Application of MICP in Water Stability and Hydraulic Erosion Control of Phosphogypsum Material in Slope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phosphogypsum Material
2.2. Microbial Treatment Solution
2.3. Water Stability Test
2.4. Environmental Impact Test
2.5. Artificial Slope Preparation
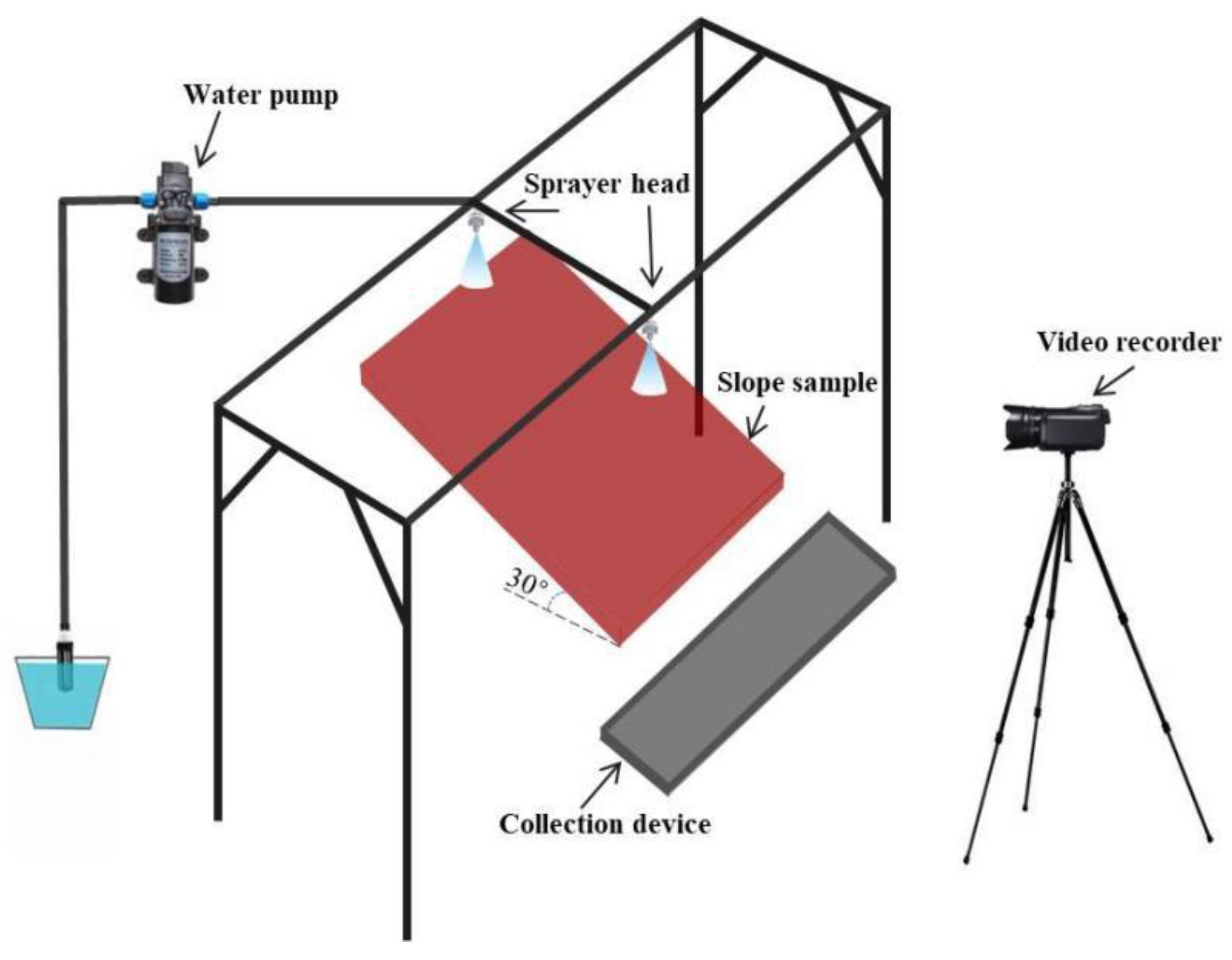
2.6. Rainfall Scouring Test
2.7. CaCO3 Content and Micromorphology
2.8. Three-Dimensional Laser Scanning Technique
3. Results and Discussion
3.1. Heavy Metal Elements of Phosphogypsum Samples
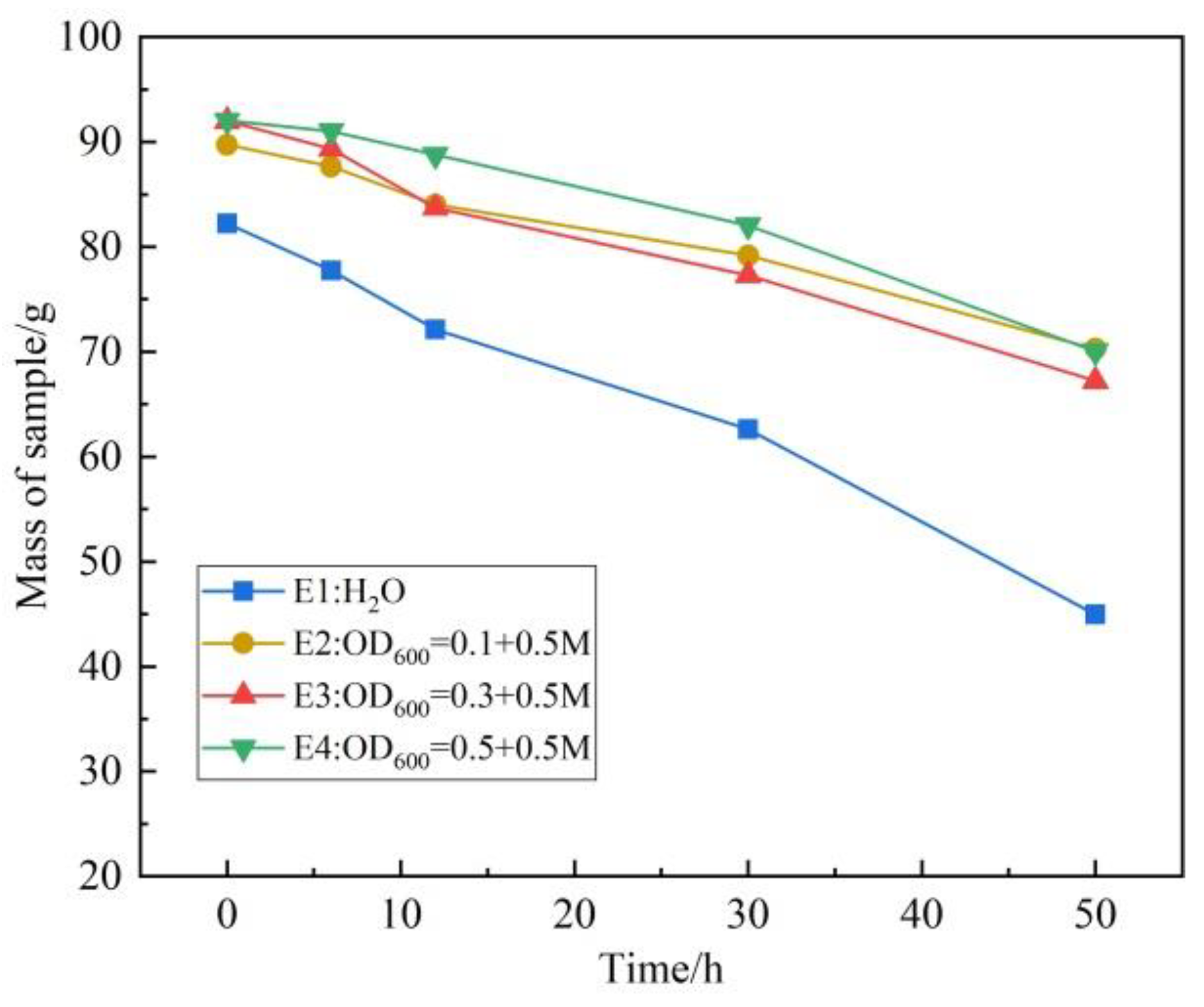
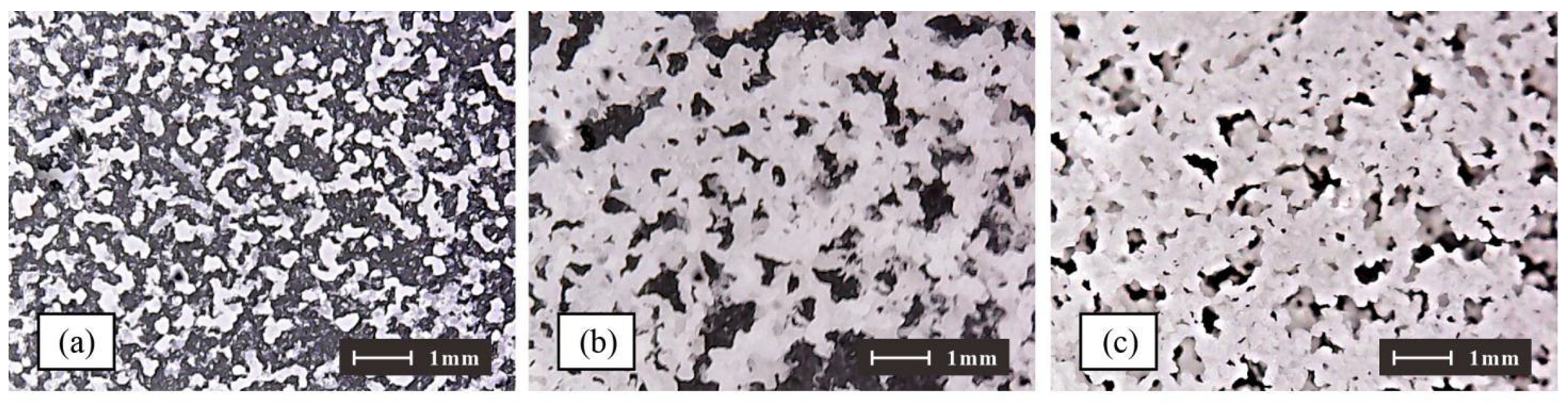
3.2. Water Stability and Permeability Characteristic of Phosphogypsum Sample
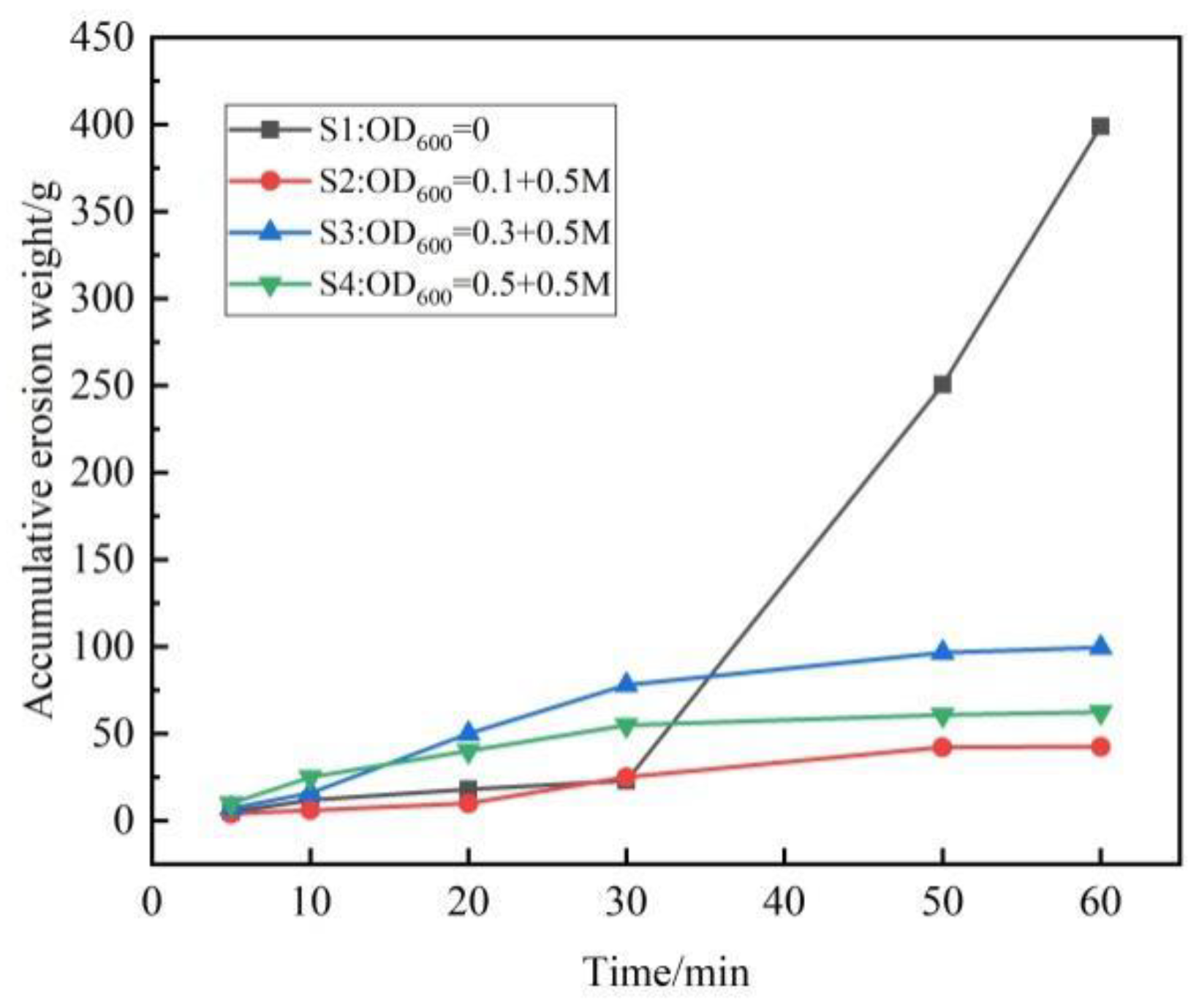
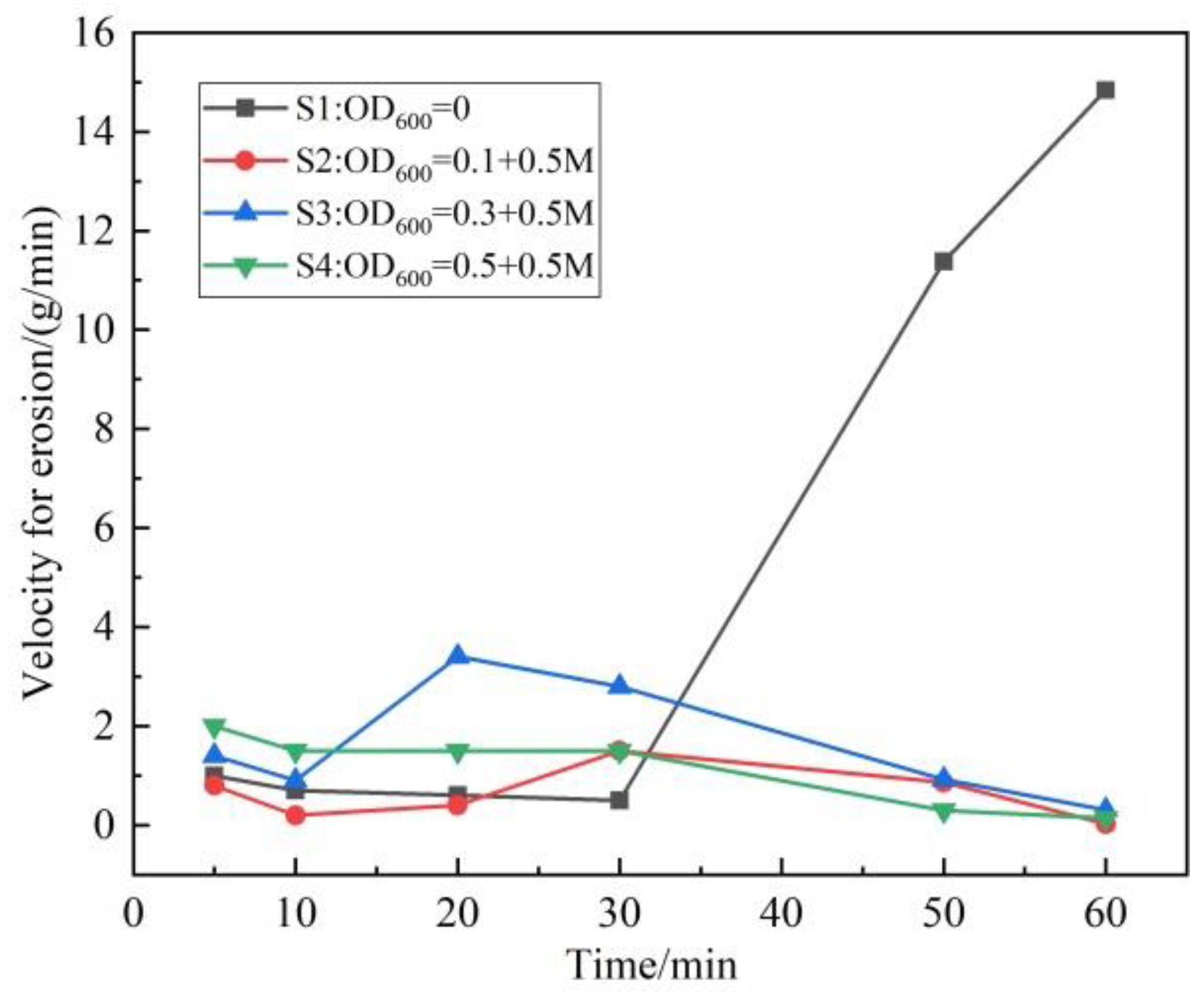
3.3. Hydraulic Erosion Condition of Scoured Slope
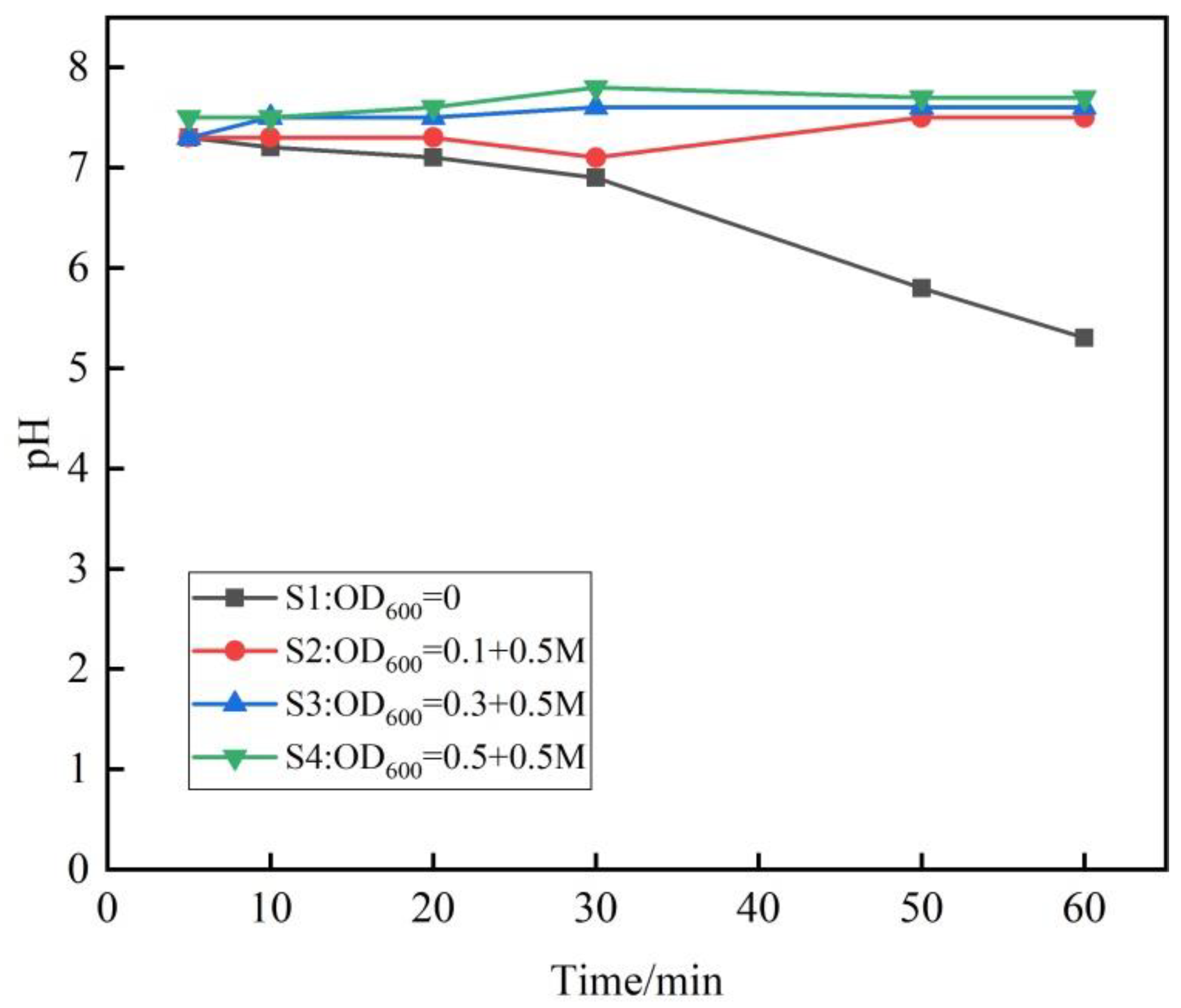
3.4. pH of Outflow Solution
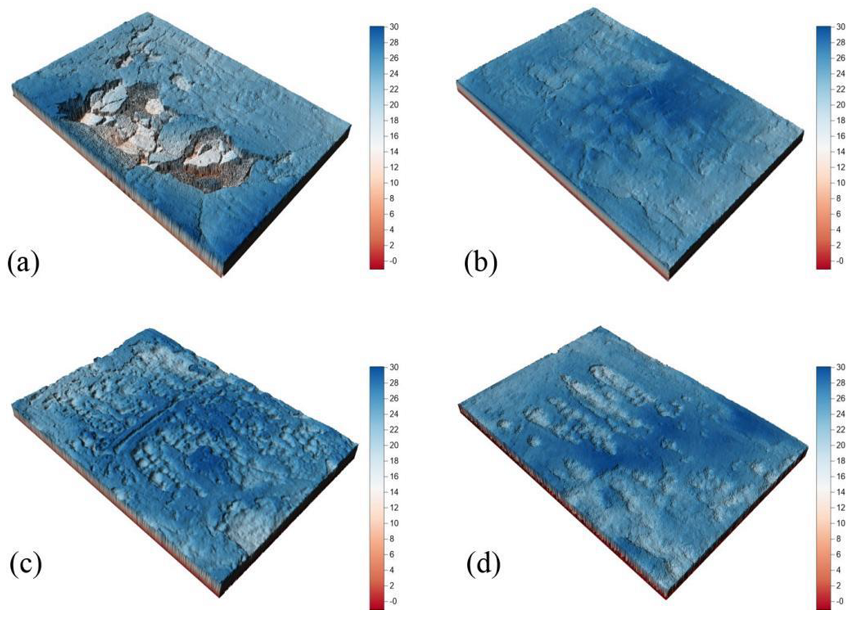
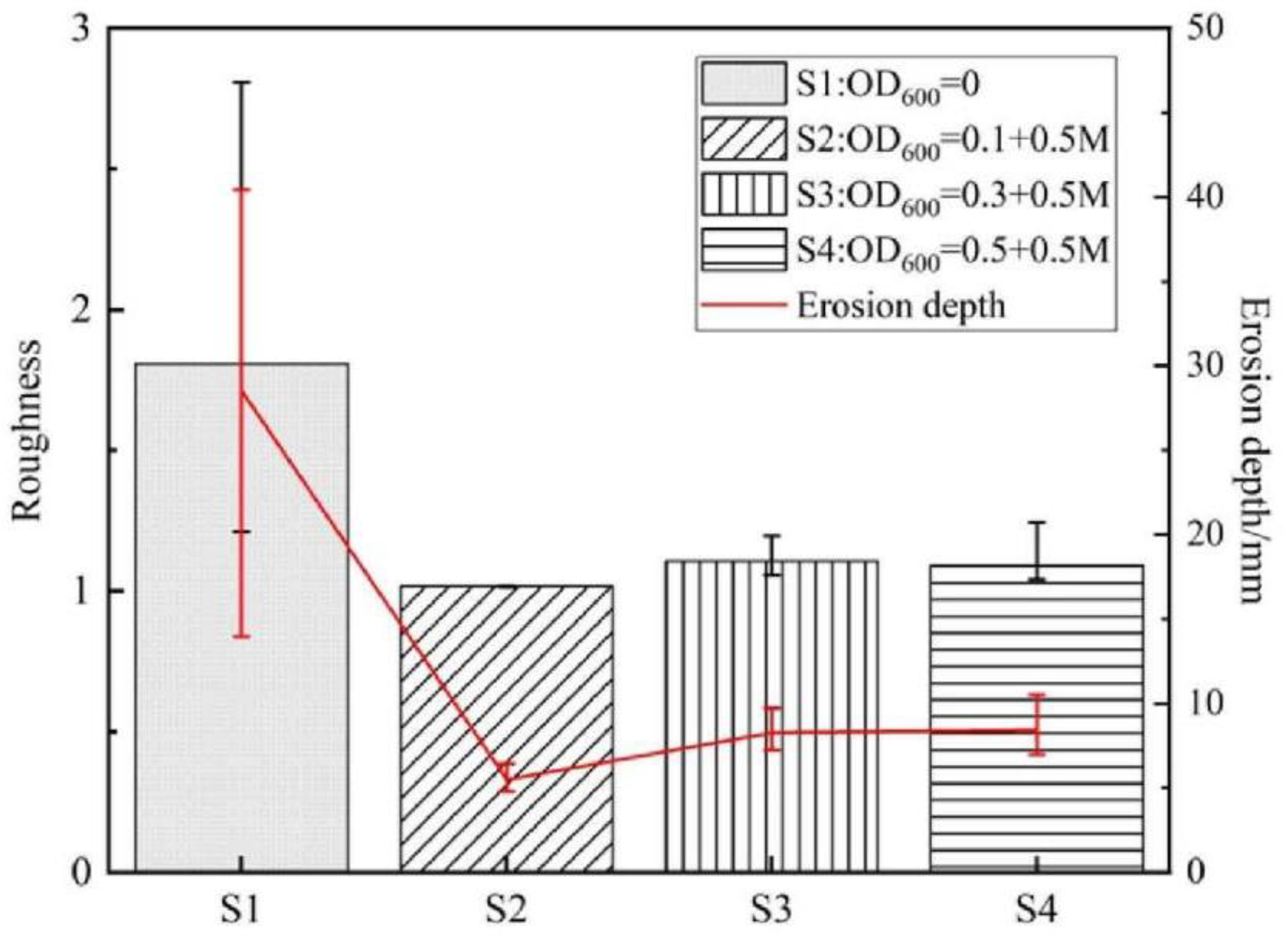
3.5. Three-Dimensional Laser Scanning Model and the Roughness of the Phosphogypsum Slope
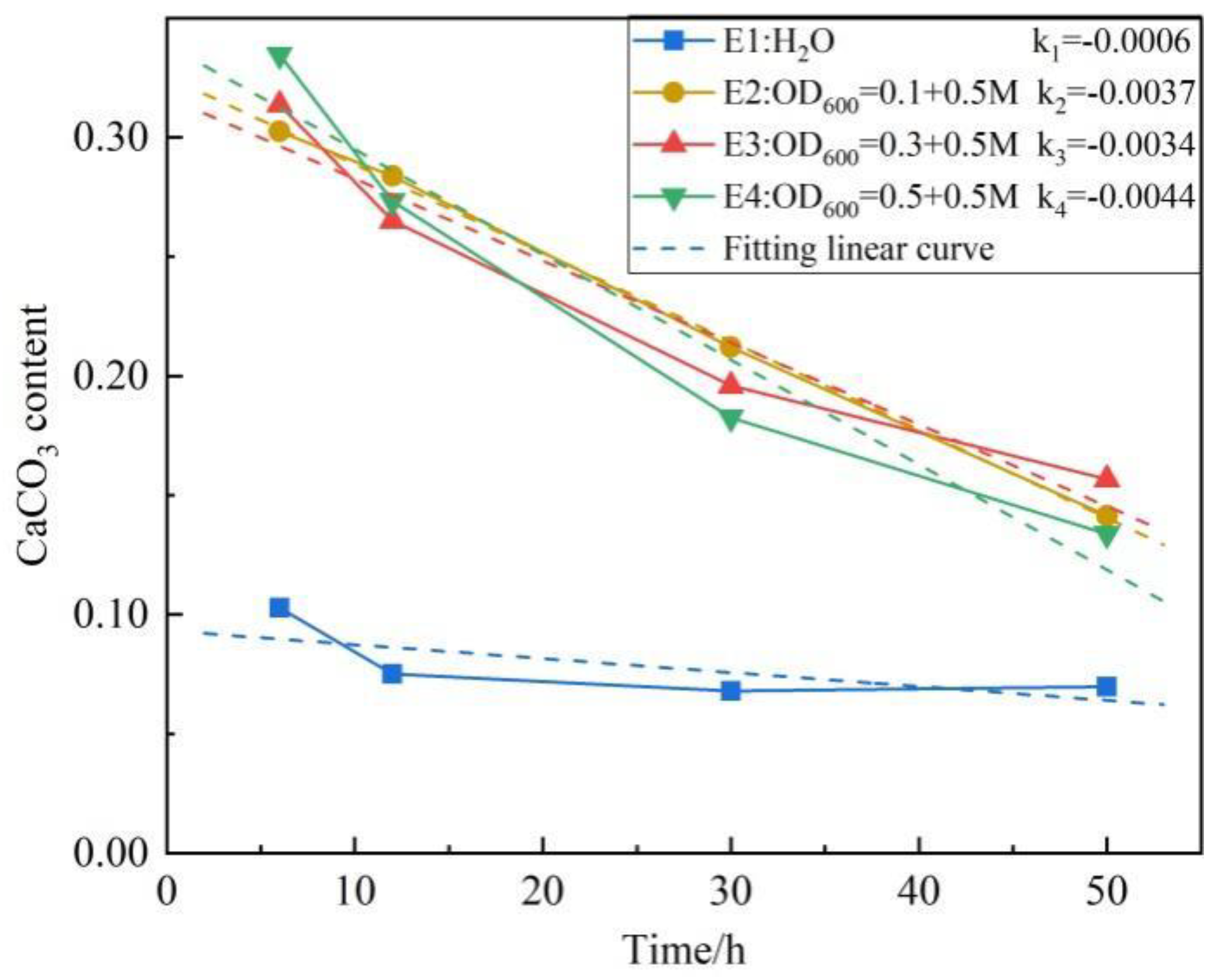
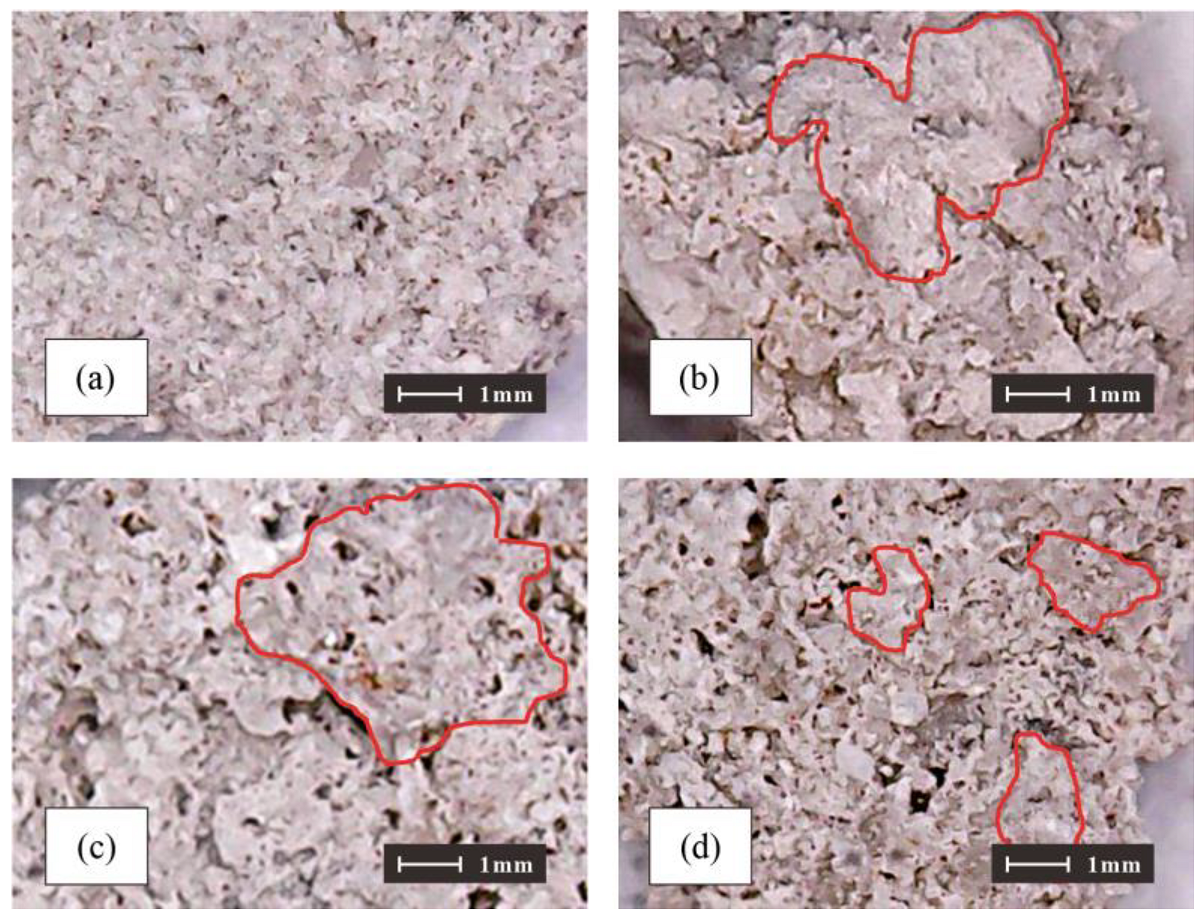
3.6. Micromorphology of the Scoured Samples and CaCO3 Crystals
4. Discussion
5. Conclusions
- The water stability and permeability of the sample treated by the microorganism are greatly improved. With the increase in the bacterial concentration, the difference between the content of CaCO3 in the outermost layer and that in the inner layer gradually increases. The degree of permeability reduction is more uneven.
- Phosphogypsum can be used as solidified material to simulate artificial slopes. The structure of the ordinary hydrated phosphogypsum slope was completely destroyed in the rainfall scouring test. When the slope is treated with OD600 = 0.1, the cementation of particles is more uniform, with fewer and smaller macropores, and the slope is eroded by sheet erosion. With the increase in the concentration of the bacterial solution, the bacterial will agglomerate and flocculate, resulting in uneven cementation and more macropores, which lead to erosion pits and gullies under rainfall scouring. The OD600 = 0.1 treated slope had the best erosion resistance and the minimum erosion loss.
- Phosphogypsum slope treated by microbial treatment can greatly improve the water erosion resistance and reduce slope erosion. The erosion degree of the microbially treated slopes is much less than that of the untreated slope. When the content of calcium carbonate is high and unevenly distributed, it can lead to deeper erosion depth.
- The structure of calcium carbonate induced by different concentrations of treatment solution is different. Under the same concentration of cementation solution, the increase in the bacterial concentration is associated with higher CaCO3 content, and the CaCO3 flocculate to form an overhead structure. The micropores in the structure easily cause the loss of fine particles.
- The pH of the modified phosphogypsum outflow solution is neutral. The heavy metal elements meet the emission standards through microbial action and fixation after microbial treatment. This article proves the effectiveness of microbially modified phosphogypsum and provides a preliminary test basis for the utilization of phosphogypsum material in the reinforcement of slopes.
Author Contributions
Funding
Conflicts of Interest
References
- Lan, W.T.; Wu, A.X.; Wang, Y.M.; Li, J.Q. Test study on factors affecting the filling strength of hemihydrate phosphogypsum. J. Harbin. Inst. Technol. 2019, 51, 128–135. (In Chinese) [Google Scholar] [CrossRef]
- Ma, B.G.; Zhu, L.; Su, Y.; Jin, Z.H.; Gao, C.; Sun, Z.D.; Peng, Y. Mechanical Performance of the Composites of β-hemihydrate Phosphogypsum and α-hemihydrate Gypsum. J. Wuhan Univ. Technol. 2017, 39, 1–8. (In Chinese) [Google Scholar] [CrossRef]
- Zeng, L.L.; Bian, X.; Zhao, L.; Wang, Y.J.; Hong, Z.-S. Effect of phosphogypsum on physiochemical and mechanical behaviour of cement stabilized dredged soil from Fuzhou, China. Geomech. Energy. Environ. 2020, 25, 100195. [Google Scholar] [CrossRef]
- Tayibi, H.; Mohamed, C.; López, F.; Alguacil, F.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Y. Development and Application of Phosphogypsum in Plasterboard. Mater. Sci. 2019, 9, 69–78. (In Chinese) [Google Scholar] [CrossRef]
- Smadi, M.; Haddad, R.; Akour, A. Potential use of phosphogypsum in concrete-Treatise on Current Research. Cem. Concr. Res. 1999, 29, 1419–1425. [Google Scholar] [CrossRef]
- Ölmez, H.; Erdem, E. The effects of phosphogypsum on the setting and mechanical properties of Portland cement and trass cement. Cem. Concr. Res. 1989, 19, 377–384. [Google Scholar] [CrossRef]
- Değirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Du, T.T.; Li, Z.Q.; Zhou, Y.X.; Lu, K.J.; Yue, R.Q. A Study on The Application of Cement Phosphogypsum Stabilized Material in Pavement Base. Highway 2018, 2, 189–195. (In Chinese) [Google Scholar]
- Erdem, E.; Ölmez, H. The mechanical properties of supersulphated cement containing phosphogypsum. Cem. Concr. Res. 1993, 23, 115–121. [Google Scholar] [CrossRef]
- Lu, W.D.; Ma, B.G.; Su, Y.; He, X.Y.; Jin, Z.H.; Qi, H.H. Low-Energy Consumption Preparation of Fine Waterproof Cementitious Material with High-Volume Phosphogypsum. J. Mater. Civ. Eng. 2020, 32, 04020329. [Google Scholar] [CrossRef]
- Dang, W.; Liu, Z.; He, X.; Liu, Q. Mixture ratio of phosphogypsum in backfilling. Min. Technol. 2013, 122, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Kandil, A.-H.T.; Cheira, M.F.; Gado, H.S.; Soliman, M.H.; Akl, H.M. Ammonium sulfate preparation from phosphogypsum waste. J. Rad. Res. Appl. Sci. 2017, 10, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Puppala, A.; Intharasombat, N.; Vempati, R. Experimental studies on ettringite-induced heaving in soils. J. Geotech. Geoenviron. Eng. 2005, 131, 325–337. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Zhao, Q. Study on lime–fly ash–phosphogypsum binder. Constr. Build. Mater. 2007, 21, 1480–1485. [Google Scholar] [CrossRef]
- Jiang, N.J.; Tang, C.S.; Yin, L.Y.; Xie, Y.H.; Shi, B. Applicability of Microbial Calcification Method for Sandy-Slope Surface Erosion Control. J. Mater. Civ. Eng. 2019, 31, 04019250. [Google Scholar] [CrossRef]
- Cochrane, B.H.W.; Reichert, J.M.; Eltz, F.L.F.; Darrell, N. Controlling soil erosion and runoff with polyacrylamide and phosphogypsum on subtropical soil. Trans. ASAE 2005, 48, 149–154. [Google Scholar] [CrossRef]
- Norton, L.D. Mineralogy of high calcium/sulfur−containing coal combustion by−products and their effect on soil surface sealing. Agric. Util. Urban Ind. By−Prod. 1995, 58, 87–106. [Google Scholar] [CrossRef]
- Basha, E.A.; Hashim, R.; Mahmud, H.B.; Muntohar, A.S. Stabilization of residual soil with rice husk ash and cement. Constr. Build. Mater. 2005, 19, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, P. Soil Stabilization with Cement and Lime: State of the Art Review; Her Majesty’s Stationery Office: London, UK, 1993. [Google Scholar]
- Karol, R.H. Chemical Grouting and Soil Stabilization; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. 2006, 132, 1382–1392. [Google Scholar] [CrossRef]
- Karatas, I. Microbiological Improvement of the Physical Properties of Soils; Arizona State University: Phoenix, AZ, USA, 2008. [Google Scholar]
- Ramakrishnan, S.K.; Panchalan, R.K.; Bang, S.S. Improvement of concrete durability by bacterial mineral precipitation. In Proceedings of the 11th International Conference on Fracture, Turin, Italy, 20–25 March 2005; pp. 2095–2100. [Google Scholar]
- Stocks Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Boil Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Chou, C.; Seagren, E.; Asce, A.; Aydilek, A.; Asce, M.; Lai, M. Biocalcification of Sand through Ureolysis. J. Geotech. Geoenviron. 2011, 137, 1179–1189. [Google Scholar] [CrossRef] [Green Version]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Oliveira, P.; Da Costa, M.; Costa, J.; Nobre, M. Comparison of the Ability of Two Bacteria to Improve the Behavior of Sandy Soil. J. Mater. Civ. Eng. 2015, 27, 06014025. [Google Scholar] [CrossRef]
- Whiffin, V.; van Paassen, L.; Harkes, M.P. Microbial Carbonate Precipitation as a Soil Improvement Technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V.; Li, B. Microbial method for construction of an aquaculture pond in sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.; Booster, J.; Whiffin, V.; van Loosdrecht, M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Boquet, E.; Boronat, A.; Ramos-Cormenzana, A. Production of Calcite (Calcium Carbonate) Crystals by Soil Bacteria is a General Phenomenon. Nature 1973, 246, 527–529. [Google Scholar] [CrossRef]
- Ferris, F.G.; Stehmeier, L.; Kantzas, A.; Mourits, F.M. Bacteriogenic Mineral Plugging. J. Can. Pet. Technol. 1996, 35, 56–61. [Google Scholar] [CrossRef]
- Liu, S.H.; Du, K.; Huang, W.; Wen, K.J.; Amini, F.; Li, L. Improvement of erosion-resistance of bio-bricks through fiber and multiple MICP treatments. Constr. Build. Mater. 2020, 271, 121573. [Google Scholar] [CrossRef]
- Qian, C.X.; Wang, J.Y.; Wang, R.X.; Cheng, L. Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater. Sci. Eng. 2009, 29, 1273–1280. [Google Scholar] [CrossRef]
- Bang, S.; Lippert, J.; Yerra, U.; Mulukutla, S.; Ramakrishnan, V. Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int. J. Smart Nano Mater. 2010, 1, 28–39. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, X.H. A performance study of high-strength microbial mortar produced by low pressure grouting for the reinforcement of deteriorated masonry structures. Constr. Build. Mater. 2013, 41, 505–515. [Google Scholar] [CrossRef]
- Kantzas, A.; Stehmeier, L.; Marentette, D.F.; Ferris, F.; Jha, K.N.; Maurits, F.M. A Novel Method of Sand Consolidation Through Bacteriogenic Mineral Plugging. In Proceedings of the Annual Technical Meeting, Calgary, AB, Canada, 6–9 June 1992. [Google Scholar]
- Van Paassen, L.; Harkes, M.P.; Van Zwieten, G.; Van der zon, W.; Van der Star, W.; Van Loosdrecht, M. Scale up of BioGrout: A biological ground reinforcement method. In Proceedings of the 17th International Conference on Soil Mechanics & Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; pp. 2328–2333. [Google Scholar]
- Van Paassen, L.A.; Ghose, R.; Van der Linden, T.J.; Van der Star, W.R.; Van Loosdrecht, M.C. Quantifying biomediated ground improvement by ureolysis: Large-scale biogrout experiment. J. Geotech. Geoenviron. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. 2001, 98, 3–9. [Google Scholar] [CrossRef]
- Maleki, M.; Ebrahimi, S.; Asadzadeh, F.; Emami Tabrizi, M. Performance of microbial-induced carbonate precipitation on wind erosion control of sandy soil. Int. J. Environ. Sci. Technol. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Gao, Y.F.; Tang, X.Y.; Chu, J.; He, J. Microbially Induced Calcite Precipitation for Seepage Control in Sandy Soil. Geomicrobiol. J. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Mitchell, J.; Santamarina, J. Biological Considerations in Geotechnical Engineering. J. Geotech. Geoenviron. 2005, 131, 1222–1233. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef]
- Pei, X.J.; Yang, Q.W.; Xu, Q.; Zhang, X.C. Research on glue reinforcement mechanism and scouring resistant properties of soil slope by modified carboxymethyl cellulose. Chin. J. Rock. Mech. Eng. 2016, 35, 2316–2327. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.; Chaosheng, T.; Liyang, Y.; Bo, L.; Chao, L.; Dianlong, W.; Xiaohua, P.; Hanlin, W.; Bin, S. Experimental study on surface erosion resistance and mechanical behavior of MICP-FRtreated calcareous sand. Chin. J. Geotech. Eng. 2021, 43, 1941–1949. (In Chinese) [Google Scholar]
- Bizjak, K. Determining the surface roughness coefficient by 3D Scanner. Geologija 2010, 53, 147–152. [Google Scholar] [CrossRef]
- Tatone, B.; Grasselli, G. A method to evaluate the three-dimensional roughness of fracture surfaces in brittle geomaterials. Rev. Sci. Instrum. 2009, 80, 125110. [Google Scholar] [CrossRef]
- Qian, C.X.; Wang, M.M.; Xu, Y.B. Current situation of soil contamination by heavy metals and research progress in bio-remediation technique. J. Southeast Univ. Nat. Sci. Ed. 2013, 43, 669–674. [Google Scholar] [CrossRef]
- Ferris, F.; Phoenix, V.; Fujita, Y.; Smith, R.W. Kinetics of Calcite Precipitation Induced by Ureolytic Bacteria at 10 to 20 °C in Artificial Groundwater. Geochim. Cosmochim. Acta 2003, 67, 1701–1722. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, M.L.; Hii, S.L. An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. World Acad. Sci. Eng. Technol. 2012, 62, 723–729. [Google Scholar]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, A. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Ercole, C.; Cacchio, P.; Botta, A.; Centi, V.; Lepidi, A. Bacterially Induced Mineralization of Calcium Carbonate: The Role of Exopolysaccharides and Capsular Polysaccharides. Microsc. Microanal. Off. J. Microsc. Soc. Am. Microbeam Anal. Soc. Microsc. Soc. Can. 2007, 13, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okwadha, G.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, S.; Cord-Ruwisch, R. Calcium Carbonate Crystals Formation by Ureolytic Bacteria Isolated from Australian Soil and Sludge. J. Adv. Sci. Eng. Res. 2012, 2, 12–26. [Google Scholar]
- Gandhi, K.; Kumar, R.; Ramkrishna, D. Some Basic Aspects of Reaction Engineering of Precipitation Processes. Ind. Eng. Chem. Res. 1995, 34, 3223–3230. [Google Scholar] [CrossRef]
- Somani, R.; Patel, K.; Mehta, A.; Jasra, R.V. Examination of the Polymorphs and Particle Size of Calcium Carbonate Precipitated Using Still Effluent (i.e., CaCl2 + NaCl Solution) of Soda Ash Manufacturing Process. Ind. Eng. Chem. Res. 2006, 45, 5223–5230. [Google Scholar] [CrossRef]
- Snoeyink, V.S.; Jenkins, D. Water Chemistry; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Soon, N.; Lee, L.; Tan, C.; Siew Ling, H. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. 2014, 140, 12–26. [Google Scholar] [CrossRef]




| Liquid Limit (%) | Plastic Limit (%) | Plastic Index (%) | Maximum Dry Density (g/cm3) | Optimum Moisture Content (%) | Specific Gravity (g/cm3) | Particle Size Distribution | ||
|---|---|---|---|---|---|---|---|---|
| d10 | d30 | d60 | ||||||
| 29.9 | 19.6 | 10.3 | 1.41 | 20.7 | 2.4 | 1.15 | 5.61 | 27.39 |
| CaO | SO3 | SiO2 | P2O5 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | F | Crystal Water | Organism | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt (%) | 30.12 | 40.33 | 9.71 | 0.70 | 0.20 | 0.09 | 0.01 | 0.22 | 0.24 | 0.20 | 18.10 | 0.08 |
| Element | Untreated Phosphogypsum | Microbially Treated Phosphogypsum | Critical Value |
|---|---|---|---|
| Se | <0.01 | <0.01 | 0.01 |
| Cd | <0.003 | <0.003 | 0.003 |
| Pb | <0.05 | <0.05 | 0.05 |
| Ni | <0.01 | <0.01 | 0.01 |
| Hg | <0.01 | <0.01 | 0.01 |
| As | <0.1 | <0.01 | 0.01 |
| Cr | <0.01 | <0.01 | 0.01 |
| Be | 0.045 | <0.005 | 0.005 |
| Ba | <0.004 | <0.004 | 0.004 |
| Ag | <0.1 | <0.1 | 0.1 |
| Cu | <0.01 | <0.01 | 0.01 |
| Zn | 0.181 | <0.006 | 0.006 |
| Sample | K1 | K2 | K3 | AVE | MAX | MIN | CV |
|---|---|---|---|---|---|---|---|
| OD600 = 0 | 1.49 × 103 | 1.15 × 103 | 7.92 × 104 | 1.14 × 103 | 1.49 × 103 | 8.02 × 104 | 0.61 |
| OD600 = 0.1 + 0.5M | 6.21 × 104 | 7.32 × 104 | 5.02 × 104 | 6.18 × 104 | 7.32 × 104 | 5.02 × 104 | 0.37 |
| OD600 = 0.3 + 0.5M | 8.79 × 104 | 6.66 × 104 | 1.06 × 103 | 8.68 × 104 | 1.06 × 103 | 6.66 × 104 | 0.45 |
| OD600 = 0.5 + 0.5M | 9.88 × 104 | 8.75 × 104 | 3.78 × 104 | 7.47 × 104 | 9.88 × 104 | 3.78 × 104 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, Z.; Wang, S.; Zhou, Y.; Li, D.; Fu, L. Application of MICP in Water Stability and Hydraulic Erosion Control of Phosphogypsum Material in Slope. Appl. Sci. 2022, 12, 1783. https://doi.org/10.3390/app12041783
Tian Y, Li Z, Wang S, Zhou Y, Li D, Fu L. Application of MICP in Water Stability and Hydraulic Erosion Control of Phosphogypsum Material in Slope. Applied Sciences. 2022; 12(4):1783. https://doi.org/10.3390/app12041783
Chicago/Turabian StyleTian, Yifan, Zhiqing Li, Shuangjiao Wang, Yingxin Zhou, Dandan Li, and Le Fu. 2022. "Application of MICP in Water Stability and Hydraulic Erosion Control of Phosphogypsum Material in Slope" Applied Sciences 12, no. 4: 1783. https://doi.org/10.3390/app12041783





