Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of Ag/CuO NPs
2.2. Experimental Design and Statistical Analysis
2.3. Ag/CuO NPs Characterization
2.4. Biological Activities of the Ag/CuO NPs
3. Results
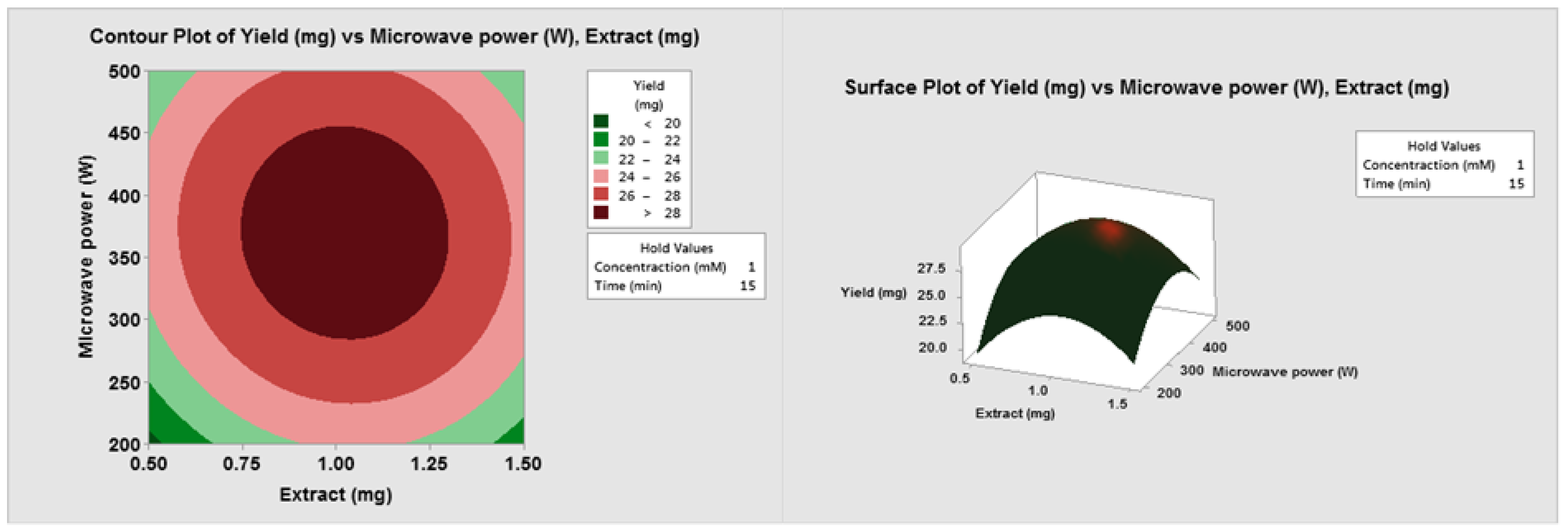
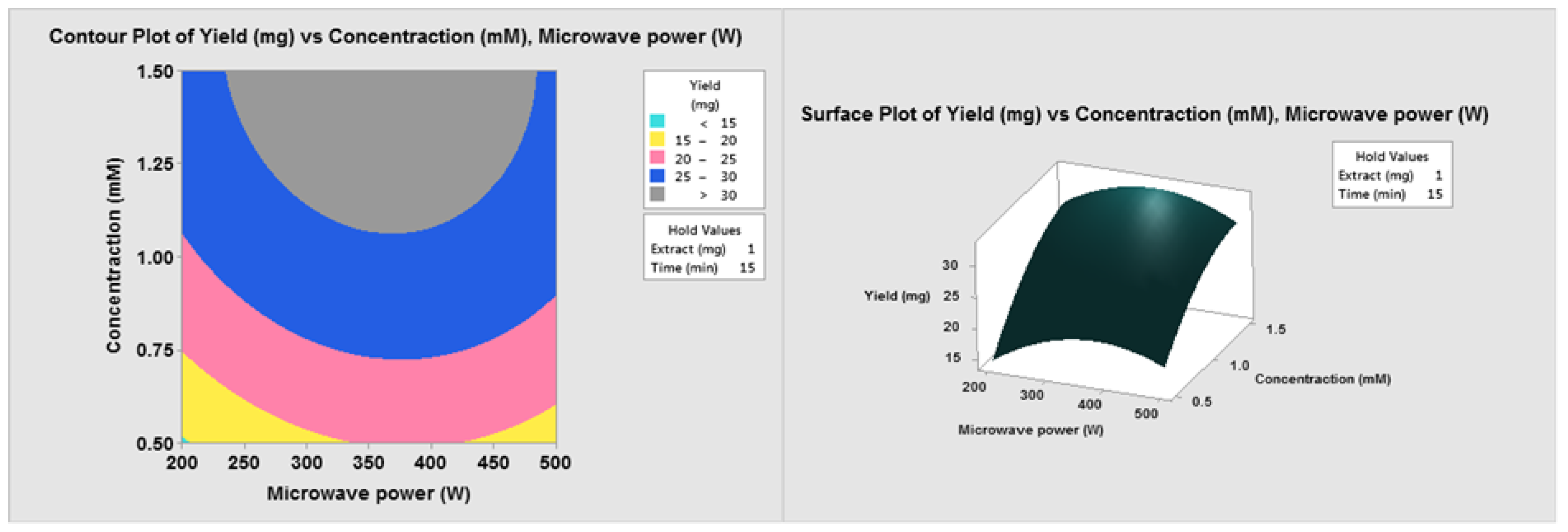
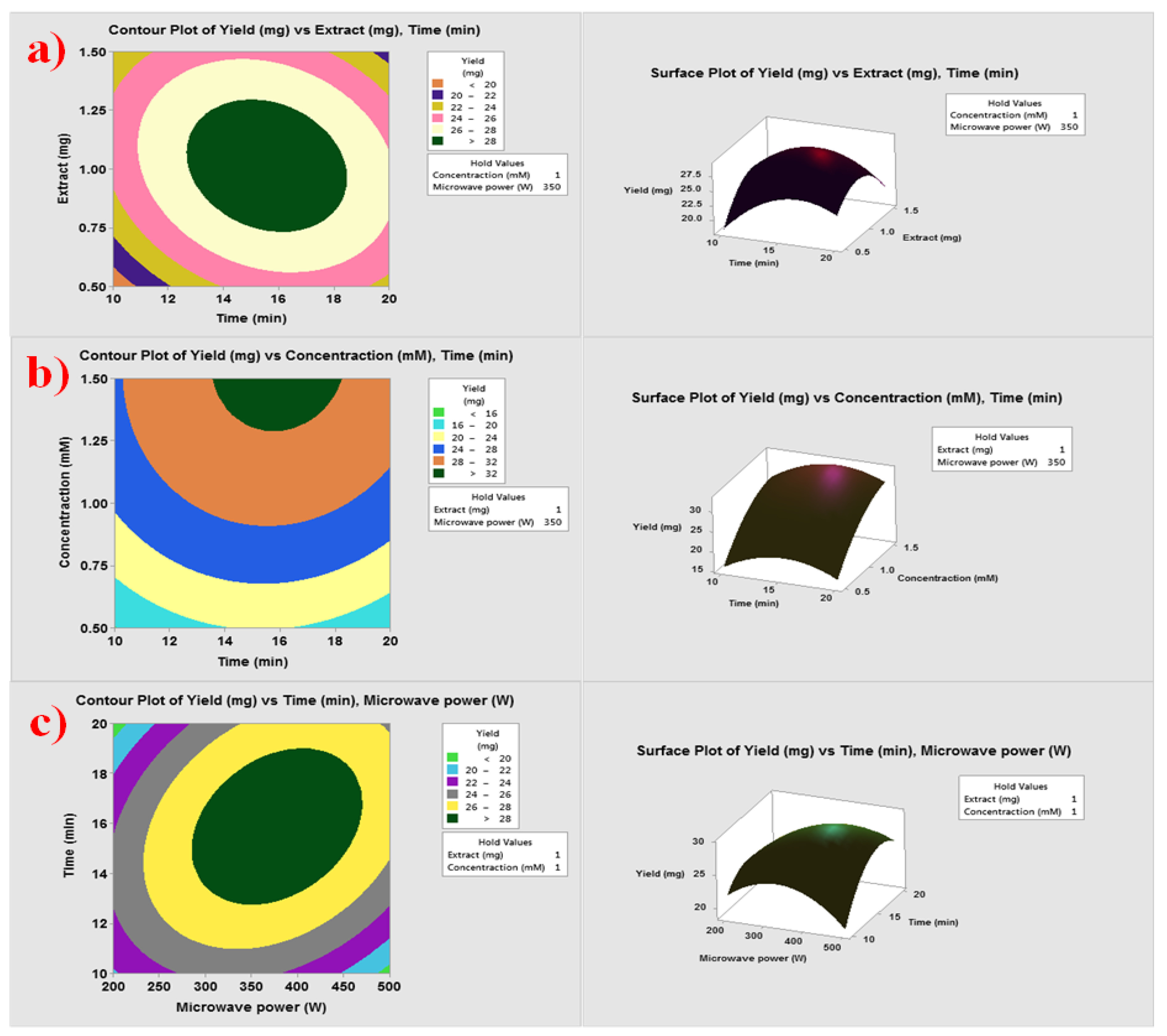
3.1. Optimization of Operating Variables
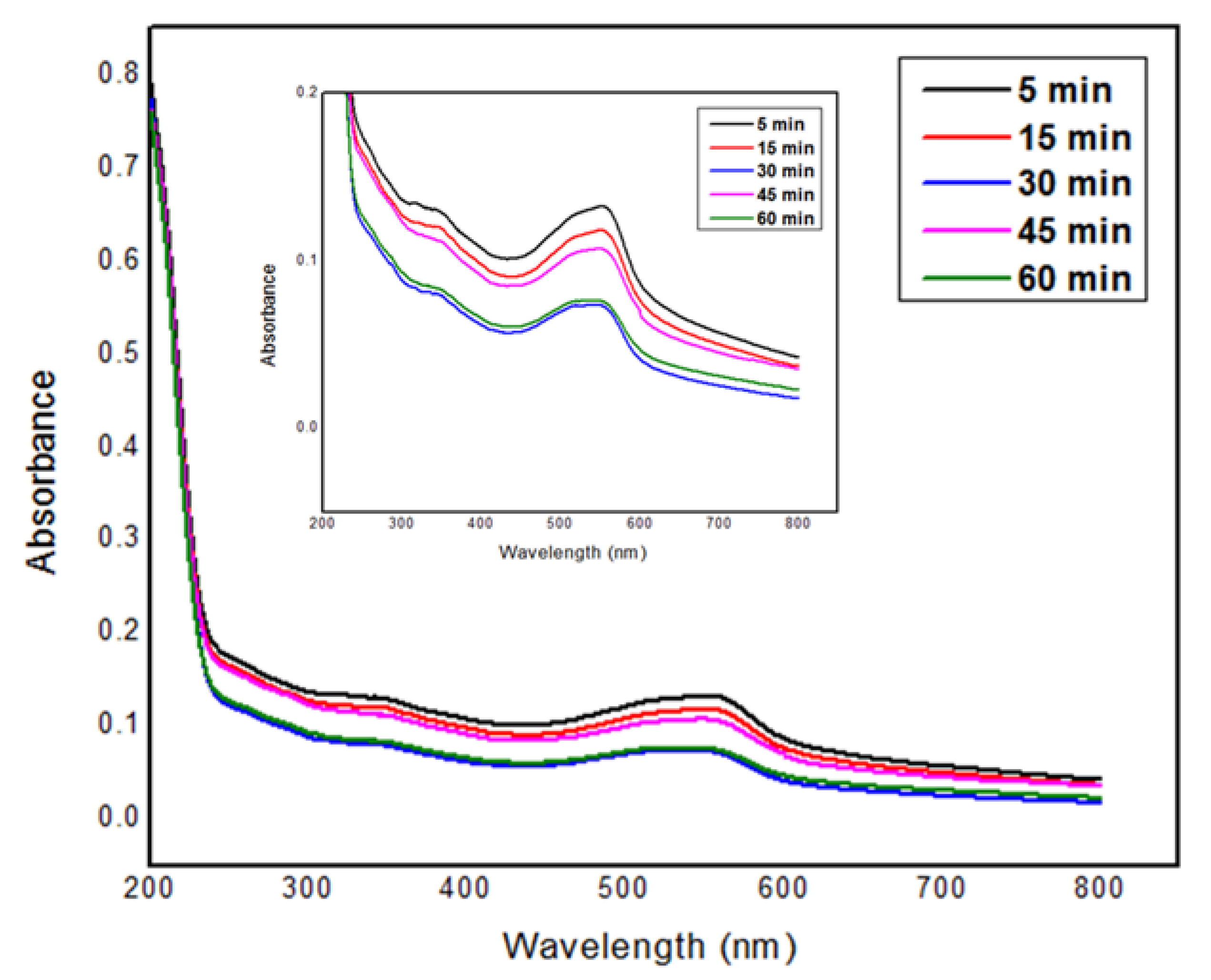
3.2. UV-Vis Spectroscopy
3.3. FT-IR Analysis
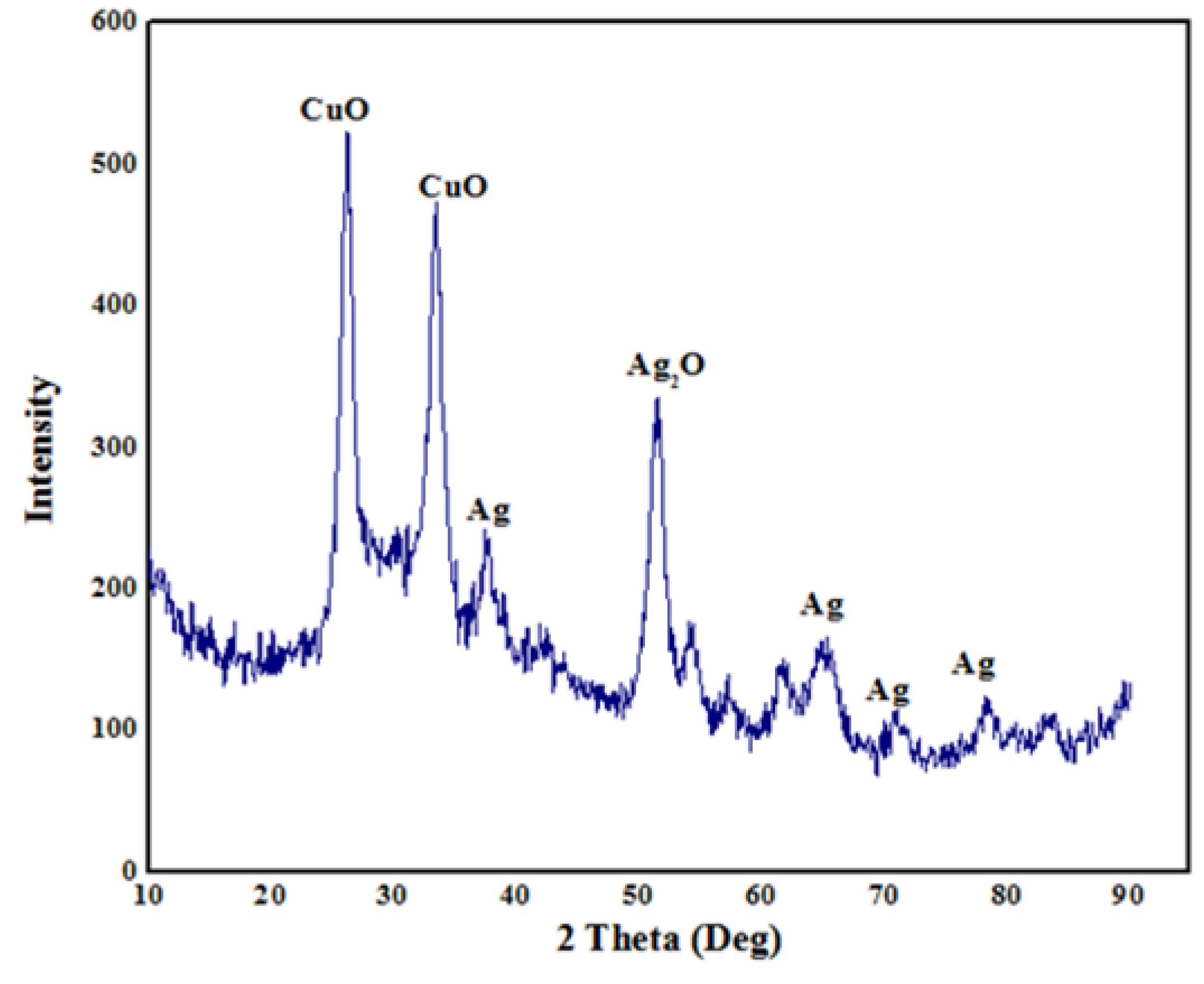
3.4. XRD Analysis
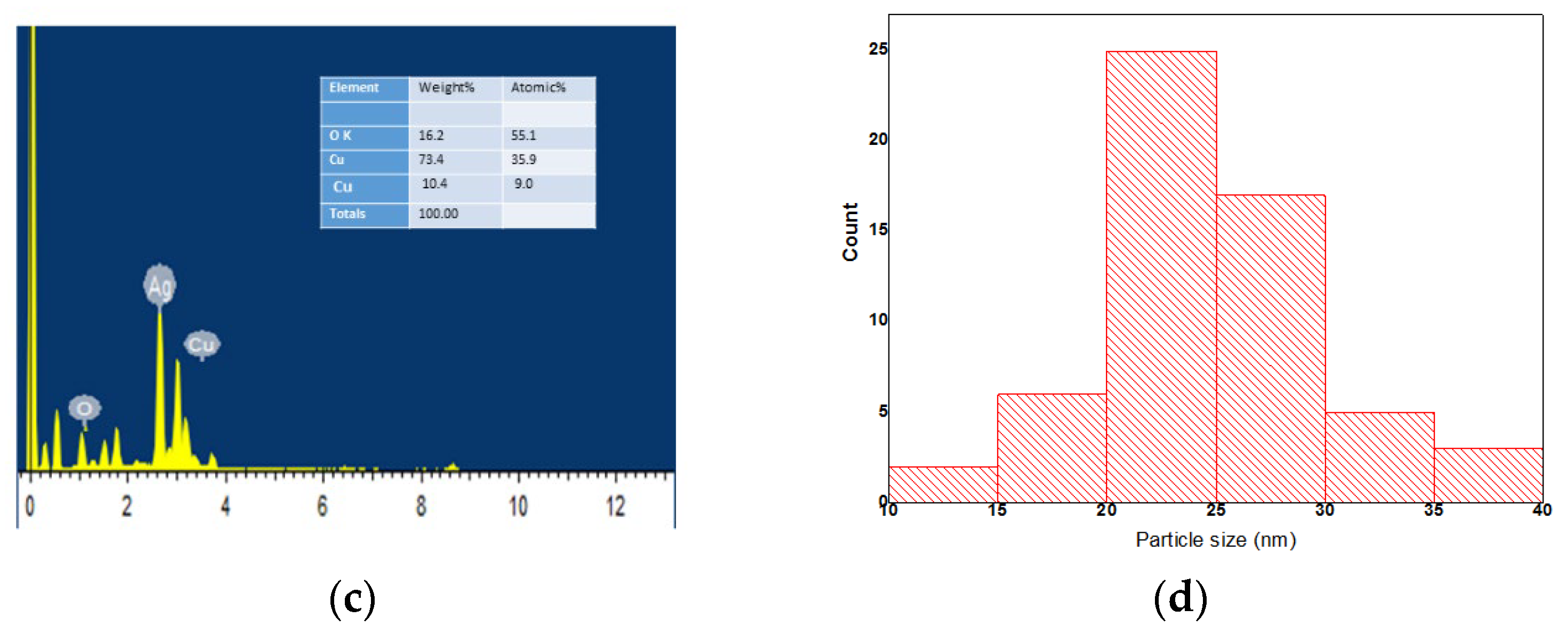
3.5. Morphological Analysis
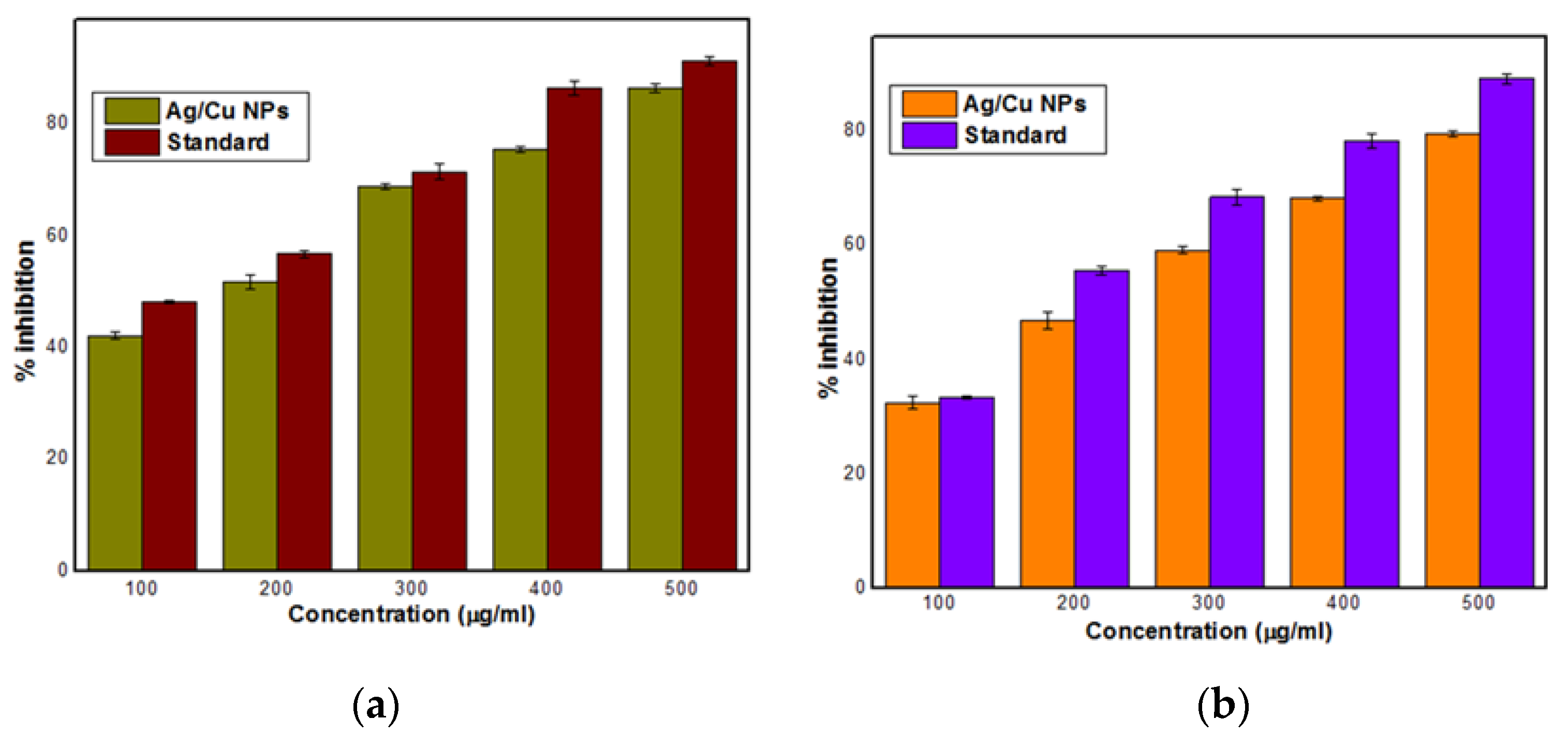
3.6. Antioxidant Activity
3.7. Antibacterial Activity
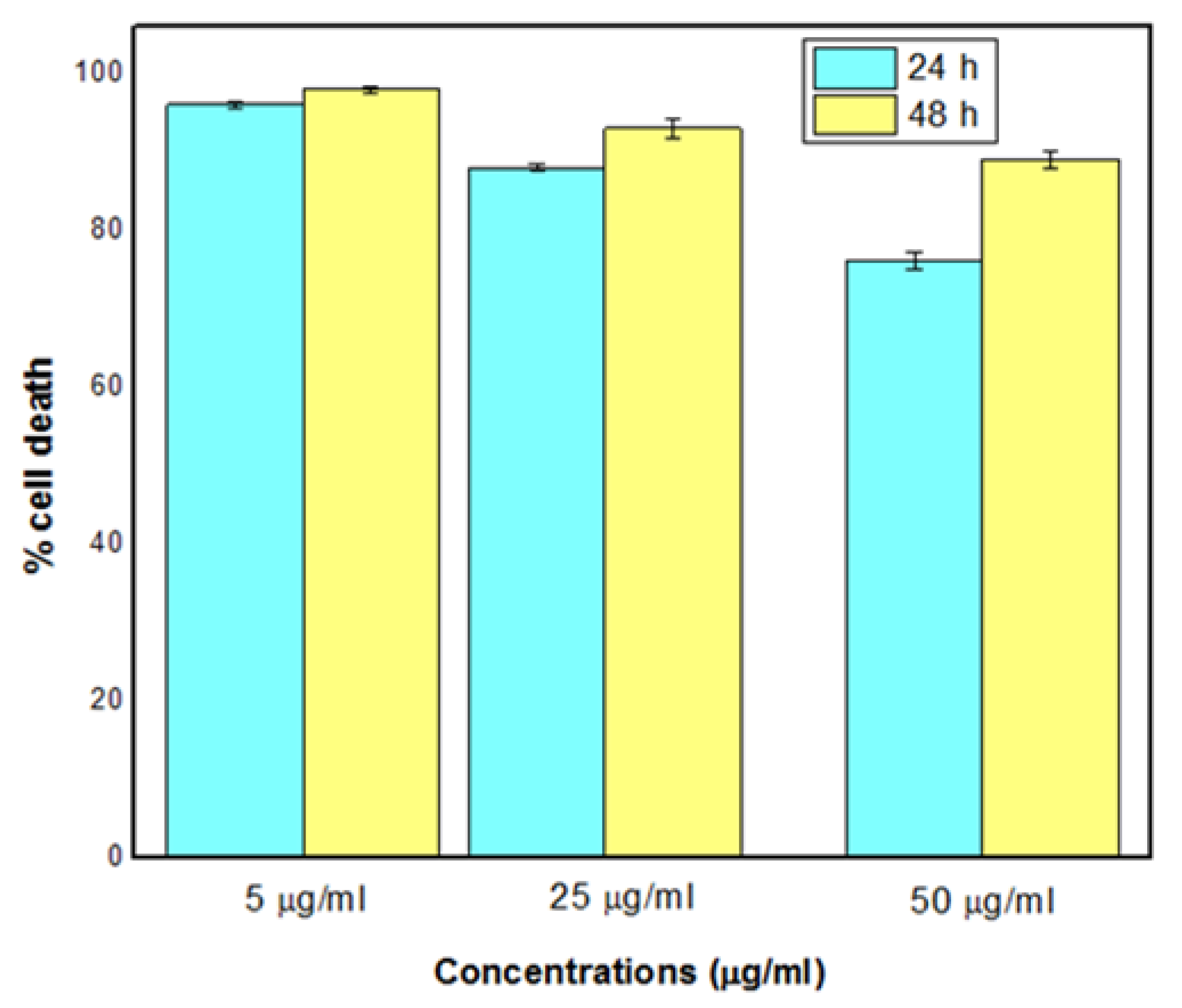
3.8. Cytotoxicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Dutta, D.; Das, B.M. Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: A review on synthesis and applications of green nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100418. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Vahabi, K.; Mansoori, G.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma Reesei (A Route for Large-Scale Production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2021, 1–9. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef]
- Patel, M.; Surti, M.; Siddiqui, A.J.; Adnan, M. Fungi and metal nanoparticles. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 861–890. [Google Scholar]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crop. Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Azmath, P.; Baker, S.; Rakshith, D.; Satish, S. Mycosynthesis of silver nanoparticles bearing antibacterial activity. Saudi Pharm. J. 2016, 24, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Guilger-Casagrande, M.; Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen, F.; Dawoud, T.; AlNadhari, S. Ecofriendly and low-cost synthesis of ZnO nanoparticles from Acremonium potronii for the photocatalytic degradation of azo dyes. Environ. Res. 2021, 202, 111700. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Jiang, L.; Zeng, G.; Wang, D.; Tang, W.; Liang, J.; Wang, H.; He, D.; Liu, Z.; Tang, L. Bimetallic nanoparticles/metal-organic frameworks: Synthesis, applications and challenges. Appl. Mater. Today 2020, 19, 100564. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mandal, T.K.; Baek, K.-H. Bimetallic and Trimetallic Nanoparticles for Active Food Packaging Applications: A Review. Food Bioprocess Technol. 2019, 13, 30–44. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Zhang, H.; Haba, M.; Okumura, M.; Akita, T.; Hashimoto, S.; Toshima, N. Novel Formation of Ag/Au Bimetallic Nanoparticles by Physical Mixture of Monometallic Nanoparticles in Dispersions and Their Application to Catalysts for Aerobic Glucose Oxidation. Langmuir 2013, 29, 10330–10339. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chen, S.-Y.; Song, J.-M.; Chen, I.-G.; Lee, H.-Y. Thermal stability of Cu@Ag core–shell nanoparticles. Corros. Sci. 2013, 74, 123–129. [Google Scholar] [CrossRef]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanoparticle Res. 2013, 15, 1537. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.H.; Yeo, S.C.; Lee, H.M. Structural stability of AgCu bimetallic nanoparticles and their application as a catalyst: A DFT study. Catal. Today 2012, 185, 94–98. [Google Scholar] [CrossRef]
- Cai, Y.; Piao, X.; Gao, W.; Zhang, Z.; Nie, E.; Sun, Z. Large-scale and facile synthesis of silver nanoparticles via a microwave method for a conductive pen. RSC Adv. 2017, 7, 34041–34048. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, H.; Anjum, T.; Riaz, S.; Naseem, S. Microwave-Assisted Green Synthesis and Characterization of Silver Nanoparticles Using Melia azedarach for the Management of Fusarium Wilt in Tomato. Front. Microbiol. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Tamilvanan, A.; Balamurugan, K.; Mohanraj, T.; Selvakumar, P.; Madhankumar, B. Parameter optimization of copper nanoparticle synthesis by electrodeposition process using RSM and CS. Mater. Today Proc. 2021, 45, 751–756. [Google Scholar] [CrossRef]
- Tangsiri, R.; Nezamzadeh-Ejhieh, A. Cadmium sulfide nanoparticles: Synthesis, brief characterization and experimental design by response surface methodology (RSM) in the photodegradation of ranitidine hydrochloride. Chem. Phys. Lett. 2020, 758, 137919. [Google Scholar] [CrossRef]
- Ameen, F.; Hadi, S.; Moslem, M.; Al-Sabri, A.; Yassin, M.A. Biodegradation of engine oil by fungi from mangrove habitat. J. Gen. Appl. Microbiol. 2015, 61, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Ameen, F.; Dawoud, T.M.; Alshehrei, F.; Alsamhary, K.; Almansob, A. Decolorization of acid blue 29, disperse red 1 and congo red by different indigenous fungal strains. Chemosphere 2021, 271, 129532. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.C.; Tortella, G.; Rubilar, O. Extracellular Biosynthesis of Copper and Copper Oxide Nanoparticles by Stereum hirsutum, a Native White-Rot Fungus from Chilean Forests. J. Nanomater. 2015, 2015, 57. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, P.S.; Gupta, A.K.; Sulaiman, A. Multivariate optimization of process parameters in the synthesis of calcined Ca–Al (NO3) LDH for defluoridation using 33 factorial, central composite and Box–Behnken design. J. Environ. Sci. Health Part A 2016, 51, 86–96. [Google Scholar] [CrossRef]
- Torabfam, M.; Jafarizadeh-Malmiri, H. Microwave-enhanced silver nanoparticle synthesis using chitosan biopolymer: Optimization of the process conditions and evaluation of their characteristics. Green Process. Synth. 2017, 7, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Ameen, F.; Stephenson, S.L.; AlNadhari, S.; Yassin, M.A. Isolation, identification and bioactivity analysis of an endophytic fungus isolated from Aloe vera collected from Asir desert, Saudi Arabia. Bioprocess Biosyst. Eng. 2021, 44, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Fowsiya, J.; Madhumitha, G. Preliminary phytochemical analysis, Antioxidant and cytotoxicity test of Carissa edulis Vahl dried fruits. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 263, p. 22018. [Google Scholar]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana mediated green synthesis of crystalline palladium nanoparticles as potential nanodrug against multidrug resistant bacteria and cancer cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef] [PubMed]
- Al-Enazi, N.M.; Ameen, F.; Alsamhary, K.; Dawoud, T.; Al-Khattaf, F.; AlNadhari, S. Tin oxide nanoparticles (SnO2-NPs) synthesis using Galaxaura elongata and its anti-microbial and cytotoxicity study: A greenery approach. Appl. Nanosci. 2021, 1–9. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Kumar, S.V.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Nallaswamy, V.D. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Xiao, X.; He, E.-J.; Lu, X.-R.; Wu, L.-J.; Fan, Y.-Y.; Yu, H.-Q. Evaluation of antibacterial activities of silver nanoparticles on culturability and cell viability of Escherichia coli. Sci. Total Environ. 2021, 794, 148765. [Google Scholar] [CrossRef]
- Vanamala, K.; Tatiparti, K.; Bhise, K.; Sau, S.; Scheetz, M.H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Novel approaches for the treatment of methicillin-resistant Staphylococcus aureus: Using nanoparticles to overcome multidrug resistance. Drug Discov. Today 2021, 26, 31–43. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Tamboli, D.P.; Lee, D.S. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J. Hazard. Mater. 2013, 260, 878–884. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E. Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: A review. Mini. Rev. Org. Chem. 2021, 18, 725–734. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles and their Biomedical Applications—A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Singh, A.C.; Asif, M.; Bacher, G.; Danielsson, B.; Willander, M.; Bhand, S. Nanoimmunosensor based on ZnO nanorods for ultrasensitive detection of 17β-Estradiol. Biosens. Bioelectron. 2019, 126, 15–22. [Google Scholar] [CrossRef]
- Li, J.; Tang, M.; Xue, Y. Review of the effects of silver nanoparticle exposure on gut bacteria. J. Appl. Toxicol. 2019, 39, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Sharma, A.S.; Ahmad, W.; Zareef, M.; Hassan, M.; Viswadevarayalu, A.; Jiao, T.; Li, H.; Chen, Q. Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Crit. Rev. Anal. Chem. 2020, 51, 454–481. [Google Scholar] [CrossRef]
- Medina, J.; Garcia-Perez, V.I.; Zanella, R. Metallic composites based on Ag, Cu, Au and Ag-Cu nanoparticles with distinctive bactericidal effect on varied species. Mater. Today Commun. 2021, 26, 102182. [Google Scholar] [CrossRef]
- Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F. Green synthesis of bimetallic copper–silver nanoparticles and their application in catalytic and antibacterial activities. Clean Technol. Environ. Policy 2019, 22, 269–277. [Google Scholar] [CrossRef]
- Reyes-Blas, M.; Maldonado-Luna, N.M.; Rivera-Quiñones, C.M.; Vega-Avila, A.L.; Roman-Velázquez, F.R.; Perales-Perez, O.J. Single Step Microwave Assisted Synthesis and Antimicrobial Activity of Silver, Copper and Silver-Copper Nanoparticles. J. Mater. Sci. Chem. Eng. 2020, 8, 13. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Chen, Y.-C.; Kang, L.; Yu, J.; Wang, Y.; Lu, C.; Mashimo, T.; Yoshiasa, A.; Lin, C.-H. Homogeneously alloyed nanoparticles of immiscible Ag–Cu with ultrahigh antibacterial activity. Colloids Surf. B Biointerfaces 2019, 180, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag-Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Bher, A.; Jacob, H.; Auras, R. Compression molded LLDPE films loaded with bimetallic (Ag-Cu) nanoparticles and cinnamon essential oil for chicken meat packaging applications. LWT 2018, 93, 329–338. [Google Scholar] [CrossRef]
- Mamatha, G.; Sowmya, P.; Madhuri, D.; Babu, N.M.; Kumar, D.S.; Charan, G.V.; Varaprasad, K.; Madhukar, K. Antimicrobial Cellulose Nanocomposite Films with In Situ Generations of Bimetallic (Ag and Cu) Nanoparticles Using Vitex negundo Leaves Extract. J. Inorg. Organomet. Polym. Mater. 2021, 31, 802–815. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma Cacao L. Bean Shell Extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Faltane, M.; Rahman, A.; Dahoumane, S.A.; Kucknoor, A.; Jeffryes, C.S. Optimized production of antibacterial copper oxide nanoparticles in a microwave-assisted synthesis reaction using response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 170–178. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H.; Jodeiri, N. Eco-friendly microwave-enhanced green synthesis of silver nanoparticles using Aloe vera leaf extract and their physico-chemical and antibacterial studies. Green Process. Synth. 2017, 7, 231–240. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.A.; Álvarez-Romero, G.A.; Castañeda-Ovando, A.; Mendoza-Tolentino, Y.; Contreras-López, E.; Galán-Vidal, C.A.; Páez-Hernández, M.E. Optimization of microwave-solvothermal synthesis of Fe3O4 nanoparticles. Coating, modification, and characterization. Mater. Chem. Phys. 2018, 205, 113–119. [Google Scholar] [CrossRef]



| NPs Concentration (µg/mL) | Zone of Inhibition (mm) Against Bacteria | |||||
|---|---|---|---|---|---|---|
| K. pneumoniae | E. cloacae | P. aeruginosa | E. coli | E. faecalis | S. aureus | |
| 0.5 | 10 ± 1.6 | 11 ± 1.5 | 9 ± 1.6 | 6.5 ± 2.4 | 3 ± 1.5 | 6 ± 1.4 |
| 1 | 12 ± 1.5 | 13 ± 1.2 | 11 ± 1.8 | 7.6 ± 2.1 | 4 ± 1.6 | 7 ± 1.5 |
| 2.5 | 15 ± 1.7 | 15 ± 1.6 | 14 ± 2.1 | 9 ± 2.2 | 6 ± 1.6 | 9 ± 1.4 |
| 4 | 19 ± 2.1 | 18 ± 2.2 | 16 ± 2.5 | 10 ± 1.2 | 9 ± 2.3 | 10 ± 2.6 |
| 5 | 21 ± 2.2 | 22 ± 2.5 | 20 ± 2.5 | 12 ± 2.4 | 10 ± 2.6 | 15 ± 2.0 |
| Bacteria | NPs Concentration (µg/mL) | |
|---|---|---|
| MIC | MBC | |
| K. pneumoniae | 0.2 | 1 |
| E. cloacae | 0.2 | 1 |
| P. aeruginosa | 0.3 | 0.5 |
| E. coli | 0.5 | 0.6 |
| E. faecalis | 1 | 0.8 |
| S. aureus | 0.8 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, F. Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles. Appl. Sci. 2022, 12, 1384. https://doi.org/10.3390/app12031384
Ameen F. Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles. Applied Sciences. 2022; 12(3):1384. https://doi.org/10.3390/app12031384
Chicago/Turabian StyleAmeen, Fuad. 2022. "Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles" Applied Sciences 12, no. 3: 1384. https://doi.org/10.3390/app12031384
APA StyleAmeen, F. (2022). Optimization of the Synthesis of Fungus-Mediated Bi-Metallic Ag-Cu Nanoparticles. Applied Sciences, 12(3), 1384. https://doi.org/10.3390/app12031384






