Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder
Abstract
:1. Introduction
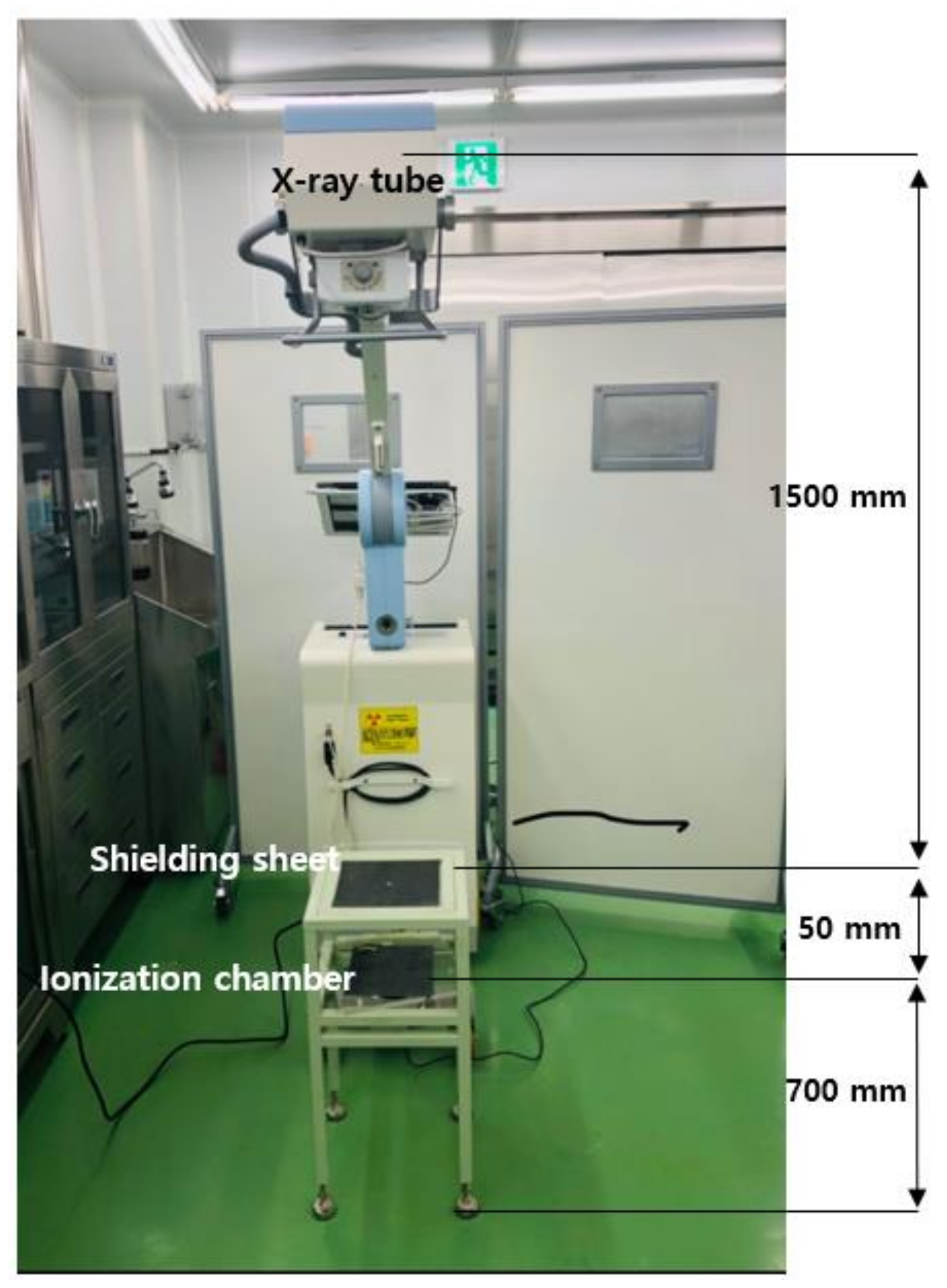
2. Materials and Methods
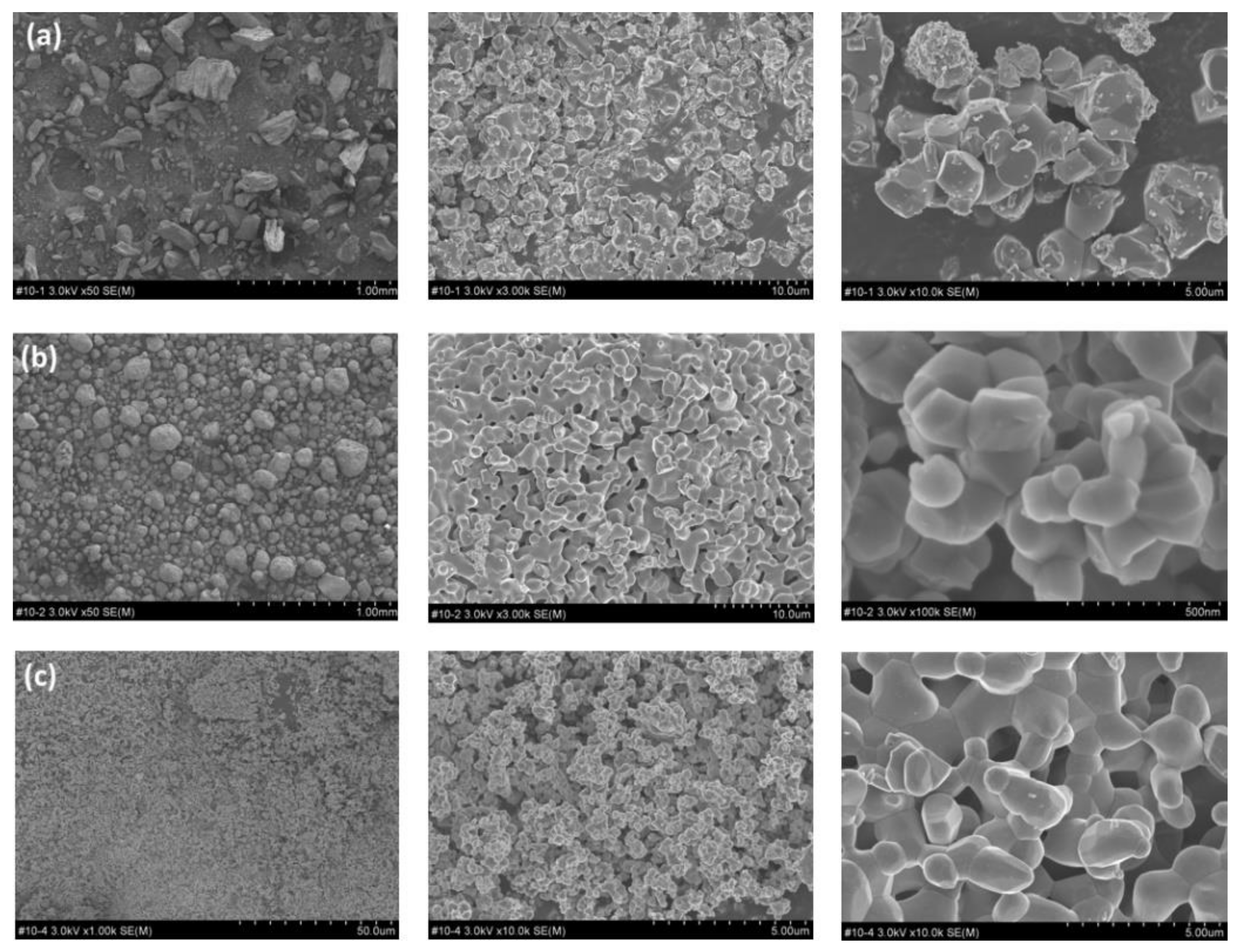
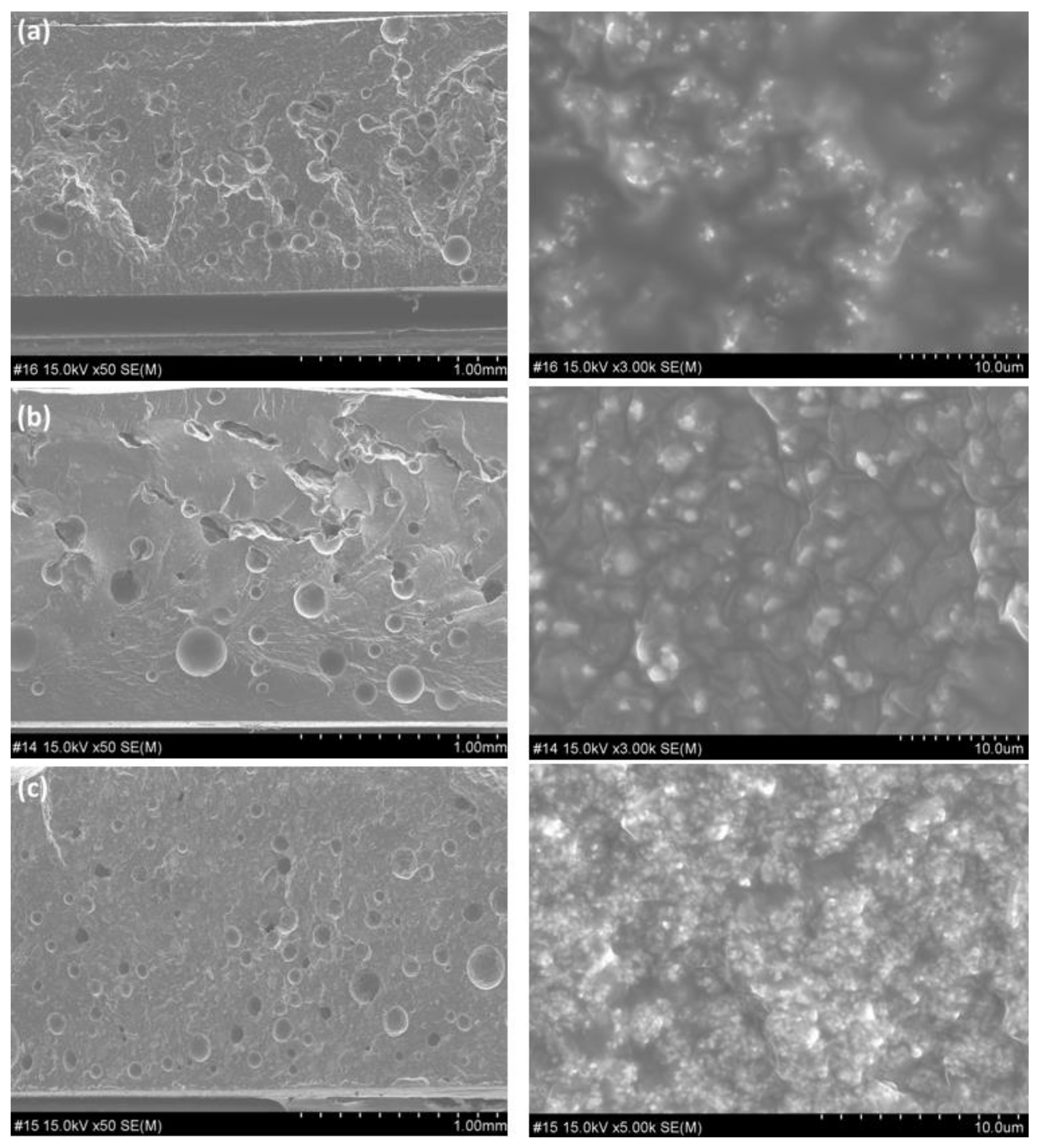
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahadevappa, M. Radiation dose management for fluoroscopically guided interventional medical procedures. National Council on Radiation Protection and Measurements. Int. J. Med. Physiol. 2010, 21, 5789–5790. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; UNSCEAR 2008 REPORT 1; UNSCEAR: New York, NY, USA, 2010. [Google Scholar]
- López, P.O.; Dauer, L.T.; Loose, R.; Martin, C.J.; Miller, D.L.; Vañó, E.; Doruff, M.; Padovani, R.; Massera, C.; Yoder, C. ICRP Publication 139, 2017 Occupational Radiological Protection in Interventional Procedures. Ann. ICRP 2017, 47, 1–118. [Google Scholar] [CrossRef]
- Dominique, A.; Marc, P.; Fabien, B.; Redha, T.; Guillaume, P. Wearing lead aprons in surgical operating rooms: Ergonomic injuries evidenced by IR Thermography. J. Surg. Res. 2017, 209, 227–233. [Google Scholar] [CrossRef]
- Zuguchi, M.; Chida, K.; Taura, M.; Inaba, Y.; Ebata, A.; Yamada, S. Usefulness of non-lead aprons in radiation protection for physicians performing interventional procedures. Radiat. Prot. Dosim. 2008, 131, 531–534. [Google Scholar] [CrossRef]
- Marichal, D.A.; Anwar, T.; Kirsch, D.; Clements, J.; Carlson, L.; Savage, C.; Rees, C.R. Comparison of a Suspended Radiation Protection System versus Standard Lead Apron for Radiation Exposure of a Simulated interventionalist. J. Vasc. Interv. Radiol. 2011, 22, 437–442. [Google Scholar] [CrossRef]
- Livingstone, R.S.; Varghese, A.; Keshava, S.N. A study on the use of radiation-protective apron among interventionists in radiology. J. Clin. Imaging Sci. 2008, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Boyd, C.; Dawson, J. Lightweight lead aprons: The emperor’s new clothes in the angiography suite? Eur. J. Endovasc. Surg. 2019, 57, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Moro, L.N.; Narayan, Y. Perceived physical stress at work and musculoskeletal discomfort in X-ray technologists. Ergonomics 2004, 47, 189–201. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, H.; Lu, S.; Jia, Q. Optimization of radiation shielding material aiming at compactness, lightweight, and low activation for a vehicle-mounted accelerator-driven DT neutron source. Appl. Radiat. Isot. 2018, 135, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Soylu, H.M.; Lambrecht, F.Y.; Ersöz, O.A. Gamma radiation shielding efficiency of a new lead-free composite material. J. Radioanal. Nucl. Chem. 2015, 305, 529–534. [Google Scholar] [CrossRef]
- Çetin, H.; Yurt, A.Y.; Yüksel, S.H. The Absorption properties of lead-free garments for use in radiation protection. Radiat. Prot. Dosim. 2017, 173, 345–350. [Google Scholar] [CrossRef] [PubMed]
- AbuAlRoos, N.J.; Azman, M.N.; Amin, N.A.B.; Zainon, R. Tungsten-based material as promising new lead-free gamma radiation shielding material in nuclear medicine. Phys. Med. 2020, 78, 48–57. [Google Scholar] [CrossRef]
- Adlienė, D.; Gilys, L.; Griškonis, E. Development and characterization of new tungsten and tantalum containing composites for radiation shielding in medicine. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 467, 21–26. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, S.G.; Seo, Y.S. Enhanced X-ray Shielding Ability of Polymer–Nonleaded Metal Composites by Multilayer Structuring. Ind. Eng. Chem. Res. 2015, 54, 5968–5973. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Q.; Zheng, W.; Zheng, Y.; Okosi, N.; Wang, Z.; Su, M. Enhanced radiation shielding with conformal light-weight nanoparticle–polymer composite. ACS Appl. Mater. 2018, 10, 35510–35515. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build Mater. 2018, 20, 751–764. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Li, H.; Chen, T.; Ye, Y.; Zheng, H. Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2502–2530. [Google Scholar] [CrossRef]
- Chung, C.W. Possibility to recycle domestic waste shells-with emphasis on oyster shells. Mag. Concr. Res. 2016, 11, 11–16. [Google Scholar] [CrossRef]
- Owuamanam, S.; Cree, D. Progress of bio-calcium carbonate waste Eggshell and seashell fillers in polymer Composites: A review. Compos. Sci. Technol. 2020, 4, 70. [Google Scholar] [CrossRef]
- Elsheikh, N.A.A. Monte Carlo modelling of a neutron-induced gamma-ray sensor for landmine or explosive detection. J. Radiat. Res. Appl. Sci. 2018, 11, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Chiou, I.J.; Chen, C.H.; Li, Y.H. Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Constr. Build Mater. 2014, 64, 480–487. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Calcined oyster shell powder as an expansive additive in cement mortar. Materials 2019, 12, 1322. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, P.; Pan, T.; Liao, Y.; Zhao, H. Evaluation of the eco-friendly crushed waste oyster shell mortars containing supplementary cementitious materials. J. Clean. Prod. 2019, 237, 1–33. [Google Scholar] [CrossRef]
- Rahman, T.; Nurdiana, J. The effect of HDPE plastic fibres on concrete performance. Resour. Recycl. 2020, 5, 1–16. [Google Scholar] [CrossRef]
- Kim, S.C. Development of air pressure mirroring particle dispersion method for producing high density tungsten medical radiation shielding film. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Maghrabi, H.A.; Vijayan, A.; Mohaddes, F.; Deb, P.W.; Wang, L. Evaluation of X-ray radiation shielding performance of barium sulphate-coated fabrics. Fibers Polym. 2016, 17, 2047–2054. [Google Scholar] [CrossRef]
- Korean Standards Association. Testing Method of Lead Equivalent for X-Ray Protective Devices; KSA 4025; Korean Standards Association: Seoul, Korea, 2017. [Google Scholar]
- Kaur, T.; Sharma, J.; Singh, T. Experimental evaluation of gamma rays shielding parameters for Zn-Cd-Sn-Pb quaternary alloy. Radiat. Phys. Chem. 2019, 156, 193–198. [Google Scholar] [CrossRef]
- Hubbell, J.H. Photon Mass Attenuation and Energy absorption Coefficients from 1 keV to 20 MeV. Int. Appl. Radiat. Isot. 1982, 33, 1269–1290. [Google Scholar] [CrossRef]
- Pingale, P.L. Formulation, evaluation of fast dissolving tablet of anti-HIV drugs as fixed dose combination: Use of freeze-dried powder of Annona reticulata and comparison WITH synthetic superdisintegrants. J. Pharm. Sci. Innov. 2019, 8, 38–41. [Google Scholar] [CrossRef]
- Xie, T.; Xu, K.; Yang, B.; He, Y. Effect of pore size and porosity distribution on radiation absorption and thermal performance of porous solar energy absorber. Sci. China Technol. Sci. 2019, 62, 2213–2225. [Google Scholar] [CrossRef]
- AbuAlRoos, N.J.; Amin, N.A.B.; Zainon, R. Conventional and new lead-free radiation shielding materials for radiation protection in nuclear medicine: A review. Radiat. Phys. Chem. 2019, 165, 108439. [Google Scholar] [CrossRef]
- Johansen, S.; Hauge, I.H.R.; Hogg, P.; England, A.; Lança, L.; Gunn, C.; Sanderud, A. Are Antimony-Bismuth Aprons as Efficient as Lead Rubber Aprons in Providing Shielding against Scattered Radiation? J. Med. Imaging Radiat. Sci. 2018, 49, 201–206. [Google Scholar] [CrossRef]
- Mori, H.; Koshida, K.; Ishigamori, O.; Matsubara, K. Evaluation of the effectiveness of X-ray protective aprons in experimental and practical fields. Radiol. Phys. Technol. 2014, 7, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, K.; Luo, W.; Dong, X.; Wang, C.; Wu, G.; Jiang, M.; Zha, Y. A new lead-free radiation shielding material for radiotherapy. Radiat. Prot. Dosim. 2009, 133, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Lee, N.J.; Ahn, J.W. Sustainable management of oyster shell by-products and recent research techniques. J. Energ. Eng. 2018, 27, 1–11. [Google Scholar] [CrossRef]
- Kwon, H.B.; Lee, C.W.; Jun, B.S.; Weon, S.Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 2017, 34, 225–230. [Google Scholar] [CrossRef]
- Lu, J.C.; Li, X.; Hao, Y.; Wang, Y.C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2017, 172, 1978–1985. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, J.M.; Saleh, H. Gamma-ray energy factor calculations and shielding effects of some Jordanian building structures. Radiat. Phys. Chem. 2015, 110, 87–95. [Google Scholar] [CrossRef]



| Thickness (mm) | Weight (g) | Density (g/cm3) | |
|---|---|---|---|
| Oyster shell sheet without heat treatment | 0.32 | 23.39 | 8.12 |
| Oyster shell sheet with plastic working at 600 °C | 0.31 | 28.12 | 10.07 |
| Oyster shell sheet with plastic working at 1200 °C | 0.32 | 31.25 | 10.85 |
| X-ray Tube Voltage (kVp) | Effective X-ray Energy (keV) | Mean Exposure (μR) | Shielding Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Nothing | 0 °C | 600 °C | 1200 °C | 0 °C | 600 °C | 1200 °C | ||
| 40 | 24.6 | 106.90 | 87.72 | 84.47 | 67.01 | 17.94 | 20.98 | 37.32 |
| 60 | 28.7 | 381.63 | 334.07 | 320.83 | 278.97 | 12.46 | 15.93 | 26.90 |
| 80 | 32.5 | 799.70 | 738.57 | 702.13 | 630.80 | 7.64 | 12.20 | 21.12 |
| 100 | 48.5 | 1318.33 | 1199.00 | 1180.67 | 1079.67 | 9.05 | 10.44 | 18.10 |
| 120 | 54.9 | 1648.33 | 1510.33 | 1484.67 | 1372.67 | 8.37 | 9.93 | 16.72 |
| X-ray Tube Voltage (kVp) | Effective X-ray Energy (keV) | Mean Exposure (μR) | Shielding Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nothing | Pb | Oyster Shell (1200 °C) | Barium Sulfate | Bismuth Oxide | Tungsten | Pb | Oyster Shell | Barium Sulfate | Bismuth Oxide | Tungsten | ||
| 40 | 24.6 | 106.90 | 0 | 67.01 | 61.04 | 41.69 | 19.23 | 100 | 37.32 | 42.90 | 61.00 | 82.01 |
| 60 | 28.7 | 381.63 | 6.47 | 278.97 | 236.51 | 160.15 | 114.36 | 98.30 | 26.90 | 38.03 | 58.04 | 70.03 |
| 80 | 32.5 | 799.70 | 48.87 | 630.80 | 550.80 | 463.81 | 351.76 | 93.89 | 21.12 | 31.12 | 42.00 | 56.01 |
| 100 | 48.5 | 1318.33 | 144.83 | 1078.67 | 1001.93 | 870.03 | 672.26 | 89.01 | 18.10 | 24.00 | 34.01 | 49.01 |
| 120 | 54.9 | 1648.33 | 207.43 | 1372.67 | 1318.66 | 1120.76 | 906.05 | 87.42 | 16.72 | 20.00 | 32.01 | 45.03 |
| Type of Shellfish | Elemental Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| CaO | Na2O | CI/MnO | SiO2 | SO3 | AI2O3 | Fe2O3 | |
| Clams | 94.97 | 1.01 | - | 1.83 | 0.52 | 0.45 | 0.58 |
| Cockles | 95.95 | - | - | 1.68 | 0.65 | 0.58 | 0.59 |
| Manila clams | 92.79 | 1.13 | 0/0.13 | 3.09 | 0.59 | 0.98 | 0.63 |
| Oysters | 84.59 | 5.92 | 5.15/0 | 1.63 | 1.19 | 0.45 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-C. Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder. Appl. Sci. 2022, 12, 968. https://doi.org/10.3390/app12030968
Kim S-C. Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder. Applied Sciences. 2022; 12(3):968. https://doi.org/10.3390/app12030968
Chicago/Turabian StyleKim, Seon-Chil. 2022. "Process Technology for Development and Performance Improvement of Medical Radiation Shield Made of Eco-Friendly Oyster Shell Powder" Applied Sciences 12, no. 3: 968. https://doi.org/10.3390/app12030968






