Making Sense of Light: The Use of Optical Spectroscopy Techniques in Plant Sciences and Agriculture
Abstract
:1. Introduction
2. Historical Perspective
3. Main Techniques
3.1. Elastic Spectroscopy
3.2. Other Spectroscopic Techniques
4. Current Applications and Future Perspectives
4.1. Elastic Spectroscopy
4.2. Other Methods
5. Emerging Technologies for Portable Spectroscopy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Ali, M.M.; Bachik, N.A.; Muhadi, N.; Yusof, T.N.T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108. [Google Scholar] [CrossRef]
- Berauer, B.J.; Wilfahrt, P.A.; Reu, B.; Schuchardt, M.A.; Garcia-Franco, N.; Zistl-Schlingmann, M.; Dannenmann, M.; Kiese, R.; Kühnel, A.; Jentsch, A. Predicting forage quality of species-rich pasture grasslands using vis-NIRS to reveal effects of management intensity and climate change. Agric. Ecosyst. Environ. 2020, 296, 106929. [Google Scholar] [CrossRef]
- Payne, W.Z.; Kurouski, D. Raman-Based Diagnostics of Biotic and Abiotic Stresses in Plants. A Review. Front. Plant Sci. 2021, 11, 616672. [Google Scholar] [CrossRef]
- Fang, S.; Cui, R.; Wang, Y.; Zhao, Y.; Yu, K.; Jiang, A. Application of multiple spectral systems for the tree disease detection: A review. Appl. Spectrosc. Rev. 2021, 1–27. [Google Scholar] [CrossRef]
- Fahlgren, N.; Gehan, M.A.; Baxter, I. Lights, camera, action: High-throughput plant phenotyping is ready for a close-up. Curr. Opin. Plant Biol. 2015, 24, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High Throughput In vivo Analysis of Plant Leaf Chemical Properties Using Hyperspectral Imaging. Front. Plant Sci. 2017, 8, 1348. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Asaari, M.S.M.; Herrero-Langreo, A.; Lohumi, S.; Diezma, B.; Scheunders, P. Close range hyperspectral imaging of plants: A review. Biosyst. Eng. 2017, 164, 49–67. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 1–22. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Walsh, K.; McGlone, V.; Han, D. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]
- Arias, F.; de Panamá, E.D.U.T.; Zambrano, M.; Broce, K.; Medina, C.; Pacheco, H.; Nunez, Y.; Ciencias, I.Y.T.-A. Hyperspectral imaging for rice cultivation: Applications, methods and challenges. AIMS Agric. Food 2021, 6, 273–307. [Google Scholar] [CrossRef]
- Newton, I. Opticks: Or, A Treatise of the Reflections, Refractions, Inflexions and Colours of Light; Printed for Sam. Smith, and Benj. Wal-ford: London, UK, 1704; p. 458. [Google Scholar]
- Thomas, N.C. The early history of spectroscopy. J. Chem. Educ. 1991, 68. [Google Scholar] [CrossRef]
- Hopkinson, F.; Rittenhouse, D. An optical problem, proposed by Mr. Hopkinson, and solved by Mr. Rittenhouse. Trans. Am. Phil. Soc. 1786, 2, 201–206. [Google Scholar] [CrossRef]
- Von Fraunhofer, J. Neue Modifikation des Lichtes durch gegenseitige Einwirkung und Beugung der Strahlen, und Gesetze derselben (New modification of light by the mutual influence and the diffraction of [light] rays, and the laws thereof). Denkschr. Königlichen Akad. Wiss. Zu München (Mem. R. Acad. Sci. Munich) 1821, 8, 3–76. [Google Scholar]
- Wollaston, W.H. A Method of examining refractive and dispersive powers, by prismatic reflection. Philos. Trans. R. Soc. Lond. 1802, 92, 365–380. [Google Scholar]
- Fraunhofer, J. Bestimmung des Brechungs- und des Farben-Zerstreuungs—Vermögens verschiedener Glasarten, in Bezug auf die Vervollkommnung achromatischer Fernröhre (Determination of the refractive and color-dispersing power of different types of glass, in relation to the improvement of achromatic telescopes). Annal. Phys. 1817, 56, 264–313. [Google Scholar]
- Ångström, A.J. Recherches sur le Spectre Solaire; W. Schultz: Uppsala, Sweden, 1868; p. 78. [Google Scholar]
- Masters, B.R. A Brief History of Spectral Analysis and Astrospectroscopy. Opt. Photon- News 2009, 20, 34–39. [Google Scholar] [CrossRef]
- Kirchhoff; Bunsen. XXIV. On chemical analysis by spectrum-observations. Q. J. Chem. Soc. 1861, 13, 270–289. [Google Scholar] [CrossRef]
- Mark, H.; Bradley, M.S. Review of New Spectroscopic Instrumentation. Spectroscopy 2021, 36, 28–47. Available online: https://www.spectroscopyonline.com/view/2021-review-of-new-spectroscopic-instrumentation (accessed on 5 October 2021).
- What Is Dynamic Mechanical Analysis (DMA)? Available online: https://coventivecomposites.com/explainers/dynamic-mechanical-analysis-dma/ (accessed on 27 December 2020).
- Tuchin, V.V. Tissue Optics and Photonics: Light-Tissue Interaction. J. Biomed. Photon- Eng. 2015, 1, 98–134. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Thomson Brooks/Cole Publishing: Pacific Grove, CA, USA, 2007; p. 1039. [Google Scholar]
- Lu, R.; Van Beers, R.; Saeys, W.; Li, C.; Cen, H. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biol. Technol. 2019, 159, 111003. [Google Scholar] [CrossRef]
- Multispectral vs Hyperspectral Imagery Explained. Available online: https://gisgeography.com/multispectral-vs-hyperspectral-imagery-explained/ (accessed on 27 December 2020).
- Sankaran, S.; Khot, L.R.; Espinoza, C.Z.; Jarolmasjed, S.; Sathuvalli, V.R.; VanDeMark, G.J.; Miklas, P.N.; Carter, A.H.; Pumphrey, M.O.; Knowles, N.R.; et al. Low-altitude, high-resolution aerial imaging systems for row and field crop phenotyping: A review. Eur. J. Agron. 2015, 70, 112–123. [Google Scholar] [CrossRef]
- Oerke, E.-C. Remote Sensing of Diseases. Annu. Rev. Phytopathol. 2020, 58, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Roberts, J. Applications and Developments on the Use of Vibrational Spectroscopy Imaging for the Analysis, Monitoring and Characterisation of Crops and Plants. Molecules 2016, 21, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Cattaneo, T.M.P.; Stellari, A. Review: NIR Spectroscopy as a Suitable Tool for the Investigation of the Horticultural Field. Agronomy 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Cavaco, A.M.; Passos, D.; Pires, R.M.; Antunes, M.A.; Guerra, R. Nondestructive Assessment of Citrus Fruit Quality and Ripening by Visible–Near Infrared Reflectance Spectroscopy. In Citrus—Research, Development and Biotechnology [Working Title]; Sarwar Khan, M.S., Khan, I., Eds.; IntechOpen Limited: London, UK, 2021; ISBN 978-1-83968-723-5. [Google Scholar] [CrossRef]
- Hogan, H. The Food Industry’s Appetite for Hyperspectral Imaging Grows. Photonics Spectra 2021, 38–41. Available online: https://www.photonics.com/ (accessed on 1 June 2021).
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Lu, R. Innovative Hyperspectral Imaging-Based Techniques for Quality Evaluation of Fruits and Vegetables: A Review. Appl. Sci. 2017, 7, 189. [Google Scholar] [CrossRef]
- Reynolds, L.; Johnson, C.; Ishimaru, A. Diffuse reflectance from a finite blood medium: Applications to the modeling of fiber optic catheters. Appl. Opt. 1976, 15, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Langerholc, J. Beam broadening in dense scattering media. Appl. Opt. 1982, 21, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Marquet, P.; Bevilacqua, F.; Depeursinge, C.; Dehaller, E.B. Determination of reduced scattering and absorption-coefficients by a single charge-coupled-device array measurement. 1. comparison between experiments and simulations. Opt. Eng. 1995, 34, 2055–2063. [Google Scholar] [CrossRef]
- Torricelli, A.; Contini, D.; Dalla Mora, A.; Martinenghi, E.; Tamborini, D.; Villa, F.; Tosi, A.; Spinelli, L. Recent advances in time-resolved nir spectroscopy for nondestructive assessment of fruit quality. Chem. Eng. Trans. 2015, 44, 43–48. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M. Time-resolved technique for measuring optical properties and quality of food. In Light Scattering Technology for Food Property, Quality and Safety Assessment; Lu, R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 187–224. [Google Scholar]
- Pifferi, A.; Taroni, P.; Torricelli, A.; Messina, F.; Cubeddu, R.; Danesini, G. Four-wavelength time-resolved optical mammography in the 680-980-nm range. Opt. Lett. 2003, 28, 1138–1140. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Clarke, R.J.; Oprysa, A. Fluorescence and light scattering. J. Chem. Educ. 2004, 81, 705–707. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Puchalski, C. Overview of Popular Techniques of Raman Spectroscopy and Their Potential in the Study of Plant Tissues. Molecules 2021, 26, 1537. [Google Scholar] [CrossRef]
- Yu, X.L.; Hoff, L.E.; Reed, I.S.; Chen, A.M.; Stotts, L.B. Automatic target detection and recognition in multiband imagery: A uni-fied ml detection and estimation approach. IEEE Trans. Image Process. 1997, 6, 143–156. [Google Scholar]
- Galvao, L.; Formaggio, A.R.; Tisot, D.A. Discrimination of sugarcane varieties in Southeastern Brazil with EO-1 Hyperion data. Remote Sens. Environ. 2005, 94, 523–534. [Google Scholar] [CrossRef]
- Teke, M.; Deveci, H.S.; Haliloglu, O.; Gurbuz, S.Z.; Sakarya, U. A short survey of hyperspectral remote sensing applications in agriculture. In Proceedings of the 2013 6th International Conference on Recent Advances in Space Technologies (RAST), Istanbul, Turkey, 12–14 June 2013; pp. 171–176. [Google Scholar]
- Resta, V.; Utkin, A.B.; Neto, F.M.; Patrikakis, C.Z. Cultural Heritage Resilience Against Climate Change and Natural Hazards; Pisa University Press: Pisa, Italy, 2019; p. 294. [Google Scholar]
- Transon, J.; D’Andrimont, R.; Maugnard, A.; Defourny, P. Survey of Hyperspectral Earth Observation Applications from Space in the Sentinel-2 Context. Remote Sens. 2018, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Tatem, A.; Goetz, S.; Hay, S. Fifty Years of Earth-Observation Satellites. American Scientist. Available online: https://www.americanscientist.org/article/fifty-years-of-earth-observation-satellites (accessed on 17 October 2021).
- Costa, J.M.; Marques da Silva, J.; Pinheiro, C.; Barón, M.; Mylona, P.; Centritto, M.; Haworth, M.; Loreto, F.; Uzilday, B.; Turkan, I.; et al. Opportunities and Limitations of Crop Phenotyping in Southern European Countries. Front. Plant Sci. 2019, 10, 1125. [Google Scholar] [CrossRef] [Green Version]
- Carter, G.A.; Lucas, K.L.; Blossom, G.A.; Lassitter, C.L.; Holiday, D.M.; Mooneyhan, D.S.; Fastring, D.R.; Holcombe, T.R.; Griffith, J.A. Remote Sensing and Mapping of Tamarisk along the Colorado River, USA: A Comparative Use of Summer-Acquired Hyperion, Thematic Mapper and QuickBird Data. Remote Sens. 2009, 1, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Bock, C.H.; Barbedo, J.G.A.; Del Ponte, E.M.; Bohnenkamp, D.; Mahlein, A.-K. From visual estimates to fully automated sensor-based measurements of plant disease severity: Status and challenges for improving accuracy. Phytopathol. Res. 2020, 2, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Diehn, K.; Hermann, D. Hyperspectral Remote Sensing as a Management Tool for a Land Application Program; Tappi Press: Atlanta, GA, USA, 1998; pp. 549–568. [Google Scholar]
- Su, J.Y.; Liu, C.J.; Coombes, M.; Hu, X.P.; Wang, C.H.; Xu, X.M.; Li, Q.D.; Guo, L.; Chen, W.-H. Wheat yellow rust monitoring by learning from multispectral UAV aerial imagery. Comput. Electron. Agric. 2018, 155, 157–166. [Google Scholar] [CrossRef]
- Nguyen, C.; Sagan, V.; Maimaitiyiming, M.; Maimaitijiang, M.; Bhadra, S.; Kwasniewski, M.T. Early Detection of Plant Viral Disease Using Hyperspectral Imaging and Deep Learning. Sensors 2021, 21, 742. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Cui, X.; Shi, Y.; Liu, L. New Spectral Index for Detecting Wheat Yellow Rust Using Sentinel-2 Multispectral Imagery. Sensors 2018, 18, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couture, J.J.; Singh, A.; Charkowski, A.O.; Groves, R.L.; Gray, S.M.; Bethke, P.C.; Townsend, P.A. Integrating Spectroscopy with Potato Disease Management. Plant Dis. 2018, 102, 2233–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulridha, J.; Ehsani, R.; De Castro, A. Detection and Differentiation between Laurel Wilt Disease, Phytophthora Disease, and Salinity Damage Using a Hyperspectral Sensing Technique. Agriculture 2016, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, N.; Mohamadi-Monavar, H.; Azizi, A.; Ghasemi, A. Detection of Fire Blight disease in pear trees by hyperspectral data. Eur. J. Remote Sens. 2017, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, N. Application of aerial remote sensing technology for detection of fire blight infected pear trees. Comput. Electron. Agric. 2019, 168, 105147. [Google Scholar] [CrossRef]
- Cubero, S.; Lee, W.S.; Aleixos, N.; Albert, F.; Blasco, J. Automated Systems Based on Machine Vision for Inspecting Citrus Fruits from the Field to Postharvest—a Review. Food Bioprocess Technol. 2016, 9, 1623–1639. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.; Liu, L.; Wang, J. Identification of yellow rust in wheat using in-situ spectral reflectance measurements and airborne hyperspectral imaging. Precis. Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Meng, R.; Lv, Z.; Yan, J.; Chen, G.; Zhao, F.; Zeng, L.; Xu, B. Development of Spectral Disease Indices for Southern Corn Rust Detection and Severity Classification. Remote Sens. 2020, 12, 3233. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Non-destructive sensing methods for quality assessment of on-tree fruits: A review. J. Food Meas. Charact. 2018, 12, 497–526. [Google Scholar] [CrossRef]
- Yamakawa, M.; Khot, L.R.; Ehsani, R.; Kondo, N. Real-time nondestructive citrus fruit quality monitoring system: Development and laboratory testing. Agric. Eng. Int. CIGR J. 2012, 14, 117–124. [Google Scholar]
- Cubero, S.; Marco-Noales, E.; Aleixos, N.; Barbé, S.; Blasco, J. RobHortic: A Field Robot to Detect Pests and Diseases in Horticultural Crops by Proximal Sensing. Agriculture 2020, 10, 276. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Defraeye, T.; De Ketelaere, B.; Herremans, E.; Hertog, M.L.; Saeys, W.; Torricelli, A.; Vandendriessche, T.; Verboven, P. Nondestructive Measurement of Fruit and Vegetable Quality. Annu. Rev. Food Sci. Technol. 2014, 5, 285–312. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Y.; Chang, C.-Y.; Ou, C.-S.; Lien, C.-T. Detection of Insect Damage in Green Coffee Beans Using VIS-NIR Hyperspectral Imaging. Remote Sens. 2020, 12, 2348. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C.W. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Gao, H.; Meng, L.; Fu, X.; Du, X.; Wu, D.; Huang, L. Nondestructive measurement of pectin polysaccharides using hyperspectral imaging in mulberry fruit. Food Chem. 2020, 334, 127614. [Google Scholar] [CrossRef]
- Li, J.L.; Sun, D.W.; Cheng, J.H. Recent Advances in Nondestructive Analytical Techniques for Determining the Total Soluble Solids in Fruits: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.; Choy, K.L.; Wu, C.H.; Ho, G.T.S.; Lam, H. Blockchain-Driven IoT for food traceability with an integrated consensus mechanism. IEEE Access 2019, 7, 129000–129017. [Google Scholar] [CrossRef]
- Canavari, M.; Centonze, R.; Hingley, M.; Spadoni, R. Traceability as part of competitive strategy in the fruit supply chain. Br. Food J. 2010, 112, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Kondo, N. Automation on fruit and vegetable grading system and food traceability. Trends Food Sci. Technol. 2010, 21, 145–152. [Google Scholar] [CrossRef]
- Haleem, A.; Khan, S.; Khan, M.I. Traceability implementation in food supply chain: A grey-DEMATEL approach. Inf. Process. Agric. 2019, 6, 335–348. [Google Scholar] [CrossRef]
- Afonso, A.M.; Guerra, R.; Cavaco, A.M.; Pinto, P.; Andrade, A.; Duarte, A.; Power, D.M.; Marques, N.T. Identification of asymptomatic plants infected with Citrus tristeza virus from a time series of leaf spectral characteristics. Comput. Electron. Agric. 2017, 141, 340–350. [Google Scholar] [CrossRef]
- Passos, D.; Rodrigues, D.; Cavaco, A.M.; Antunes, M.D.; Guerra, R. Non-Destructive Soluble Solids Content Determination for ‘Rocha’ Pear Based on VIS-SWNIR Spectroscopy under ‘Real World’ Sorting Facility Conditions. Sensors 2019, 19, 5165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, S.; Guerra, R.; Brazio, A.; Cavaco, A.M.; Antunes, D.; Passos, D. Nondestructive simultaneous prediction of internal browning disorder and quality attributes in ‘Rocha’ pear (Pyrus communis L.) using VIS-NIR spectroscopy. Postharvest Biol. Technol. 2021, 179, 111562. [Google Scholar] [CrossRef]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit Quality Evaluation Using Spectroscopy Technology: A Review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef] [Green Version]
- Rungpichayapichet, P.; Mahayothee, B.; Nagle, M.; Khuwijitjaru, P.; Müller, J. Robust NIRS models for non-destructive prediction of postharvest fruit ripeness and quality in mango. Postharvest Biol. Technol. 2016, 111, 31–40. [Google Scholar] [CrossRef]
- Cavaco, A.M.; Pires, R.; Antunes, M.D.; Panagopoulos, T.; Brazio, A.; Afonso, A.M.; Silva, L.; Lucas, M.R.; Cadeiras, B.; Cruz, S.P.; et al. Validation of short wave near infrared calibration models for the quality and ripening of ‘Newhall’ orange on tree across years and orchards. Postharvest Biol. Technol. 2018, 141, 86–97. [Google Scholar] [CrossRef]
- Zude, M.; Pflanz, M.; Kaprielian, C.; Aivazian, B.L. NIRS as a tool for precision horticulture in the citrus industry. Biosyst. Eng. 2008, 99, 455–459. [Google Scholar] [CrossRef]
- Sánchez, M.-T.; De la Haba, M.-J.; Serrano, I.; Pérez-Marín, D. Application of NIRS for Nondestructive Measurement of Quality Parameters in Intact Oranges During On-Tree Ripening and at Harvest. Food Anal. Methods 2012, 6, 826–837. [Google Scholar] [CrossRef]
- Mark, H.; Workman, J. Chemometrics in Spectroscopy, 1st ed.; Academic Press: Cambridge, UK, 2007; 558p. [Google Scholar] [CrossRef]
- Goyal, S. Artificial Neural Networks in Fruits: A Comprehensive Review. Int. J. Image Graph. Signal Process. 2014, 5, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Wang, Q.; Li, J.; Peng, F.; Huang, W. Non-destructive prediction of soluble solids content of pear based on fruit surface feature classification and multivariate regression analysis. Infrared Phys. Technol. 2018, 92, 336–344. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Sabzi, S.; Benmouna, B.; García-Mateos, G.; Hernández-Hernández, J.L.; Molina-Martínez, J.M. Estimation of the Constituent Properties of Red Delicious Apples Using a Hybrid of Artificial Neural Networks and Artificial Bee Colony Algorithm. Agronomy 2020, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Fuentes, S.; Poblete-Echeverria, C.; Viejo, C.G.; Tongson, E.; Du, H.; Su, B. Automated Chinese medicinal plants classification based on machine learning using leaf morpho-colorimetry, fractal dimension and visible/near infrared spectroscopy. Int. J. Agric. Biol. Eng. 2019, 12, 123–131. [Google Scholar] [CrossRef]
- Daniel, M.; Guerra, R.; Brázio, A.; Rodrigues, D.; Cavaco, A.M.; Antunes, M.D.; de Oliveira, J.V. Feature discovery in NIR spectroscopy based Rocha pear classification. Expert Syst. Appl. 2021, 177, 114949. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Utkin, A.B.; Eiras-Dias, J.; Cunha, J.; Silvestre, J.; Melo-Pinto, P. Grapevine variety identification using “Big Data” collected with miniaturized spectrometer combined with support vector machines and convolutional neural networks. Comput. Electron. Agric. 2019, 163, 104855. [Google Scholar] [CrossRef]
- Diago, M.P.; Fernández-Novales, J.; Fernandes, A.M.; Melo-Pinto, P.; Tardaguila, J. Use of Visible and Short-Wave Near-Infrared Hyperspectral Imaging to Fingerprint Anthocyanins in Intact Grape Berries. J. Agric. Food Chem. 2016, 64, 7658–7666. [Google Scholar] [CrossRef]
- Yu, K.; Fang, S.; Zhao, Y. Heavy metal Hg stress detection in tobacco plant using hyperspectral sensing and data-driven machine learning methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118917. [Google Scholar] [CrossRef] [PubMed]
- Lanczos, C. Linear Differential Operators; Van Nostrad: London, UK, 1961; p. 132. [Google Scholar]
- Huang, C.H.; Singh, G.P.; Park, S.H.; Chua, N.H.; Ram, R.J.; Park, B.S. Early Diagnosis and Management of Nitrogen Deficiency in Plants Utilizing Raman Spectroscopy. Front. Plant Sci. 2020, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Sarojam, R.; Jang, I.-C.; Park, B.S.; Naqvi, N.I.; Wong, M.H.; Singh, G.P.; Ram, R.J.; Shoseyov, O.; Saito, K.; et al. Species-independent analytical tools for next-generation agriculture. Nat. Plants 2020, 6, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced Application of Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy in Plant Disease Diagnostics: A Review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef]
- Abbas, F.; Fares, A. Best management practices in Citrus production. Tree For. Sci. Biotechnol. 2009, 3, 1–11. [Google Scholar]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.; Bohlmeyer, D.; Hemmer, P.; Kolomiets, M.V.; Yuan, J.S.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393–3396. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Huang, C.H.; Singh, G.P.; Park, B.S.; Chua, N.-H.; Ram, R.J. Portable Raman leaf-clip sensor for rapid detection of plant stress. Sci. Rep. 2020, 10, 20206. [Google Scholar] [CrossRef]
- Li, F.; Lu, A.X.; Wang, J.H. Application of Raman spectroscopy in grain detection. J. Food Saf. Qual. 2016, 7, 4408–4414. [Google Scholar]
- Jin, K.X.; Wang, K.; Cui, H.S.; Yang, S.M.; Tian, G.L.; Ma, J.F. Application of Raman spectroscopy to the research on lignin. Sci. Silvae Sin. 2018, 54, 144–151. [Google Scholar]
- Angel, S.M.; Gomer, N.R.; Sharma, S.K.; McKay, C. Remote Raman spectroscopy for planetary exploration: A review. Appl. Spectrosc. 2012, 66, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Maeda, T.E.; Misra, A.K.; Muzangwa, L.G.; Berlanga, G.; Muchow, D.; Porter, J.; Sharma, S.K. Remote Raman measurements of minerals, organics, and inorganics at 430 m range. Appl. Opt. 2016, 55, 10283. [Google Scholar] [CrossRef]
- Lavrov, A.; Utkin, A.B.; da Silva, J.M.; Vilar, R.; Santos, N.M.; Alves, B. Water stress assessment of cork oak leaves and maritime pine needles based on LIF spectra. Opt. Spectrosc. 2012, 112, 271–279. [Google Scholar] [CrossRef]
- Gameiro, C.; Utkin, A.; Cartaxana, P.; da Silva, J.M.; Matos, A. The use of laser induced chlorophyll fluorescence (LIF) as a fast and non-destructive method to investigate water deficit in Arabidopsis. Agric. Water Manag. 2015, 164, 127–136. [Google Scholar] [CrossRef]
- Utkin, A.B.; Felizardo, R.; Gameiro, C.; Matos, A.R.; Cartaxana, P. Laser induced fluorescence technique for environmental appli-cations. In Proceedings of the Second International Conference on Applications of Optics and Photonics, Putrajaya, Malaysia, 22 August 2019; Costa, M., Nogueira, R.N., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2014; Volume 9286, p. 928609. [Google Scholar]
- Cabrita, M.T.; Gameiro, C.; Utkin, A.B.; Duarte, B.; Caçador, I.; Cartaxana, P. Photosynthetic pigment laser-induced fluorescence indicators for the detection of changes associated with trace element stress in the diatom model species Phaeodactylum tricornutum. Environ. Monit. Assess. 2016, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.; Cabrita, M.T.; David, A. Portuguese Polar Program: Annual Report 2019; Centro de Estudos Geográficos, Universidade de Lisboa: Lisbon, Portugal, 2020. [Google Scholar]
- Duarte, B.; Gameiro, C.; Utkin, A.B.; Matos, A.R.; Caçador, I.; Fonseca, V.; Cabrita, M.T. A multivariate approach to chlorophyll a fluorescence data for trace element ecotoxicological trials using a model marine diatom. Estuarine Coast. Shelf Sci. 2021, 250, 107170. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Utkin, A.B. Application of Laser-Induced Fluorescence in Functional Studies of Photosynthetic Biofilms. Processes 2018, 6, 227. [Google Scholar] [CrossRef] [Green Version]
- Marques da Silva, J. Monitoring photosynthesis by in vivo chlorophyll fluorescence: Application to high-throughput plant phe-notyping. In Applied Photosynthesis—New Progress; Najafpour, M.M., Ed.; InTechOpen: Rijeka, Croatia, 2016; ISBN 978-953-51-2267-8. [Google Scholar]
- Babichenko, S.M. SFS Technique; LDI Innovation: Tallinn. 2014. Available online: https://ldi-innovation.com/wp-content/uploads/data/SFStechnique.pdf (accessed on 18 March 2021).
- Babichenko, S. (Ed.) Spectral Fluorescent Signatures in Diagnostics of Water Environment; Tallinn Pedagogical Univ., Inst. of Ecology: Tallinn, Estonia, 2002; p. 193. ISBN 9985-58-208-. [Google Scholar]
- HORIBA. What Is an Excitation Emission Matrix (EEM)? Available online: https://www.horiba.com/en_en/technology/measurement-and-control-techniques/molecular-spectroscopy/fluorescence-spectroscopy/what-is-an-excitation-emission-matrix-eem/ (accessed on 3 March 2021).
- JASCO Inc. Application NoteFP-0021: High-Speed Measurement and EEM Interpretation for Olive Oil Analysis; JASCO: Easton, MD, USA, 2017; p. 4. [Google Scholar]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Carter, G.A.; Jones, J.H.; Mitchell, R.J.; Brewer, C.H. Detection of solar-excited chlorophyll a fluorescence and leaf photosynthetic capacity using a Fraunhofer line radiometer. Remote Sens. Environ. 1996, 55, 89–92. [Google Scholar] [CrossRef]
- Cogliati, S.; Verhoef, W.; Kraft, S.; Sabater, N.; Alonso, L.; Vicent, J.; Moreno, J.; Drusch, M.; Colombo, R. Retrieval of sun-induced fluorescence using advanced spectral fitting methods. Remote Sens. Environ. 2015, 169, 344–357. [Google Scholar] [CrossRef]
- Sabater, N.; Kolmonen, P.; Van Wittenberghe, S.; Arola, A.; Moreno, J. Challenges in the atmospheric characterization for the retrieval of spectrally resolved fluorescence and PRI region dynamics from space. Remote Sens. Environ. 2020, 254, 112226. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Rastogi, A.; Juszczak, R. Review of Top-of-Canopy Sun-Induced Fluorescence (SIF) Studies from Ground, UAV, Airborne to Spaceborne Observations. Sensors 2020, 20, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Suomalainen, J.; Bartholomeus, H.; Kooistra, L.; Masiliūnas, D.; Clevers, J.G. Diurnal variation of sun-induced chlorophyll fluorescence of agricultural crops observed from a point-based spectrometer on a UAV. Int. J. Appl. Earth Obs. Geoinf. 2020, 96, 102276. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2018, 148, 1–14. [Google Scholar] [CrossRef]
- Hirschfeld, T. Fellgett’s Advantage in uv-VIS Multiplex Spectroscopy. Appl. Spectrosc. 1976, 30, 68–69. [Google Scholar] [CrossRef]
- Tran, N.-T.; Fukuzawa, M. A Portable Spectrometric System for Quantitative Prediction of the Soluble Solids Content of Apples with a Pre-calibrated Multispectral Sensor Chipset. Sensors 2020, 20, 5883. [Google Scholar] [CrossRef] [PubMed]
- Esquerre, C.A.; Achata, E.M.; García-Vaquero, M.; Zhang, Z.; Tiwari, B.K.; O’Donnell, C.P. Use of an NIR MEMS spectrophotometer and visible/NIR hyperspectral imaging systems to predict quality parameters of treated ground peppercorns. LWT 2020, 131, 109761. [Google Scholar] [CrossRef]
- Wiedemair, V.; Langore, D.; Garsleitner, R.; Dillinger, K.; Huck, C. Investigations into the Performance of a Novel Pocket-Sized Near-Infrared Spectrometer for Cheese Analysis. Molecules 2019, 24, 428. [Google Scholar] [CrossRef] [Green Version]
- Zgouz, A.; Héran, D.; Barthès, B.; Bastianelli, D.; Bonnal, L.; Baeten, V.; Lurol, S.; Bonin, M.; Roger, J.-M.; Bendoula, R.; et al. Dataset of visible-near infrared handheld and micro-spectrometers—comparison of the prediction accuracy of sugarcane properties. Data Brief 2020, 31, 106013. [Google Scholar] [CrossRef]

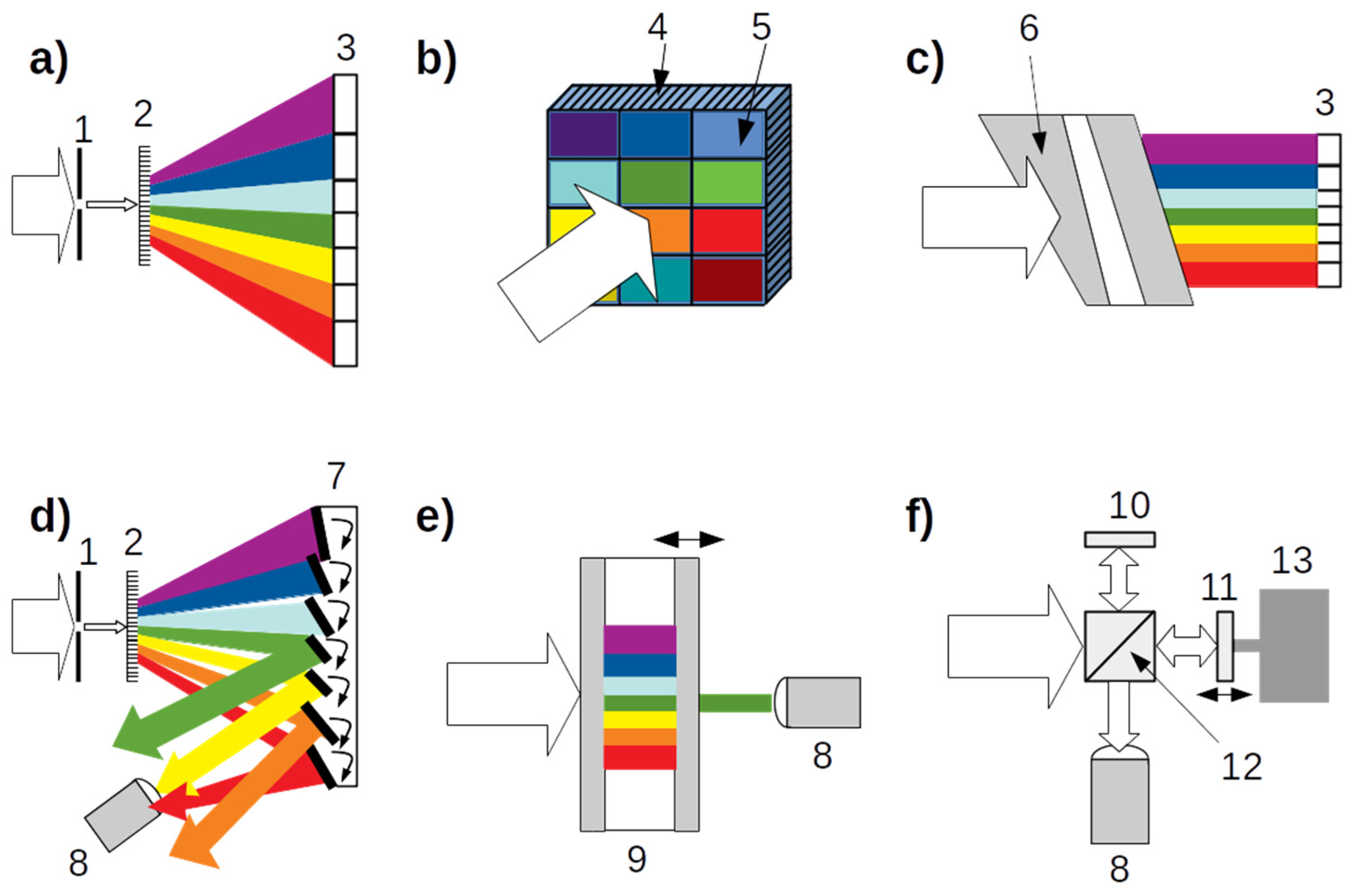
| Spectrometer Type | Jacquinot’s Advantage | Fellgett’s Advantage | Point or Array Detector | Achievable Spectral Resolution |
|---|---|---|---|---|
| (a) Dispersive | N | N | Array | High |
| (b) Multi-filter | Y | N | Array | Low |
| (c) LFV | Y | N | Array | Medium |
| (d) DMD | N | Y | Point | Medium |
| (e) Fabry–Pérot | Y | N | Point | Medium |
| (f) FTIR | Y | Y | Point | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaco, A.M.; Utkin, A.B.; Marques da Silva, J.; Guerra, R. Making Sense of Light: The Use of Optical Spectroscopy Techniques in Plant Sciences and Agriculture. Appl. Sci. 2022, 12, 997. https://doi.org/10.3390/app12030997
Cavaco AM, Utkin AB, Marques da Silva J, Guerra R. Making Sense of Light: The Use of Optical Spectroscopy Techniques in Plant Sciences and Agriculture. Applied Sciences. 2022; 12(3):997. https://doi.org/10.3390/app12030997
Chicago/Turabian StyleCavaco, Ana M., Andrei B. Utkin, Jorge Marques da Silva, and Rui Guerra. 2022. "Making Sense of Light: The Use of Optical Spectroscopy Techniques in Plant Sciences and Agriculture" Applied Sciences 12, no. 3: 997. https://doi.org/10.3390/app12030997
APA StyleCavaco, A. M., Utkin, A. B., Marques da Silva, J., & Guerra, R. (2022). Making Sense of Light: The Use of Optical Spectroscopy Techniques in Plant Sciences and Agriculture. Applied Sciences, 12(3), 997. https://doi.org/10.3390/app12030997









