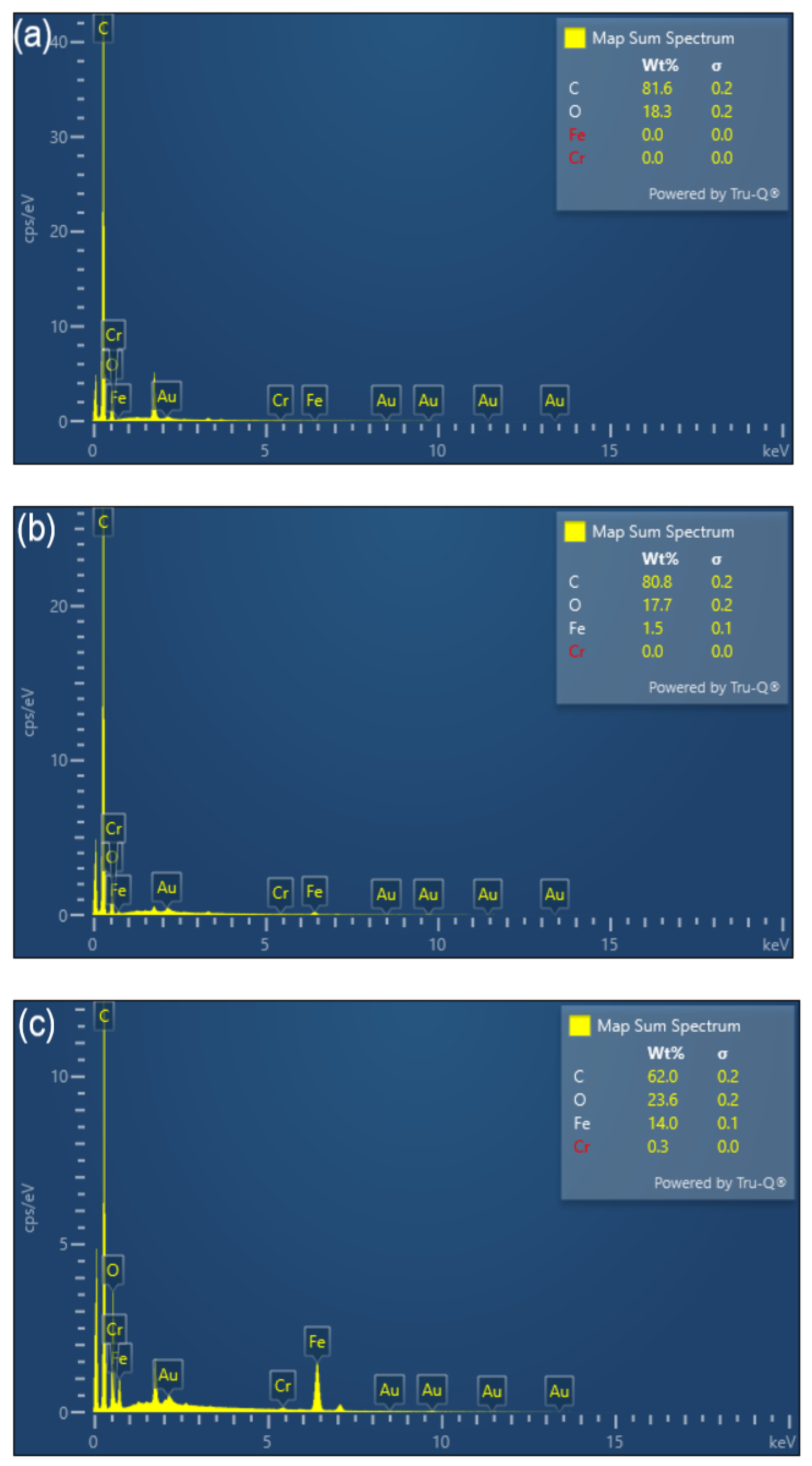
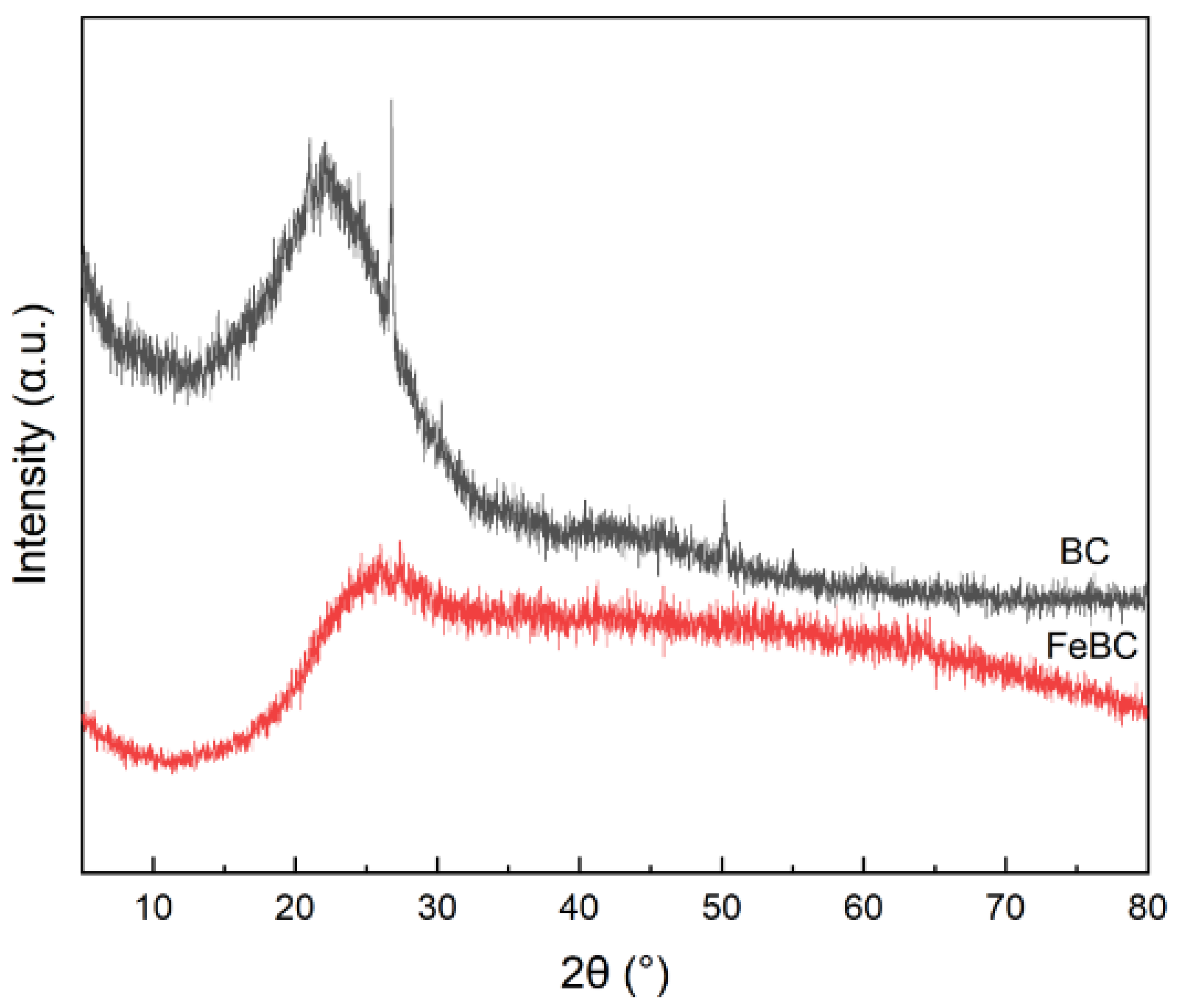
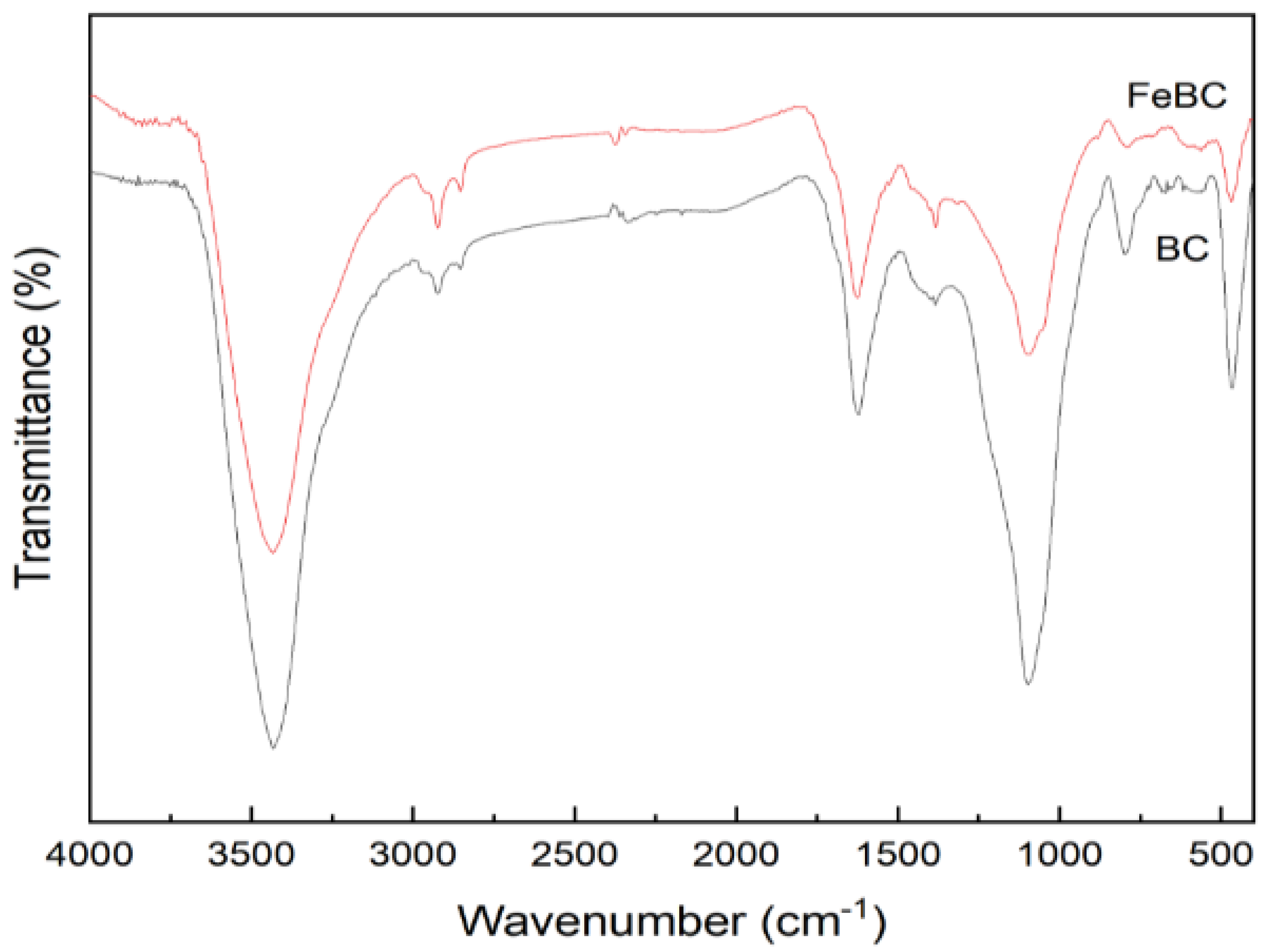


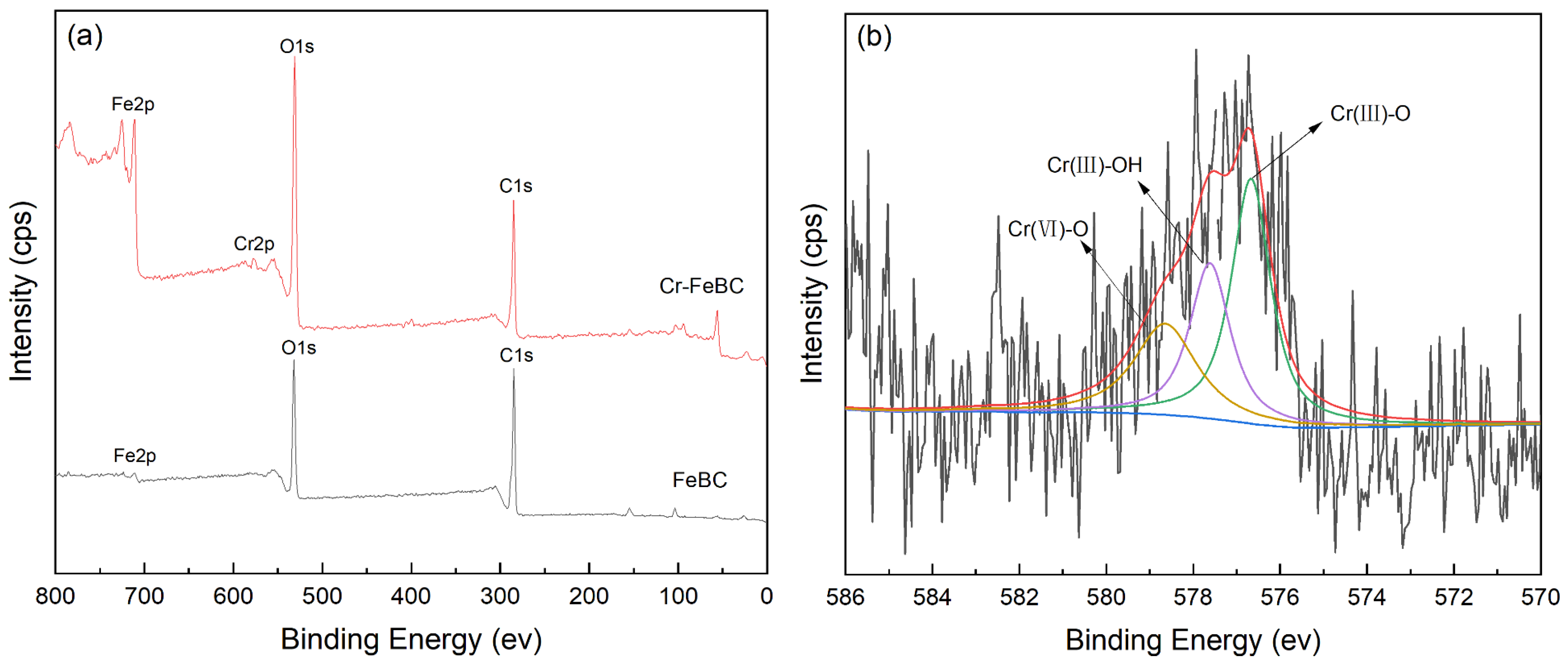
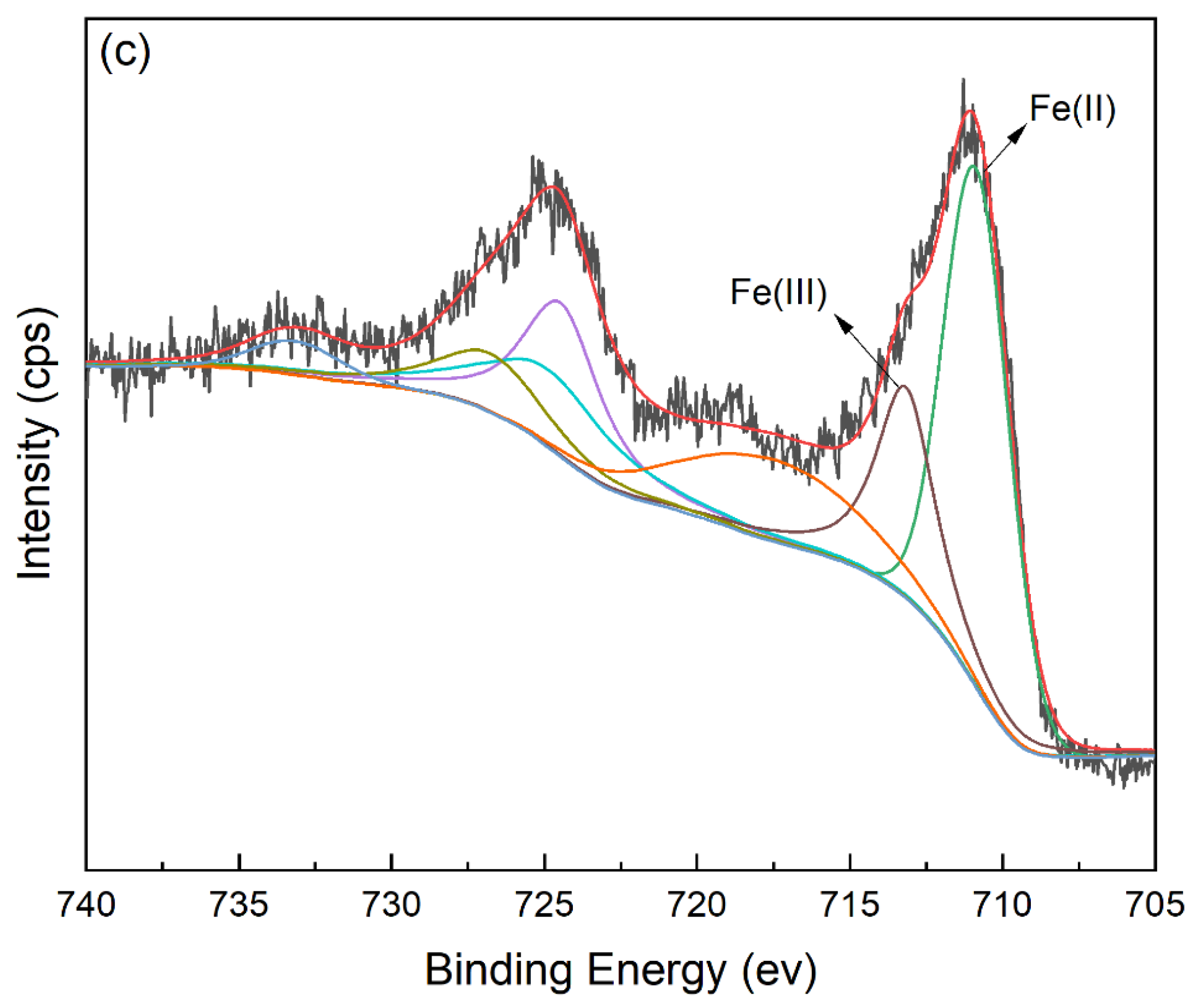
| Sample | Specific Surface Area /m2·g−1 | Pore Volume /cm3·g−1 | Average Pore Diameter /nm |
|---|---|---|---|
| BC | 26.75 | 0.0072 | 5.11 |
| FeBC | 44.69 | 0.0351 | 5.50 |
| Temperature/K | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm/mg·g−1 | KL/L·mg−1 | R2 | KF | n | R2 | |
| 298 | 2.7840 | 0.6001 | 0.9950 | 1.4606 | 5.8720 | 0.9310 |
| 308 | 3.0921 | 0.8398 | 0.9972 | 1.8098 | 6.8917 | 0.9304 |
| Initial Cr(VI) Concentration/mg·L−1 | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe/mg·g−1 | K1/min−1 | R2 | qe/mg·g−1 | K2/g−1·mg−1 min−1 | R2 | |
| 10 | 0.2066 | 0.0085 | 0.9219 | 1.2211 | 0.1403 | 0.9998 |
| 20 | 0.5278 | 0.0046 | 0.9329 | 1.7918 | 0.0320 | 0.9982 |
| Condition | pH | Total Cr (mg·L−1) | Cr(VI) (mg·L−1) | Ni (mg·L−1) |
|---|---|---|---|---|
| Before adsorption | 6.2 | 19.89 ± 0.35 | 10.85 ± 0.11 | 0.48 ± 0.051 |
| After adsorption | 7.8 | 1.32 ± 0.07 | 0.35 ± 0.076 | 0.27 ± 0.021 |
| Standard limit | 6–9 | 1.5 | 0.5 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, R.; Bu, J.; Ren, G.; Zhang, Z.; Li, K.; Ding, A. Mechanism of Removal of Hexavalent Chromium from Aqueous Solution by Fe-Modified Biochar and Its Application. Appl. Sci. 2022, 12, 1238. https://doi.org/10.3390/app12031238
Pan R, Bu J, Ren G, Zhang Z, Li K, Ding A. Mechanism of Removal of Hexavalent Chromium from Aqueous Solution by Fe-Modified Biochar and Its Application. Applied Sciences. 2022; 12(3):1238. https://doi.org/10.3390/app12031238
Chicago/Turabian StylePan, Run, Jiangping Bu, Guoyu Ren, Zihao Zhang, Kexin Li, and Aifang Ding. 2022. "Mechanism of Removal of Hexavalent Chromium from Aqueous Solution by Fe-Modified Biochar and Its Application" Applied Sciences 12, no. 3: 1238. https://doi.org/10.3390/app12031238
APA StylePan, R., Bu, J., Ren, G., Zhang, Z., Li, K., & Ding, A. (2022). Mechanism of Removal of Hexavalent Chromium from Aqueous Solution by Fe-Modified Biochar and Its Application. Applied Sciences, 12(3), 1238. https://doi.org/10.3390/app12031238






