Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
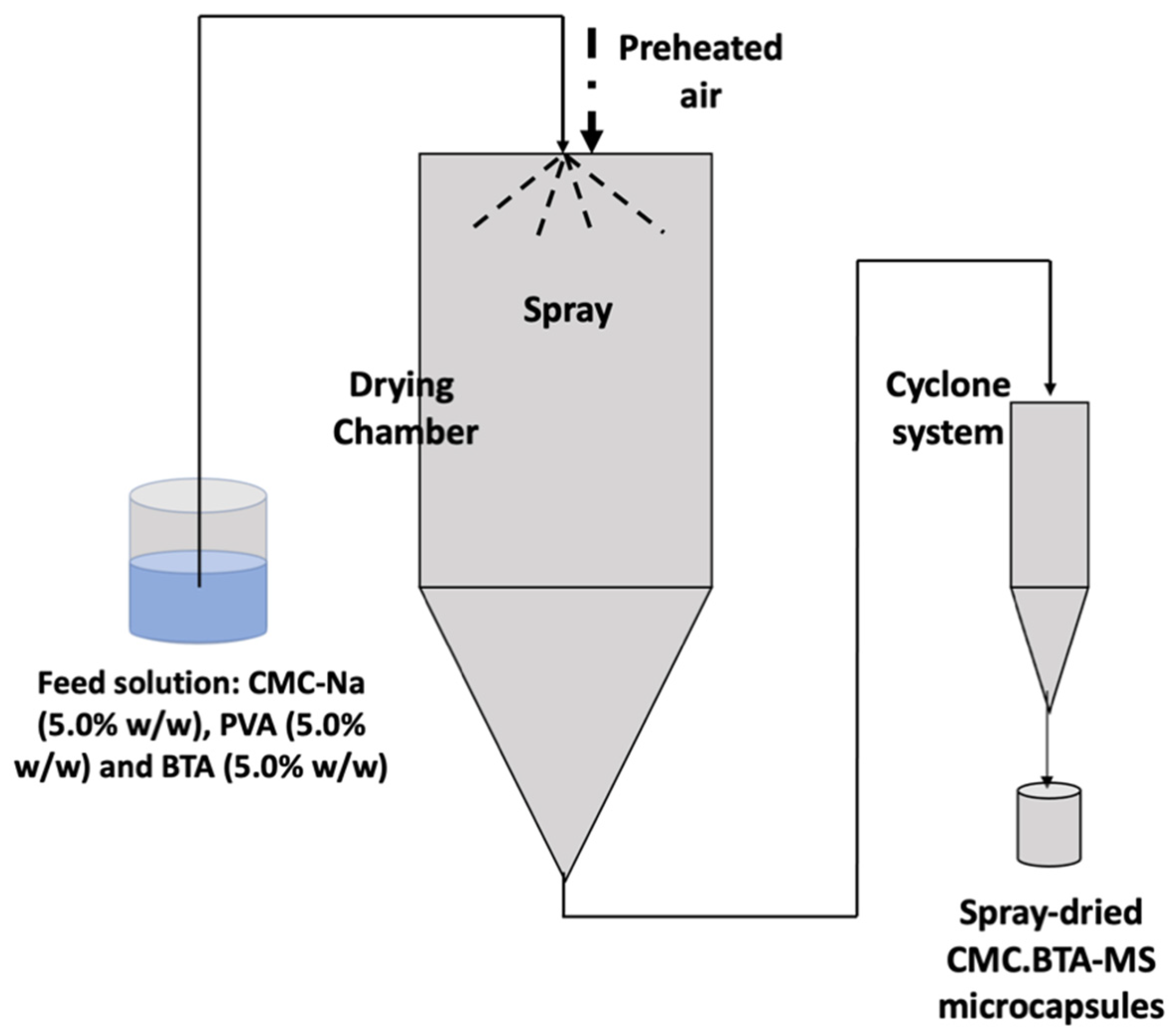
2.2. Preparation of Carboxymethylcellulose Microspheres Loaded with BTA
2.3. Characterization of Spray-Dried Microspheres
2.3.1. Moisture Content
2.3.2. Microparticle Morphology and Size Distribution
2.3.3. BTA Release Studies
Encapsulation Efficiency and Loading Capacity
3. Results and Discussion
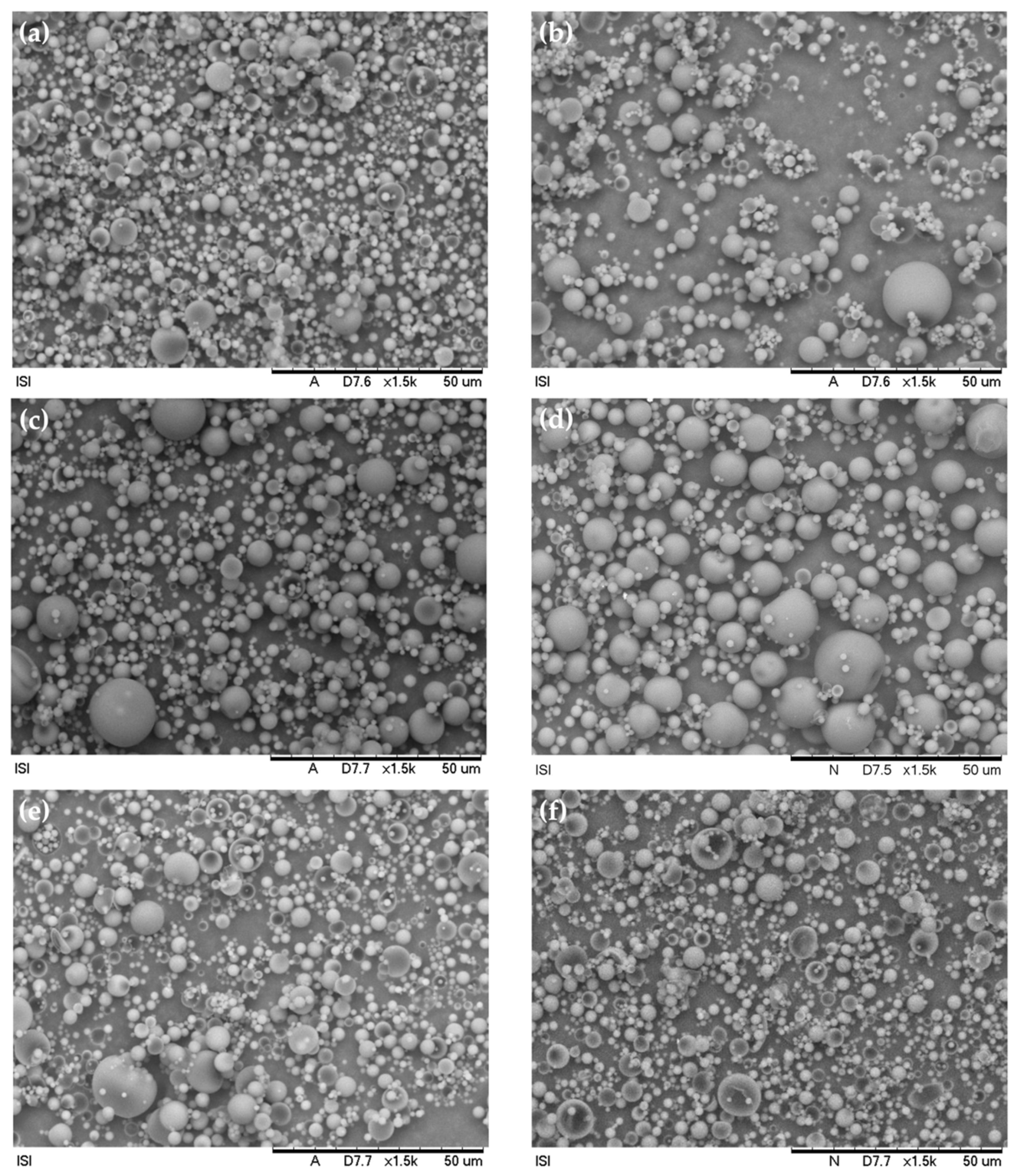
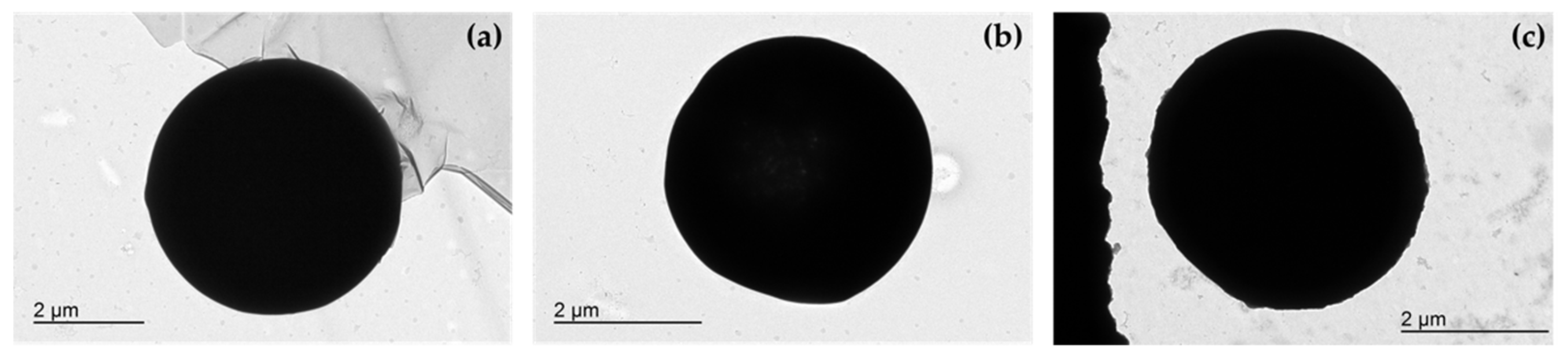
3.1. Microsphere Morphological Characterization
3.2. Particle Size
3.3. Moisture Content
3.4. Yield
3.5. BTA Release Studies from Carboxymethyl Cellulose Microspheres
3.5.1. Encapsulation Efficiency (EE%) and Loading Capacity (LC%)
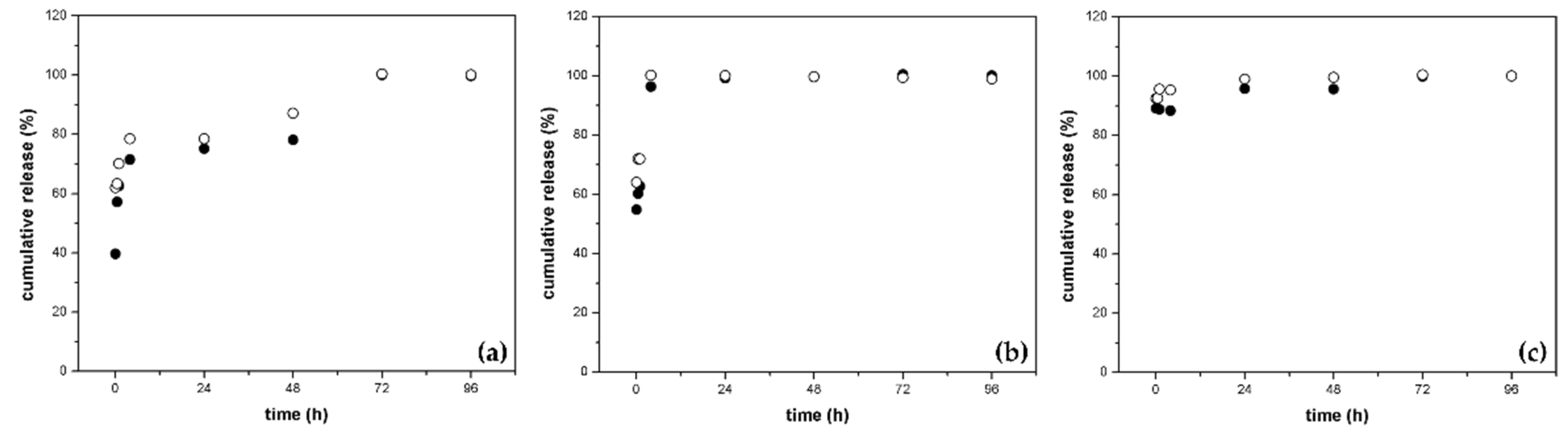
3.5.2. Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, X.; Wang, J.; Hu, W. Inhibition Behavior of Cu-Benzoltriazole-Calcium Alginate Gel Beads by Piercing and Solidification. Mater. Des. 2017, 126, 322–330. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” Coatings for Active Corrosion Protection Based on Multi-Functional Micro and Nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Qian, M.; Mcintosh Soutar, A.; Tan, X.H.; Zeng, X.T.; Wijesinghe, S.L. Two-Part Epoxy-Siloxane Hybrid Corrosion Protection Coatings for Carbon Steel. Thin Solid Film. 2009, 517, 5237–5242. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and Smart Coatings for Corrosion Protection: A Review of Recent Advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential Use of Smart Coatings for Corrosion Protection of Metals and Alloys: A Review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Rajan, R.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Developments in Smart Anticorrosive Coatings with Multifunctional Characteristics. Prog. Org. Coat. 2017, 111, 294–314. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would like to Know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef]
- Leal, D.A.; Riegel-Vidotti, I.C.; Ferreira, M.G.S.; Marino, C.E.B. Smart Coating Based on Double Stimuli-Responsive Microcapsules Containing Linseed Oil and Benzotriazole for Active Corrosion Protection. Corros. Sci. 2018, 130, 56–63. [Google Scholar] [CrossRef]
- Maia, F.; Yasakau, K.A.; Carneiro, J.; Kallip, S.; Tedim, J.; Henriques, T.; Cabral, A.; Venâncio, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion Protection of AA2024 by Sol–Gel Coatings Modified with MBT-Loaded Polyurea Microcapsules. Chem. Eng. J. 2016, 283, 1108–1117. [Google Scholar] [CrossRef]
- Choi, H.; Kim, K.Y.; Park, J.M. Encapsulation of Aliphatic Amines into Nanoparticles for Self-Healing Corrosion Protection of Steel Sheets. Prog. Org. Coat. 2013, 76, 1316–1324. [Google Scholar] [CrossRef]
- Cotting, F.; Aoki, I.V. Smart Protection Provided by Epoxy Clear Coating Doped with Polystyrene Microcapsules Containing Silanol and Ce (III) Ions as Corrosion Inhibitors. Surf. Coat. Technol. 2016, 303, 310–318. [Google Scholar] [CrossRef]
- Kopeć, M.; Szczepanowicz, K.; Mordarski, G.; Podgórna, K.; Socha, R.P.; Nowak, P.; Warszyński, P.; Hack, T. Self-Healing Epoxy Coatings Loaded with Inhibitor-Containing Polyelectrolyte Nanocapsules. Prog. Org. Coat. 2015, 84, 97–106. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of Spray Drying: A Critical Review on Drying of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Zuo, J.; Zhan, J.; Luo, C.; Dong, B.; Xing, F.; Chen, D. Characteristics and Release Property of Polylactic Acid/Sodium Monofluorophosphate Microcapsules Prepared by Spray Drying. Adv. Powder Technol. 2017, 28, 2805–2811. [Google Scholar] [CrossRef]
- Calegari, F.; da Silva, B.C.; Tedim, J.; Ferreira, M.G.S.; Berton, M.A.C.; Marino, C.E.B. Benzotriazole Encapsulation in Spray-Dried Carboxymethylcellulose Microspheres for Active Corrosion Protection of Carbon Steel. Prog. Org. Coat. 2020, 138, 105329. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with Chitosan by Spray Drying for Industry Applications—A Review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Encapsulação: Aplicação à Tecnologia de Alimentos. Alim. Nutri. 2005, 16, 89–97. [Google Scholar]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.S.R.; Gandini, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Functionalized Chitosan-Based Coatings for Active Corrosion Protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Yabuki, A.; Shiraiwa, T.; Fathona, I.W. PH-Controlled Self-Healing Polymer Coatings with Cellulose Nanofibers Providing an Effective Release of Corrosion Inhibitor. Corros. Sci. 2016, 103, 117–123. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-Derived Biopolymers: Potential Platforms for Enzyme Immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Khatri, O.P. A Scanning Electron Microscope Based New Method for Determining Degree of Substitution of Sodium Carboxymethyl Cellulose: Sem Based Method for Determining DS. J. Microsc. 2012, 246, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ré, M.-I. Formulating Drug Delivery Systems by Spray Drying. Null 2006, 24, 433–446. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Null 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of Carboxymethyl Cellulose-Chitosan Hybrid Micro- and Macroparticles for Encapsulation of Probiotic Bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermomechanical Properties of Poly(Vinyl Alcohol) Plasticized with Varying Ratios of Sorbitol. Mater. Sci. Eng. A 2011, 528, 925–930. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef]
- Mlalila, N.; Swai, H.; Kalombo, L.; Hilonga, A. Effects of Spray-Drying on w/o/w Multiple Emulsions Prepared from a Stearic Acid Matrix. NSA 2014, 7, 105. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.; Li, Q.; Che, Y.; Liu, X.; Dong, C.; Chen, X.; Wang, C. Effect of Na2CO3 on the Microstructure and Macroscopic Properties and Mechanism Analysis of PVA/CMC Composite Film. Polymers 2020, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, J.A.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Ré, M.-I. Microencapsulation by spray drying. Null 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Büchi Mini Spray Dryer B-290 Operation Manual, Training Papers Spray Drying. Available online: https://www.manualslib.com/manual/1440156/Buchi-B-290.html (accessed on 16 October 2021).
- Anandharamakrishnan, C.; Ishwarya, S.P. Introduction to Spray Drying. In Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–36. ISBN 978-1-118-86398-5. [Google Scholar]
- Díaz, I.; Chico, B.; de la Fuente, D.; Simancas, J.; Vega, J.M.; Morcillo, M. Corrosion Resistance of New Epoxy–Siloxane Hybrid Coatings. A Laboratory Study. Prog. Org. Coat. 2010, 69, 278–286. [Google Scholar] [CrossRef]
- Raupach, M.; Rößler, G. Surface Treatments and Coatings for Corrosion Protection. In Corrosion in Reinforced Concrete Structures; Woodhead Publishing: Cambridge, UK, 2005; pp. 163–189. ISBN 978-1-85573-768-6. [Google Scholar]
- Cole, I.S. Smart Coatings for Corrosion Protection: An Overview. In Handbook of Smart Coatings for Materials Protection; Woodhead Publishing: Cambridge, UK, 2014; pp. 29–55. ISBN 978-0-85709-680-7. [Google Scholar]
- Rahsepar, M.; Mohebbi, F.; Hayatdavoudi, H. Synthesis and Characterization of Inhibitor-Loaded Silica Nanospheres for Active Corrosion Protection of Carbon Steel Substrate. J. Alloy. Compd. 2017, 709, 519–530. [Google Scholar] [CrossRef]
- Thenapakiam, S.; Saravanan, M.; Pushpamalar, J.; Hong, C.L. Spray Dried Solid Dispersions of Piroxicam in Carboxymethyl Sago Cellulose Using Aqueous Solvents: A Simple, Novel and Green Approach to Produce Enteric Microparticles with Enhanced Dissolution. Dry. Technol. 2019, 37, 1191–1200. [Google Scholar] [CrossRef]
- Aguiar, J.; Costa, R.; Rocha, F.; Estevinho, B.N.; Santos, L. Design of Microparticles Containing Natural Antioxidants: Preparation, Characterization and Controlled Release Studies. Powder Technol. 2017, 313, 287–292. [Google Scholar] [CrossRef]
- Sansone, F.; Picerno, P.; Mencherini, T.; Russo, P.; Gasparri, F.; Giannini, V.; Lauro, M.R.; Puglisi, G.; Aquino, R.P. Enhanced Technological and Permeation Properties of a Microencapsulated Soy Isoflavones Extract. J. Food Eng. 2013, 115, 298–305. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef]
- Pratap Singh, A.; Siddiqui, J.; Diosady, L.L. Characterizing the PH-Dependent Release Kinetics of Food-Grade Spray Drying Encapsulated Iron Microcapsules for Food Fortification. Food Bioprocess Technol. 2018, 11, 435–446. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of Spray Drying Conditions and Feed Composition on the Physical Properties of Black Mulberry Juice Powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Maa, Y.F.; Costantino, H.R.; Nguyen, P.A.; Hsu, C.C. The Effect of Operating and Formulation Variables on the Morphology of Spray-Dried Protein Particles. Pharm. Dev. Technol. 1997, 2, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Fu, Z.Y.; Zhang, J.Y.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Microencapsulation of Radix Salvia Miltiorrhiza Nanoparticles by Spray-Drying. Powder Technol. 2008, 184, 114–121. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and Dielectric Properties of Carboxymethyl Cellulose/Polyvinyl Alcohol Blends. Phys. B Condens. Matter. 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Ishwarya, S.P. Selection of Wall Material for Encapsulation by Spray Drying. In Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 77–100. ISBN 978-1-118-86398-5. [Google Scholar]
- Tchabo, W.; Ma, Y.; Kaptso, G.K.; Kwaw, E.; Cheno, R.W.; Xiao, L.; Osae, R.; Wu, M.; Farooq, M. Process Analysis of Mulberry (Morus Alba) Leaf Extract Encapsulation: Effects of Spray Drying Conditions on Bioactive Encapsulated Powder Quality. Food Bioprocess Technol. 2019, 12, 122–146. [Google Scholar] [CrossRef]
- Li, D.X.; Oh, Y.-K.; Lim, S.-J.; Kim, J.O.; Yang, H.J.; Sung, J.H.; Yong, C.S.; Choi, H.-G. Novel Gelatin Microcapsule with Bioavailability Enhancement of Ibuprofen Using Spray-Drying Technique. Int. J. Pharm. 2008, 355, 277–284. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray Drying Formulation of Amorphous Solid Dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- De Souza Lima, R.; Ré, M.-I.; Arlabosse, P. Drying Droplet as a Template for Solid Formation: A Review. Powder Technol. 2020, 359, 161–171. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/PT/en/sds/sial/b11400 (accessed on 16 October 2021).
| Condition Number | Inlet Temperature (°C) | Drying Gas Flow Rate (L/h) |
|---|---|---|
| 1 | 170 | 440 |
| 2 | 170 | 600 |
| 3 | 180 | 440 |
| 4 | 180 | 600 |
| 5 | 190 | 440 |
| 6 | 190 | 600 |
| Condition Number | Average Diameter (μm) | Size Distribution (μm) |
|---|---|---|
| 1 | 1.7 ± 0.97 | 0.52–9.88 |
| 2 | 2.0 ± 1.2 | 0.12–11.4 |
| 3 | 2.2 ± 1.5 | 0.73–14.8 |
| 4 | 1.8 ± 1.1 | 0.38–11.5 |
| 5 | 2.0 ± 1.2 | 0.52–9.08 |
| 6 | 1.8 ± 1.0 | 0.67–7.67 |
| Condition Number | Moisture Content (%) |
|---|---|
| 1 | 2.0 ± 0.23 |
| 2 | 2.3 ± 0.12 |
| 3 | 1.9 ± 0.32 |
| 4 | 2.0 ± 0.48 |
| 5 | 1.9 ± 0.02 |
| 6 | 1.8 ± 0.07 |
| Condition Number | Outlet Temperature (°C) | Product Yield (%) | Encapsulation Efficiency (%) * | Loading Capacity (gBTA/gmicroparticle) * |
|---|---|---|---|---|
| 1 | 86 ± 2 | 63.4 ± 1.4 | 39.0 | 15.6 |
| 2 | 82 ± 2 | 60.3 ± 1.2 | 35.9 | 14.3 |
| 3 | 82 ± 2 | 45.2 ± 1.2 | 36.9 | 14.8 |
| 4 | 80 ± 2 | 60.2 ± 1.2 | 34.4 | 13.7 |
| 5 | 106 ± 2 | 43.5 ± 1.5 | 37.6 | 15.0 |
| 6 | 106 ± 2 | 48.5 ± 1.5 | 36.1 ± 0.4 | 14.4 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calegari, F.; Sousa, I.; Ferreira, M.G.S.; Berton, M.A.C.; Marino, C.E.B.; Tedim, J. Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres. Appl. Sci. 2022, 12, 1800. https://doi.org/10.3390/app12041800
Calegari F, Sousa I, Ferreira MGS, Berton MAC, Marino CEB, Tedim J. Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres. Applied Sciences. 2022; 12(4):1800. https://doi.org/10.3390/app12041800
Chicago/Turabian StyleCalegari, Francyelle, Isabel Sousa, Mário G. S. Ferreira, Marcos A. C. Berton, Cláudia E. B. Marino, and João Tedim. 2022. "Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres" Applied Sciences 12, no. 4: 1800. https://doi.org/10.3390/app12041800
APA StyleCalegari, F., Sousa, I., Ferreira, M. G. S., Berton, M. A. C., Marino, C. E. B., & Tedim, J. (2022). Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres. Applied Sciences, 12(4), 1800. https://doi.org/10.3390/app12041800









