Design of Titanium Alloy Femoral Stem Cellular Structure for Stress Shielding and Stem Stability: Computational Analysis
Abstract
:1. Introduction
2. Design Approach
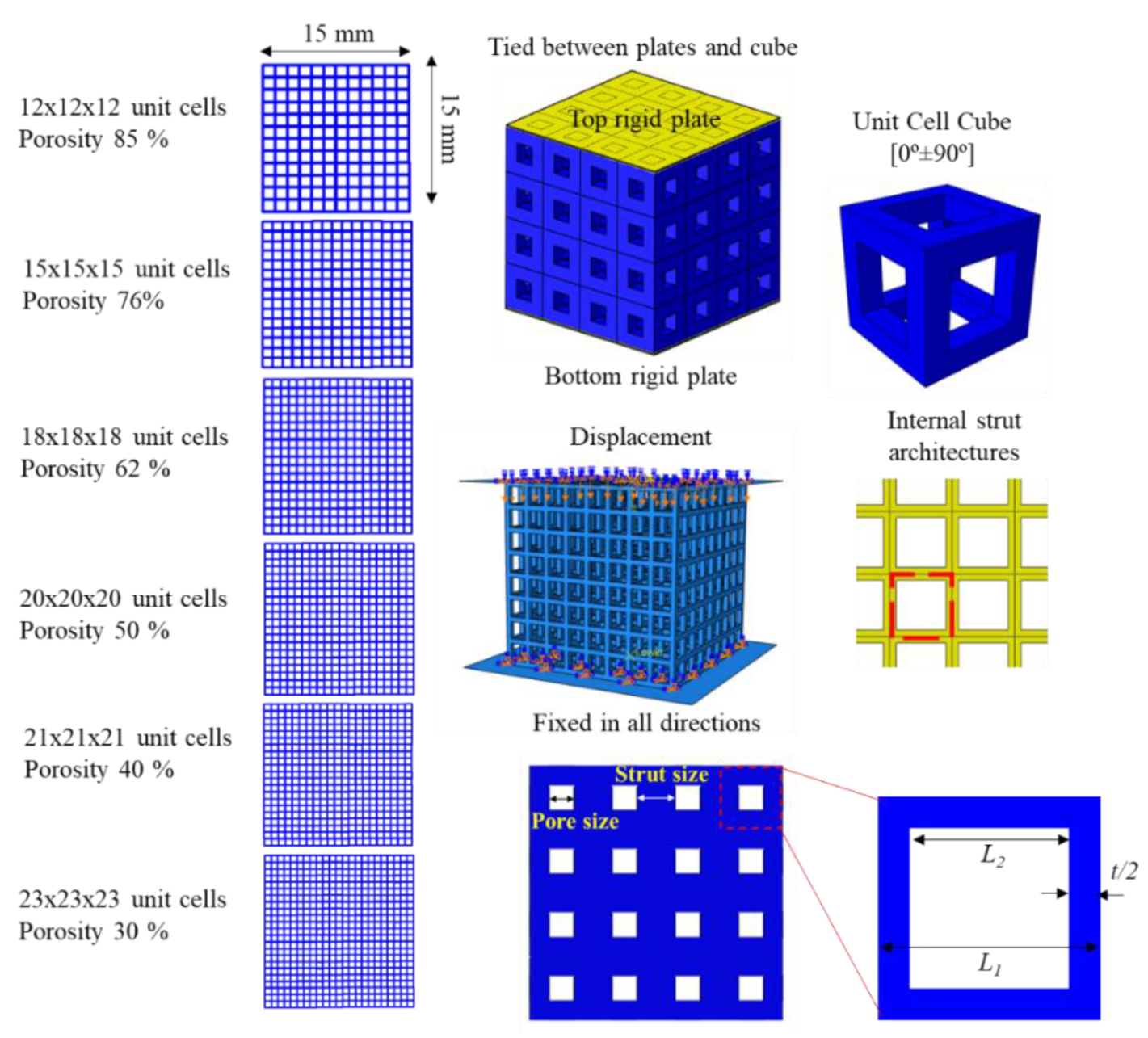
2.1. Design of Porous Microstructures
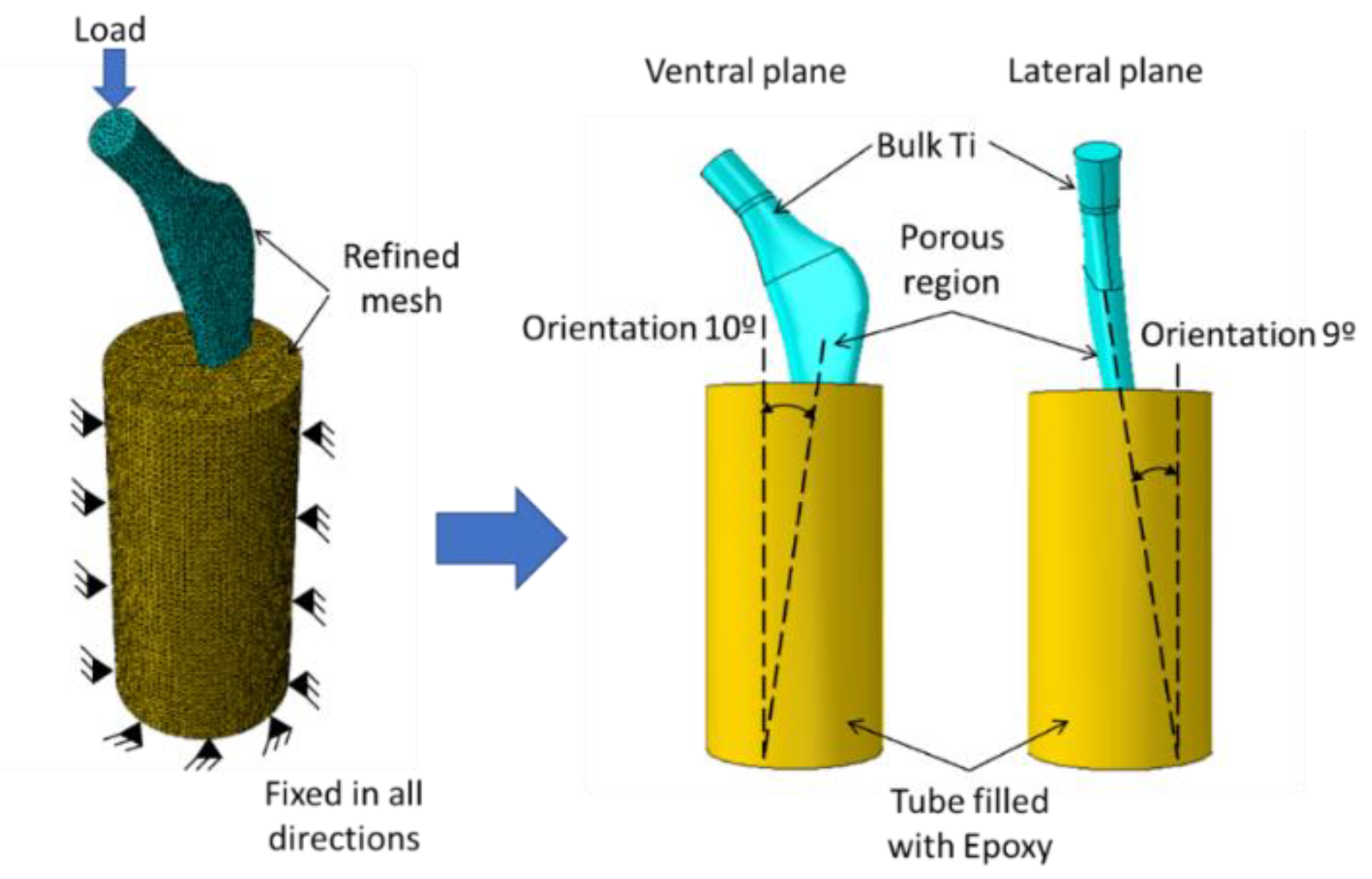
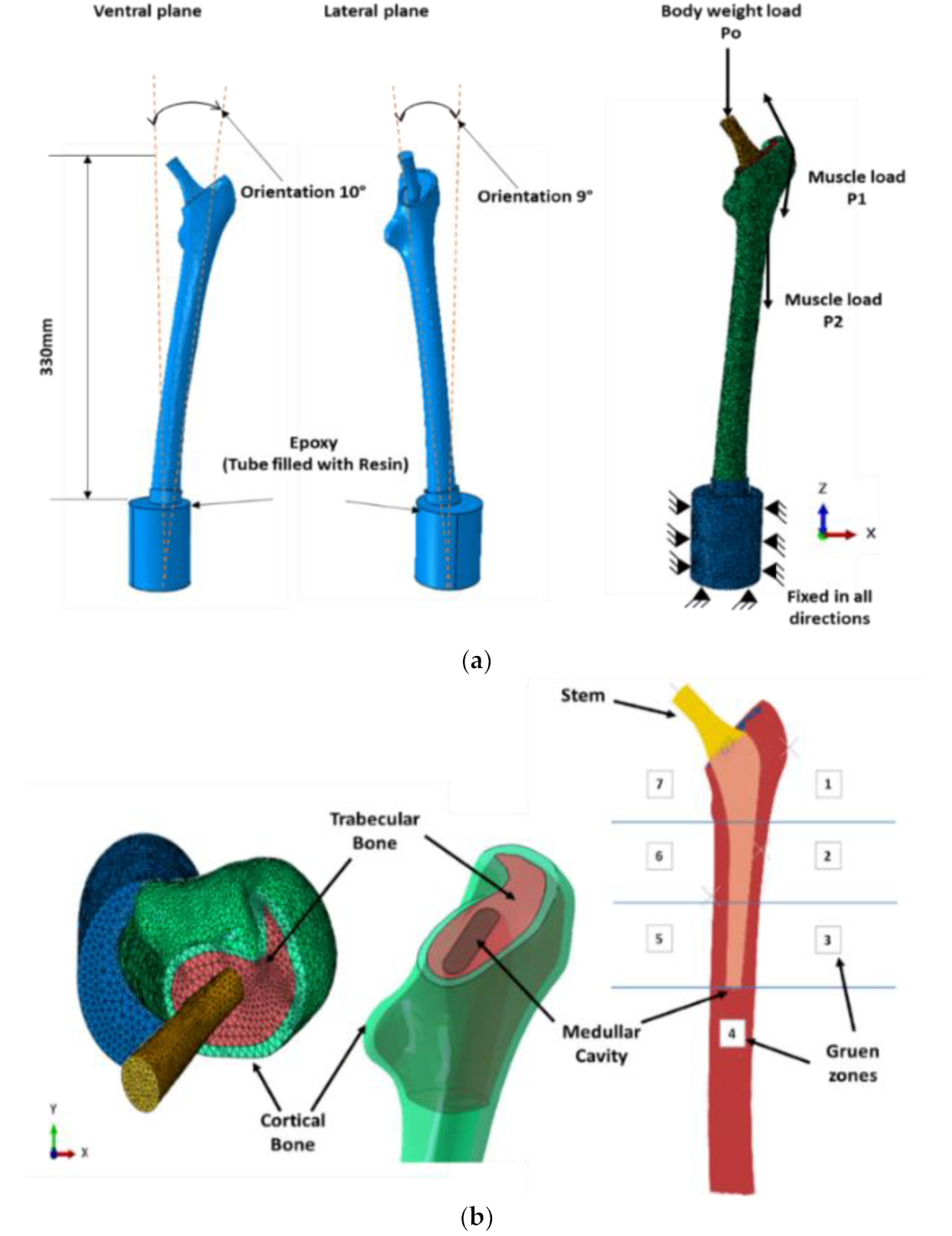
2.2. Design of Porous Stem
3. Results and Discussion
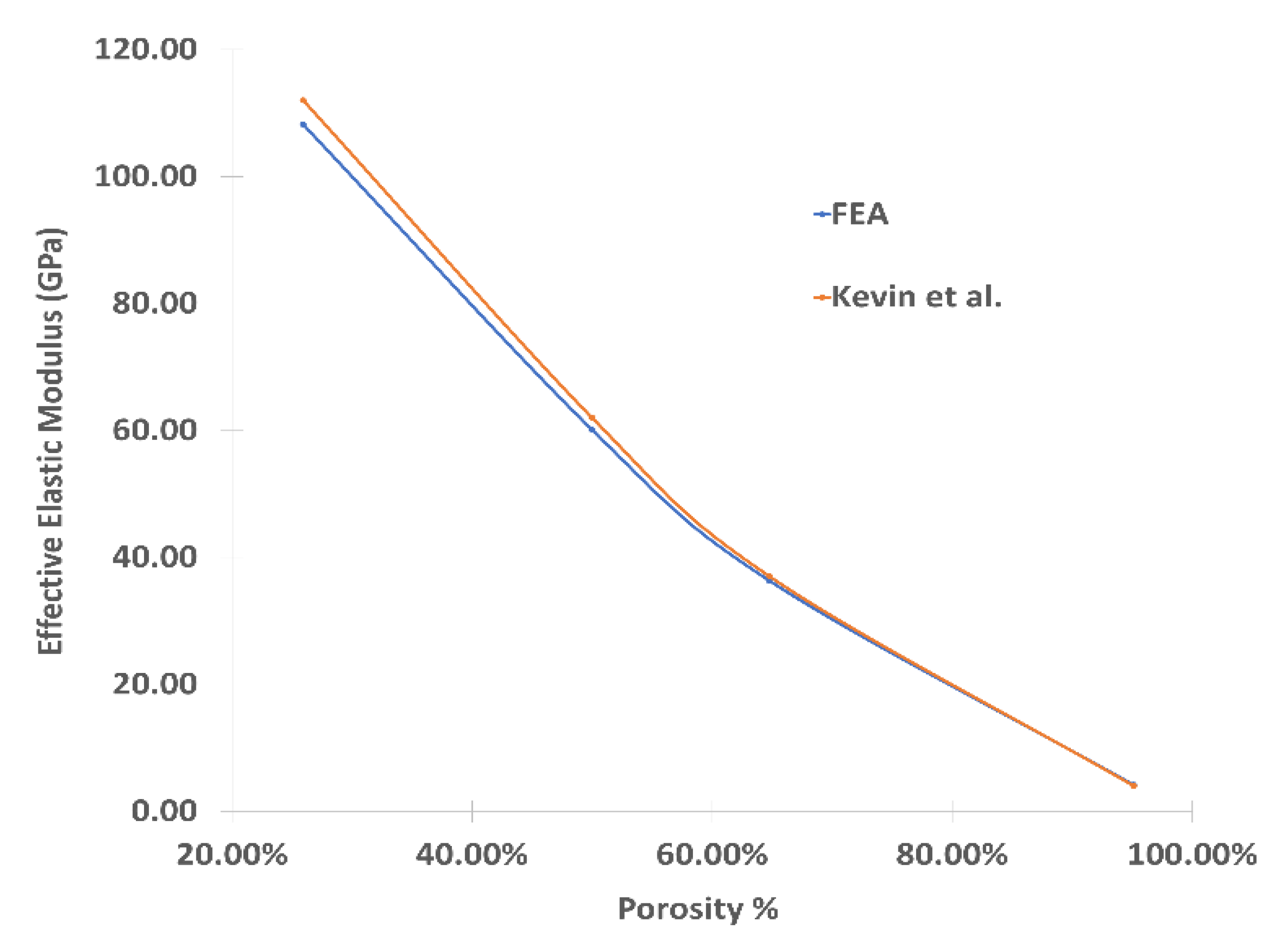
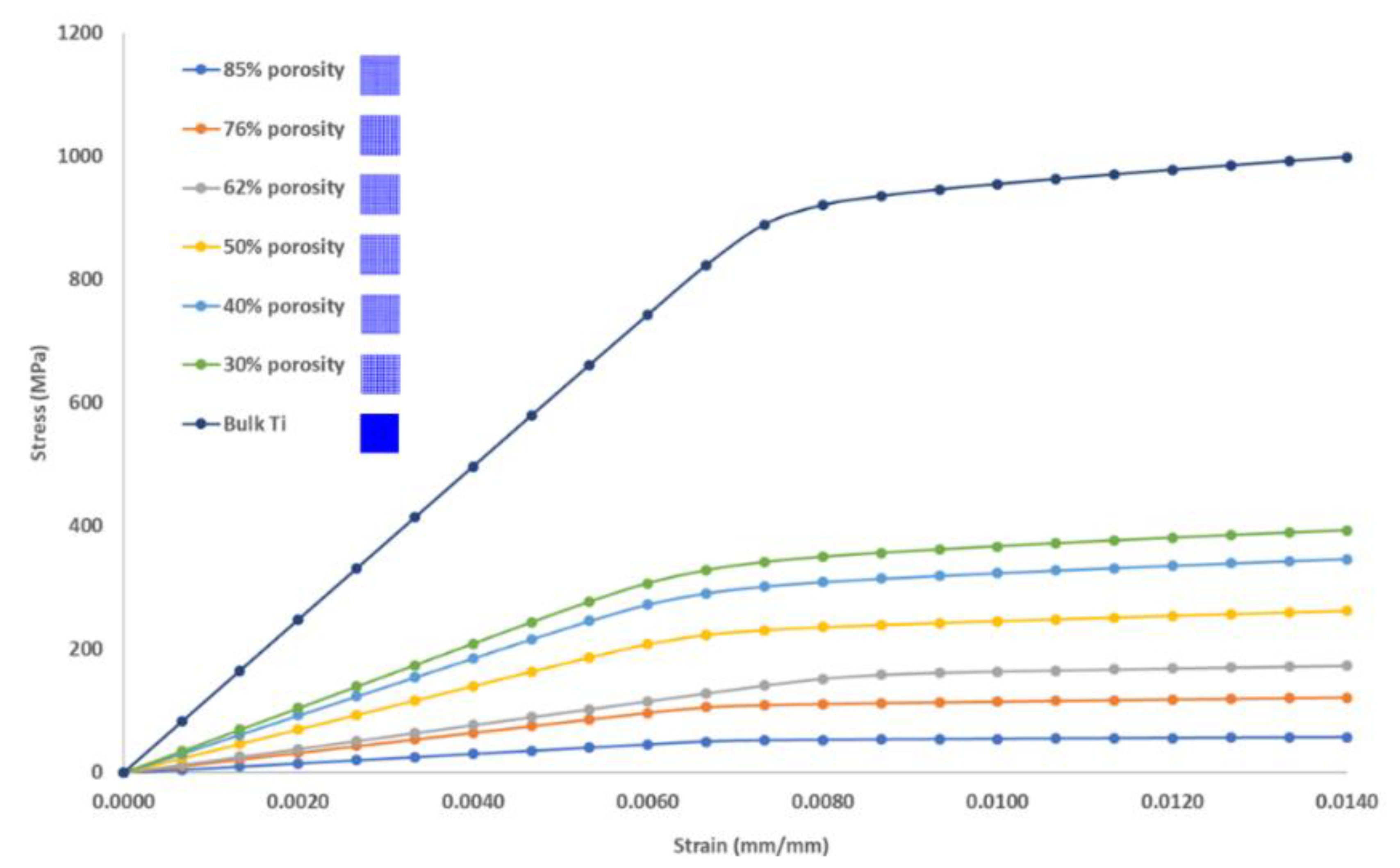
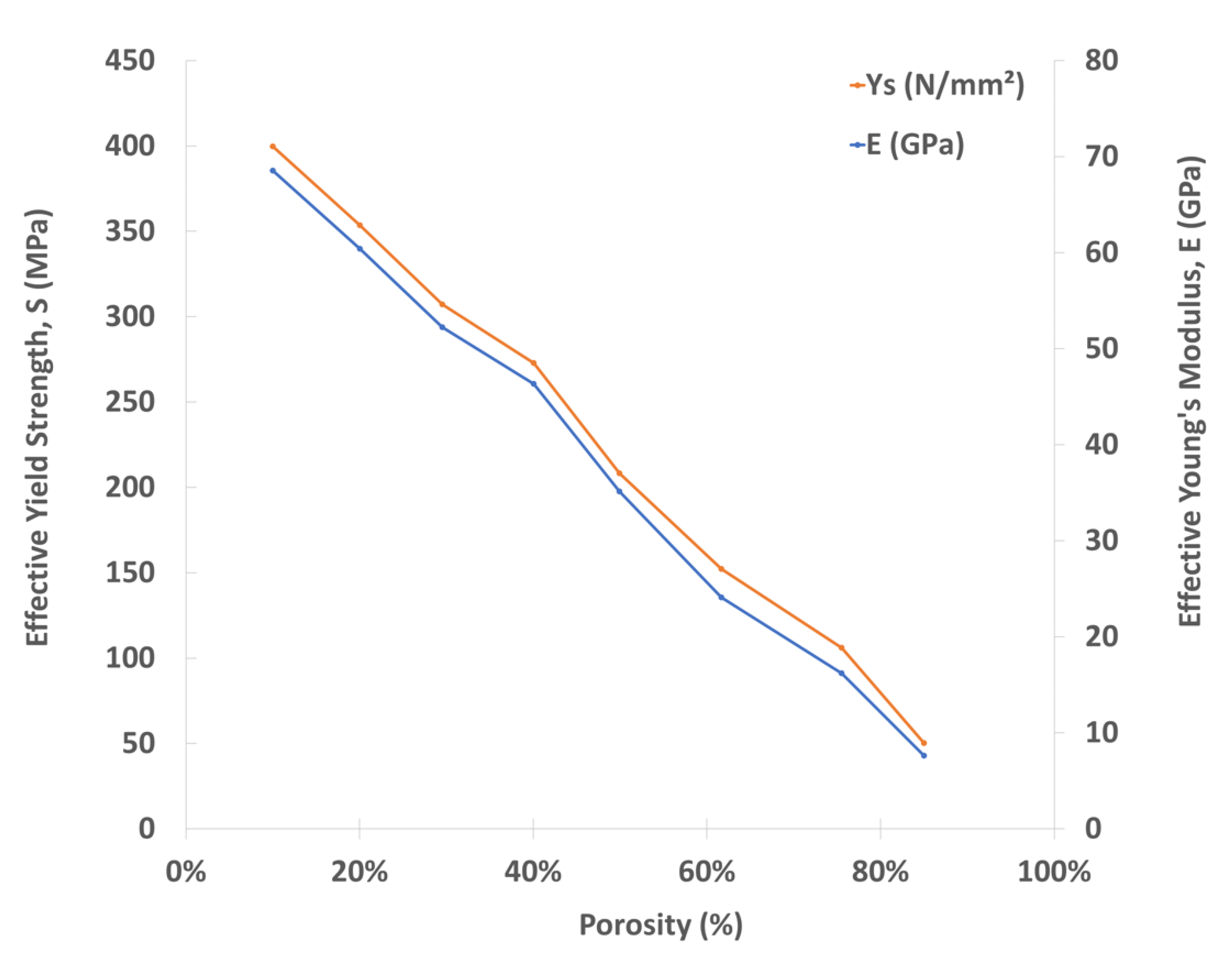
3.1. Mechanical Properties of Porous Cubic Structures
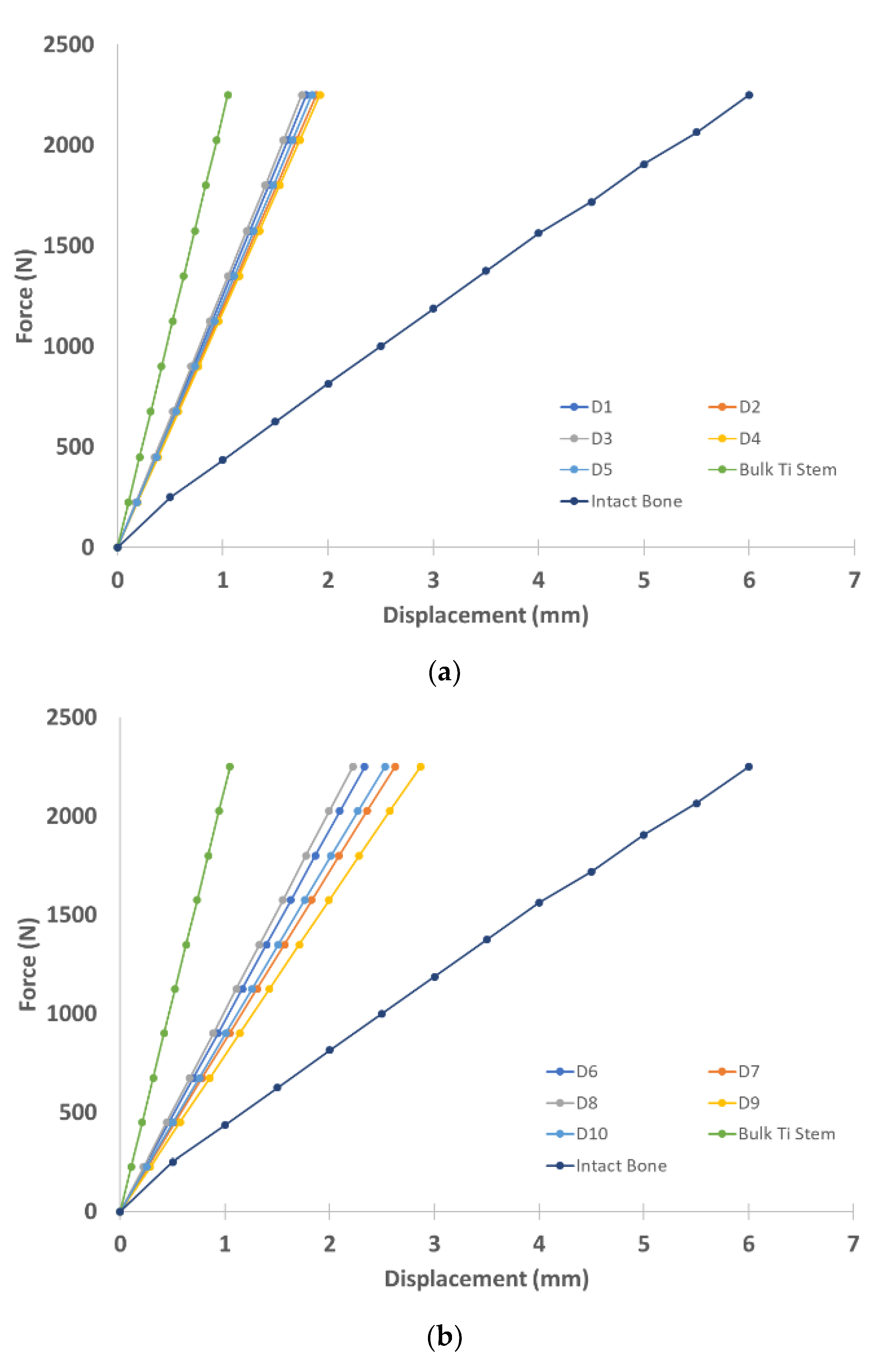
3.2. Biomechanical Performance of Stems
3.3. Assessment of Stress Shielding and Micromotions
4. Conclusions
- Stems with 70% average porosity were found to have the best match with the intact bone mechanical properties for the stem inside the epoxy model.
- Functionally graded porous stems tend to transfer higher stress values to the bone compared to the bulk stem for all physiological loads associated with the three studied walking speeds.
- Micromotion values increased as the porosity and physiological loads/walking speed increases. Hence, the patient is to be advised to walk at a low speed to prevent the stem’s micromotion, which affects bone tissue ingrowth leads to failure of the implant.
- Designs associated with stems with an average porosity of 70% at all walking speeds and 50% at 5 km/h walking speed are considered to have failed in terms of micromotion.
- FG stems of Designs 1 and 3, with 30% average porosity, and Design 8, with 50% average porosity, create moderate interfacial micromotions and enough stress transfer to the bone without yielding. Hence, these designs are recommended as the most suitable candidates to be considered for further studies (fatigue analysis).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Limitation of the Study
References
- Garrison, D. Osteoarthritis, Osteoporosis, and Exercise. Work. Health Saf. 2012, 60, 381–383. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, U.G.; Legnani, C.; Calori, G.M. Acute total hip replacement for acetabular fractures: A systematic review of the literature. Injury 2014, 45, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Parkkari, J.; Sievänen, H.; Heinonen, A.; Vuori, I.; Järvinen, M. Epidemiology of hip fractures. Bone 1996, 18, S57–S63. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Ahmad, F.; Tarlochan, F.; Mehboob, A.; Chang, S.H. A comprehensive analysis of bio-inspired design of femoral stem on primary and secondary stabilities using mechanoregulatory algorithm. Biomech. Model. Mechanobiol. 2020, 19, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H.; Ramesh, S.; Harun, W.S.W.; Kadirgama, K. A novel design, analysis and 3D printing of Ti-6Al-4V alloy bio-inspired porous femoral stem. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H. Finite element modelling and characterization of 3D cellular microstructures for the design of a cementless biomimetic porous hip stem. Mater. Des. 2018, 149, 101–112. [Google Scholar] [CrossRef]
- Alkhatib, S.E.; Mehboob, H.; Tarlochan, F. Finite Element Analysis of Porous Titanium Alloy Hip Stem to Evaluate the Biomechanical Performance During Walking and Stair Climbing. J. Bionic Eng. 2019, 16, 1103–1115. [Google Scholar] [CrossRef]
- Tarlochan, F.; Mehboob, H.; Mehboob, A.; Chang, S.-H. Influence of functionally graded pores on bone ingrowth in cementless hip prosthesis: A finite element study using mechano-regulatory algorithm. Biomech. Model. Mechanobiol. 2017, 17, 701–716. [Google Scholar] [CrossRef]
- Labek, G.; Thaler, M.; Janda, W.; Agreiter, M.; Stockl, B. Revision rates after total joint replacement. CUMULATIVE RESULTS FROM WORLDWIDE JOINT REGISTER DATASETS. J. Bone Jt. Surgery. Br. Vol. 2011, 93, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Stimac, J.D.; Boles, J.; Parkes, N.; Della Valle, A.G.; Boettner, F.; Westrich, G.H. Revision Total Hip Arthroplasty With Modular Femoral Stems. J. Arthroplast. 2014, 29, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Khatod, M.; Cafri, G.; Namba, R.S.; Inacio, M.; Paxton, E.W. Risk Factors for Total Hip Arthroplasty Aseptic Revision. J. Arthroplast. 2014, 29, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Besculides, M.C.; Gaber, L.; Liu, S.; Della Valle, A.G. Risk factors for pulmonary embolism after hip and knee arthroplasty: A population-based study. Int. Orthop. 2008, 33, 1739–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Gil, L.; Brühl, S.; Jiménez, L.; Leon, O.; Guevara, R.; Staia, M.H. Corrosion performance of the plasma nitrided 316L stainless steel. Surf. Coatings Technol. 2006, 201, 4424–4429. [Google Scholar] [CrossRef]
- Healy, W.L.; Tilzey, J.F.; Iorio, R.; Specht, L.M.; Sharma, S. Prospective, Randomized Comparison of Cobalt-Chrome and Titanium Trilock Femoral Stems. J. Arthroplast. 2009, 24, 831–836. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Met. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Chen, J.-H.; Liu, C.; You, L.; Simmons, C.A. Boning up on Wolff’s Law: Mechanical regulation of the cells that make and maintain bone. J. Biomech. 2010, 43, 108–118. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Oshkour, A.; Abu Osman, N.; Bayat, M.; Afshar, R.; Berto, F. Three-dimensional finite element analyses of functionally graded femoral prostheses with different geometrical configurations. Mater. Des. 2014, 56, 998–1008. [Google Scholar] [CrossRef]
- Hazlehurst, K.B.; Wang, C.J.; Stanford, M. A numerical investigation into the influence of the properties of cobalt chrome cellular structures on the load transfer to the periprosthetic femur following total hip arthroplasty. Med Eng. Phys. 2014, 36, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Schmidutz, F.; Agarwal, Y.; Müller, P.; Gueorguiev, B.; Richards, R.; Sprecher, C. Stress-shielding induced bone remodeling in cementless shoulder resurfacing arthroplasty: A finite element analysis and in vivo results. J. Biomech. 2014, 47, 3509–3516. [Google Scholar] [CrossRef]
- Alkhatib, S.E.; Tarlochan, F.; Mehboob, H.; Singh, R.; Kadirgama, K.; Harun, W.S.W. Finite element study of functionally graded porous femoral stems incorporating body-centered cubic structure. Artif. Organs 2019, 43, E152–E164. [Google Scholar] [CrossRef] [PubMed]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. Stiffness and strength tailoring of cobalt chromium graded cellular structures for stress-shielding reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Sharma, G.K.; Gurumoorthy, B. Iso-material contour representation for process planning of heterogeneous object model. J. Comput. Des. Eng. 2020, 7, 498–513. [Google Scholar] [CrossRef]
- Velasco, M.; Lancheros, Y.; Garzón-Alvarado, D.A. Geometric and mechanical properties evaluation of scaffolds for bone tissue applications designing by a reaction-diffusion models and manufactured with a material jetting system. J. Comput. Des. Eng. 2016, 3, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Ahn, S.-J.; Lee, H.; Noh, G. Implant placement in the removable mandibular advancement device for completely edentulous patients: A finite element study. J. Comput. Des. Eng. 2021, 8, 140–148. [Google Scholar] [CrossRef]
- Bagheri, M.A.; Rouhi, G. Design and numerical investigation of an adaptive intramedullary nail with a novel interlocking mechanism. J. Comput. Des. Eng. 2020, 7, 722–735. [Google Scholar] [CrossRef]
- Kim, W.; Veloso, A.P.; Araújo, D.; Kohles, S.S. Novel computational approaches characterizing knee physiotherapy. J. Comput. Des. Eng. 2014, 1, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.; Kim, J.-E.; Jung, J.; Oh, S.-H.; Noh, G.; Kwon, Y.-D. Effect of mandibular contouring surgery on the stress distribution during various clenching tasks. J. Comput. Des. Eng. 2021, 8, 570–580. [Google Scholar] [CrossRef]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Hedayati, R.; Hosseini-Toudeshky, H.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A. Computational prediction of the fatigue behavior of additively manufactured porous metallic biomaterials. Int. J. Fatigue 2016, 84, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Jetté, B.; Brailovski, V.; Simoneau, C.; Dumas, M.; Terriault, P. Development and in vitro validation of a simplified numerical model for the design of a biomimetic femoral stem. J. Mech. Behav. Biomed. Mater. 2018, 77, 539–550. [Google Scholar] [CrossRef]
- Simoneau, C.; Terriault, P.; Jetté, B.; Dumas, M.; Brailovski, V. Development of a porous metallic femoral stem: Design, manufacturing, simulation and mechanical testing. Mater. Des. 2017, 114, 546–556. [Google Scholar] [CrossRef]
- Oshkour, A.; Davoodi, M.; Abu Osman, N.; Yau, Y.; Tarlochan, F.; Abas, W.W. Finite element analysis of circumferential crack behavior in cement–femoral prosthesis interface. Mater. Des. 2013, 49, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Yeni, Y.N.; Wu, B.; Huang, L.; Oravec, D. Mechanical Loading Causes Detectable Changes in Morphometric Measures of Trabecular Structure in Human Cancellous Bone. J. Biomech. Eng. 2013, 135, 054505–0545055. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Nakamura, J.; Matsuura, Y.; Wako, Y.; Suzuki, T.; Hagiwara, S.; Orita, S.; Inage, K.; Kawarai, Y.; Sugano, M.; et al. Prediction of fracture load and stiffness of the proximal femur by CT-based specimen specific finite element analysis: Cadaveric validation study. BMC Musculoskelet. Disord. 2017, 18, 536. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip joint loading during walking and running, measured in two patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Heller, M.; Bergmann, G.; Kassi, J.-P.; Claes, L.; Haas, N.; Duda, G. Determination of muscle loading at the hip joint for use in pre-clinical testing. J. Biomech. 2005, 38, 1155–1163. [Google Scholar] [CrossRef]
- Cavalli, L.; Brandi, M.L. Periprosthetic bone loss: Diagnostic and therapeutic approaches. F1000Research 2014, 2, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazlehurst, K.; Wang, C.J.; Stanford, M. Evaluation of the stiffness characteristics of square pore CoCrMo cellular structures manufactured using laser melting technology for potential orthopaedic applications. Mater. Des. 2013, 51, 949–955. [Google Scholar] [CrossRef]
- Morrey, B. Cementless Femoral Fixation in Total Hip Arthroplasty. Yearb. Orthop. 2011, 2011, 148–149. [Google Scholar] [CrossRef]
- Alm, J.J.; Mäkinen, T.J.; Lankinen, P.; Moritz, N.; Vahlberg, T.; Aro, H.T. Female patients with low systemic BMD are prone to bone loss in Gruen zone 7 after cementless total hip arthroplasty. Acta Orthop. 2009, 80, 531–537. [Google Scholar] [CrossRef]
- Pitto, R.P.; Hayward, A.; Walker, C.; Shim, V.B. Femoral bone density changes after total hip arthroplasty with uncemented taper-design stem: A five year follow-up study. Int. Orthop. 2009, 34, 783–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morri, M.; Natali, E.; Tosarelli, D. At discharge gait speed and independence of patients provides a challenges for rehabilitation after total joint arthroplasty: An observational study. Arch. Physiother. 2016, 29, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhardt, D.M.J.M.; Ter Mors, T.G.; Hannink, G.; Van Susante, J.L.C. Resurfacing hip arthroplasty better preserves a normal gait pattern at increasing walking speeds compared to total hip arthroplasty. Acta Orthop. 2019, 90, 231–236. [Google Scholar] [CrossRef] [Green Version]

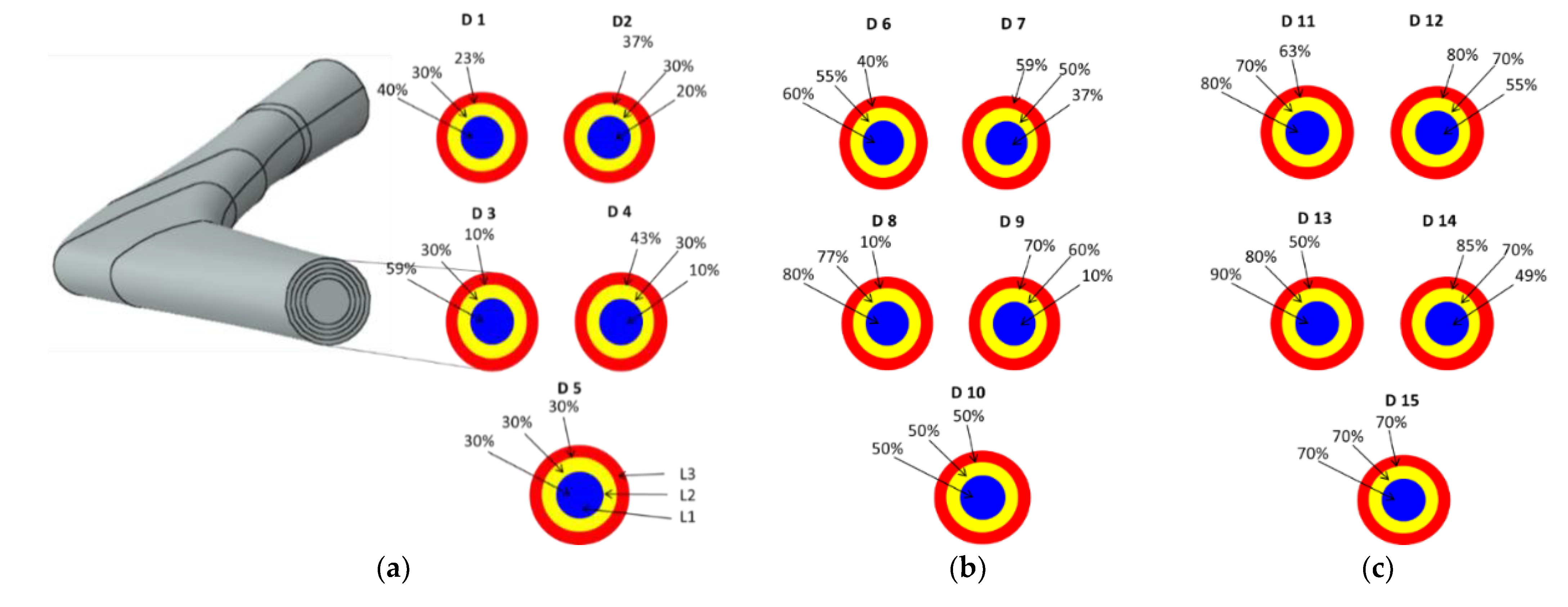

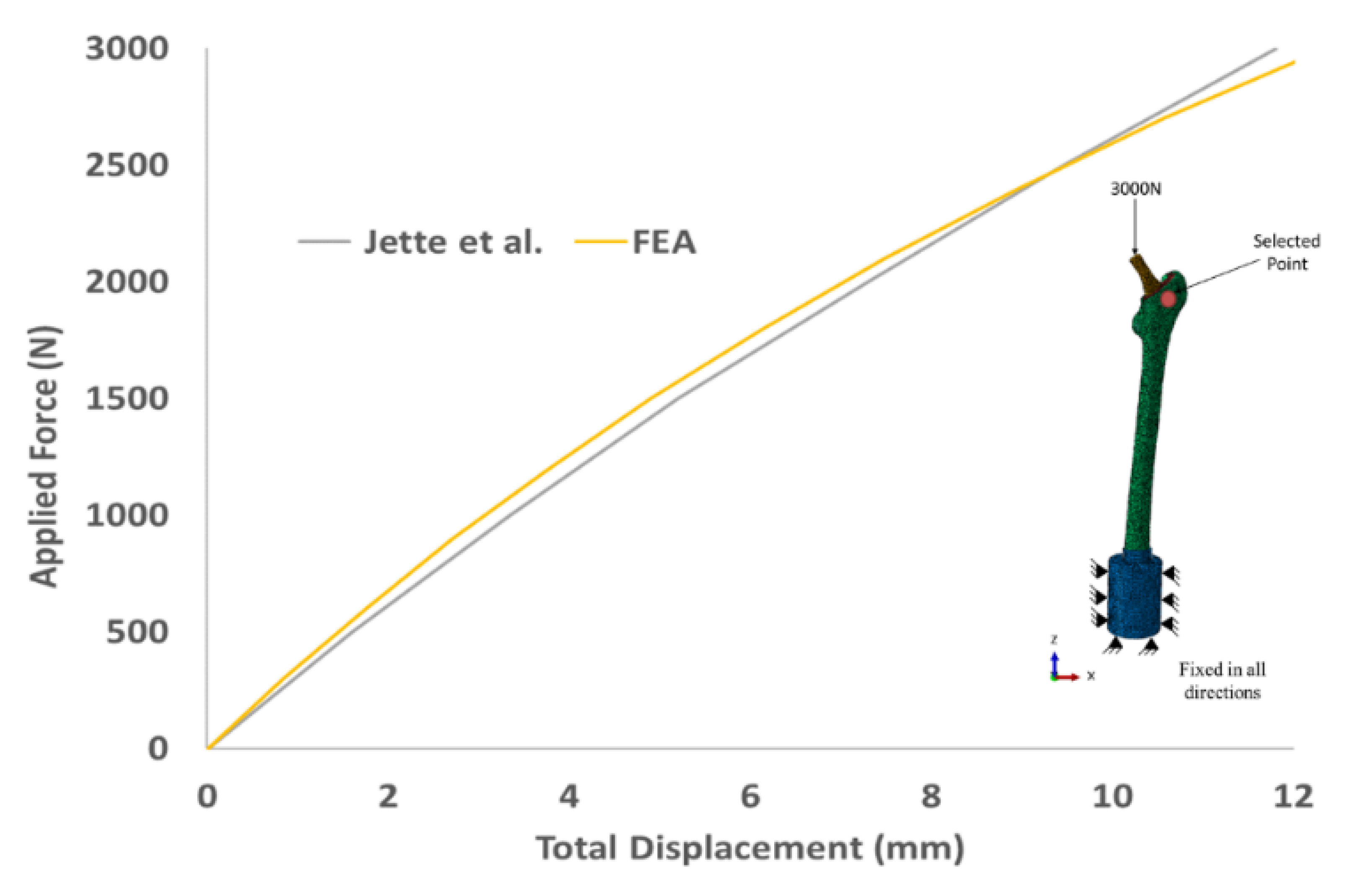


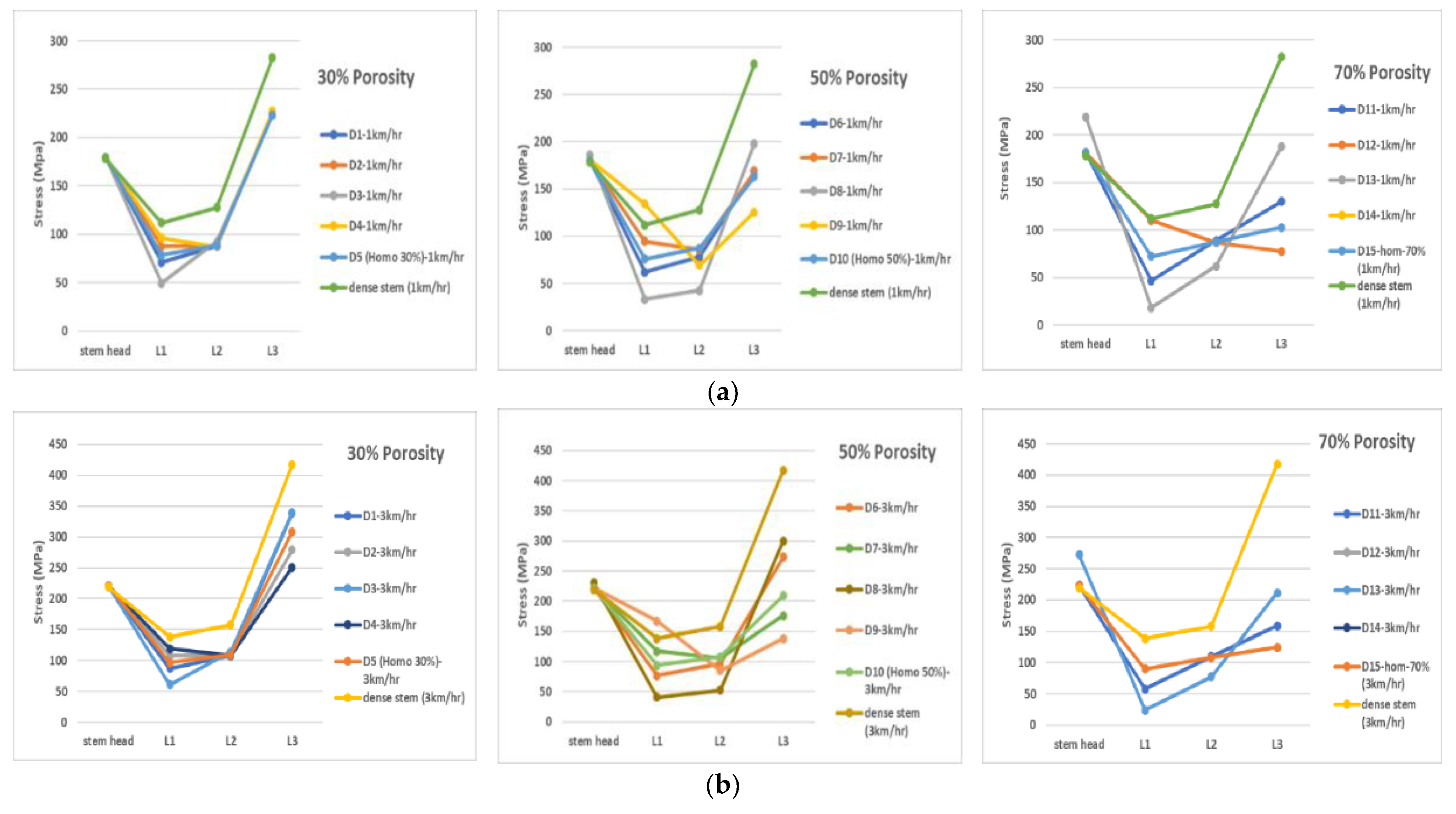

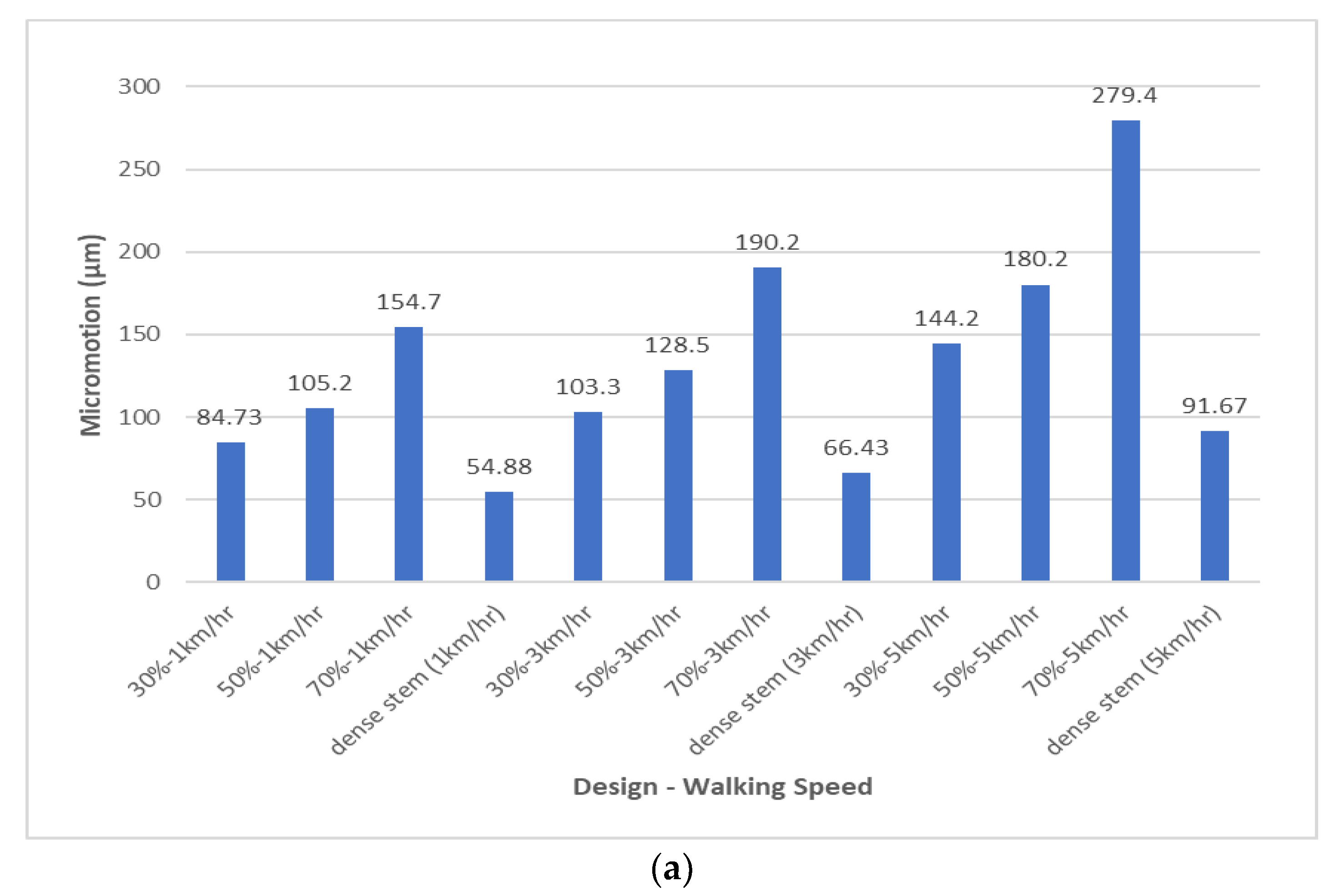

| 30% Overall Volumetric Porosity | |||||
| Stem Layers | FG-Design 1 (D1) | FG-Design 2 (D2) | FG-Design 3 (D3) | FG-Design 4 (D4) | H-Design 5 (D5) |
| Layer 1 (L1-Core) | 40% | 20% | 59% | 10% | 30% |
| Layer 2 (1 mm) (L2) | 30% | 30% | 30% | 30% | |
| Layer 3 (1 mm) (L3) | 23% | 37% | 10% | 43% | |
| 50% Overall Volumetric Porosity | |||||
| Stem Layers | FG-Design 6 (D6) | FG-Design 7 (D7) | FG-Design 8 (D8) | FG-Design 9 (D9) | H-Design 10 (D10) |
| Layer 1 (L1-Core) | 60% | 37% | 80% | 10% | 50% |
| Layer 2 (1 mm) (L2) | 55% | 50% | 77% | 60% | |
| Layer 3 (1 mm) (L3) | 40% | 59% | 10% | 70% | |
| 70% Overall Volumetric Porosity | |||||
| Stem Layers | FG-Design 11 (D11) | FG-Design 12 (D12) | FG-Design 13 (D13) | FG-Design 14 (D14) | H-Design 15 (D15) |
| Layer 1 (L1-Core) | 80% | 55% | 90% | 49% | 70% |
| Layer 2 (1 mm) (L2) | 70% | 70% | 80% | 70% | |
| Layer 3 (1 mm) (L3) | 63% | 80% | 50% | 85% | |
| Bone Material | Young’s Modulus (GPa) | Shear Modulus (GPa) | Poisson’s Ratio |
|---|---|---|---|
| Spongy bone (trabecular bone) | 0.4 | - | 0.3 |
| Cortical bone | Exx = 11.5 | Gxy = 3.6 | νxy = 0.51 |
| Eyy = 11.5 | Gyz = 3.3 | νyz = 0.31 | |
| Ezz = 17 | Gyz = 3.3 | νyz = 0.31 |
| Force (Body Weight Used BW = 900 N) | %Po | 1 km/h (Po = 293% BW) | 3 km/h (Po = 352% BW) | 5 km/h (Po = 471% BW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fx | Fy | Fz | Fx | Fy | Fz | Fx | Fy | Fz | ||
| Po | 100 | −599 | −363 | −2542 | −719 | −437 | −3054 | −962 | −584 | −4086 |
| P1 | 44 | 643 | 8.0 | 959 | 772 | 57.3 | 1152 | 1034 | 76.7 | 1542 |
| P1-tensor fascia latae, proximal part | 8 | 79.9 | 129 | 146 | 95.9 | 154 | 175 | 128 | 206 | 235.4 |
| P1-tensor fascia latae, distal part | 8 | −5.5 | −7.8 | −210 | −6.7 | −9.3 | −253 | −8.9 | −12.5 | −338 |
| P2 | 40 | −10.0 | 205 | −1030 | −12.0 | 246 | −1237 | −16.0 | 329.9 | −1656 |
| 1 km/h | ||||||||
| Design | Location | G 1 | G 2 | G 3 | G 4 | G 5 | G 6 | G 7 |
| 30% Porosity | Trabecular bone | 27.1% | 9.4% | 2.5% | 1.2% | 4.9% | 10.4% | 35.9% |
| 50% Porosity | Trabecular bone | 35.8% | 12.2% | 4.5% | 1.8% | 6.6% | 16.1% | 48.1% |
| 70% Porosity | Trabecular bone | 46.1% | 20.3% | 6.6% | 2.8% | 8.6% | 22.3% | 62.6% |
| 3 km/h | ||||||||
| Design | Location | G 1 | G 2 | G 3 | G 4 | G 5 | G 6 | G 7 |
| 30% Porosity | Trabecular bone | 21.8% | 9.5% | 2.2% | 1.5% | 5.2% | 10.2% | 36.1% |
| 50% Porosity | Trabecular bone | 31.8% | 12.3% | 4.3% | 2.2% | 7.0% | 16.0% | 48.4% |
| 70% Porosity | Trabecular bone | 41.4% | 19.8% | 6.6% | 3.5% | 9.1% | 22.1% | 63.1% |
| 5 km/h | ||||||||
| Design | Location | G 1 | G 2 | G 3 | G 4 | G 5 | G 6 | G 7 |
| 30% Porosity | Trabecular bone | 20.3% | 10.5% | 2.2% | 2.2% | 5.8% | 9.9% | 36.6% |
| 50% Porosity | Trabecular bone | 30.5% | 12.9% | 4.1% | 3.3% | 7.8% | 15.9% | 49.2% |
| 70% Porosity | Trabecular bone | 40.0% | 19.2% | 6.8% | 5.2% | 10.2% | 22.4% | 64.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Zoubi, N.F.; Tarlochan, F.; Mehboob, H.; Jarrar, F. Design of Titanium Alloy Femoral Stem Cellular Structure for Stress Shielding and Stem Stability: Computational Analysis. Appl. Sci. 2022, 12, 1548. https://doi.org/10.3390/app12031548
Al Zoubi NF, Tarlochan F, Mehboob H, Jarrar F. Design of Titanium Alloy Femoral Stem Cellular Structure for Stress Shielding and Stem Stability: Computational Analysis. Applied Sciences. 2022; 12(3):1548. https://doi.org/10.3390/app12031548
Chicago/Turabian StyleAl Zoubi, Naser Fawzi, Faris Tarlochan, Hassan Mehboob, and Firas Jarrar. 2022. "Design of Titanium Alloy Femoral Stem Cellular Structure for Stress Shielding and Stem Stability: Computational Analysis" Applied Sciences 12, no. 3: 1548. https://doi.org/10.3390/app12031548
APA StyleAl Zoubi, N. F., Tarlochan, F., Mehboob, H., & Jarrar, F. (2022). Design of Titanium Alloy Femoral Stem Cellular Structure for Stress Shielding and Stem Stability: Computational Analysis. Applied Sciences, 12(3), 1548. https://doi.org/10.3390/app12031548









