Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel
Abstract
1. Introduction
2. Experiment
2.1. Materials and Chemicals
2.2. Preparation of Tannic Acid-Loaded HAP Particles
2.2.1. Synthesis of Hydroxyapatite
2.2.2. Loading of HAP Particles with Tannic Acid
2.3. Preparation of Polyolefin-Coated Carbon Steel Samples
3. Physicochemical Study of Particles and Coated Samples
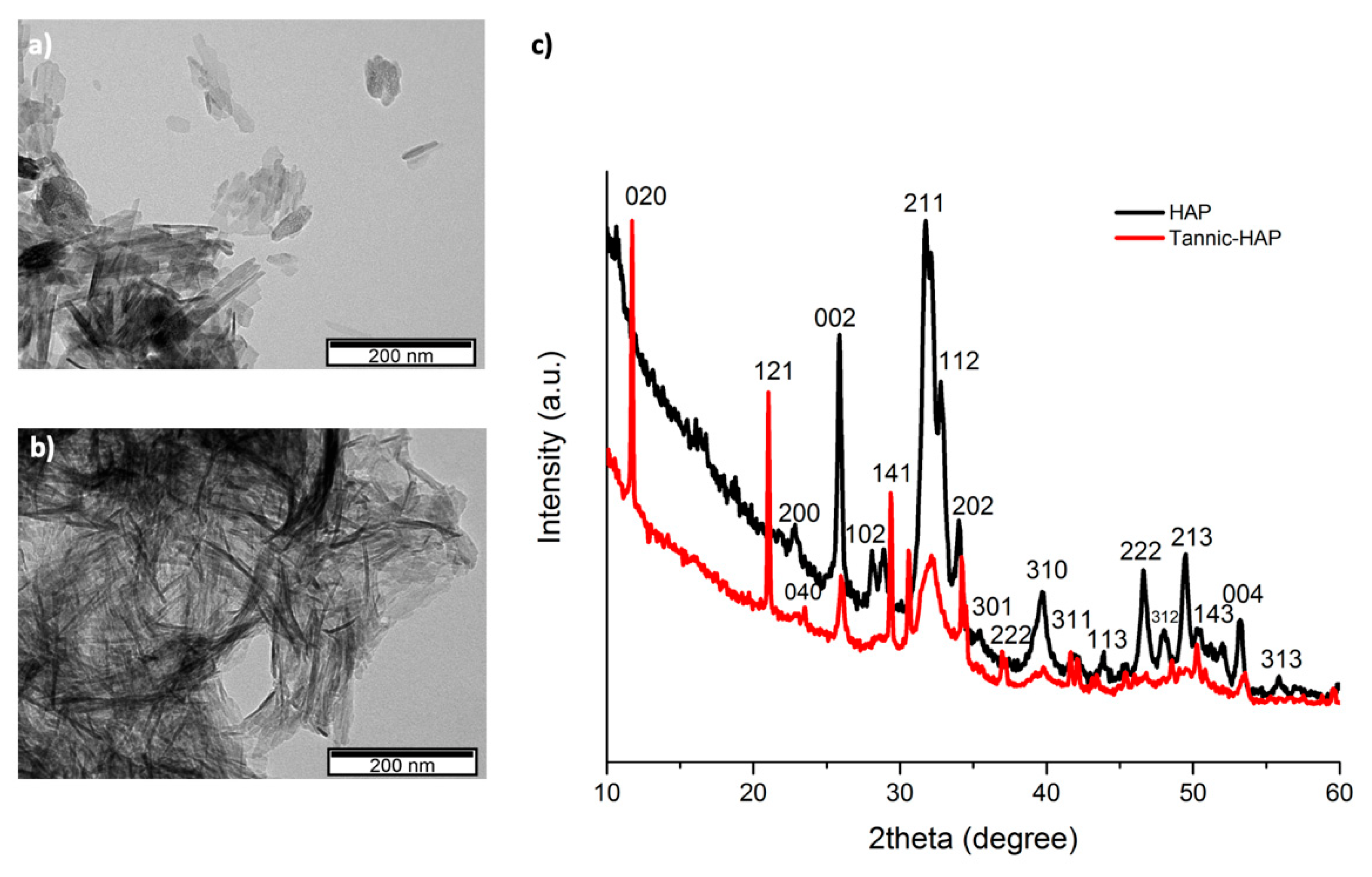
3.1. Transmission Electron Microscopy
3.2. X-ray Diffraction
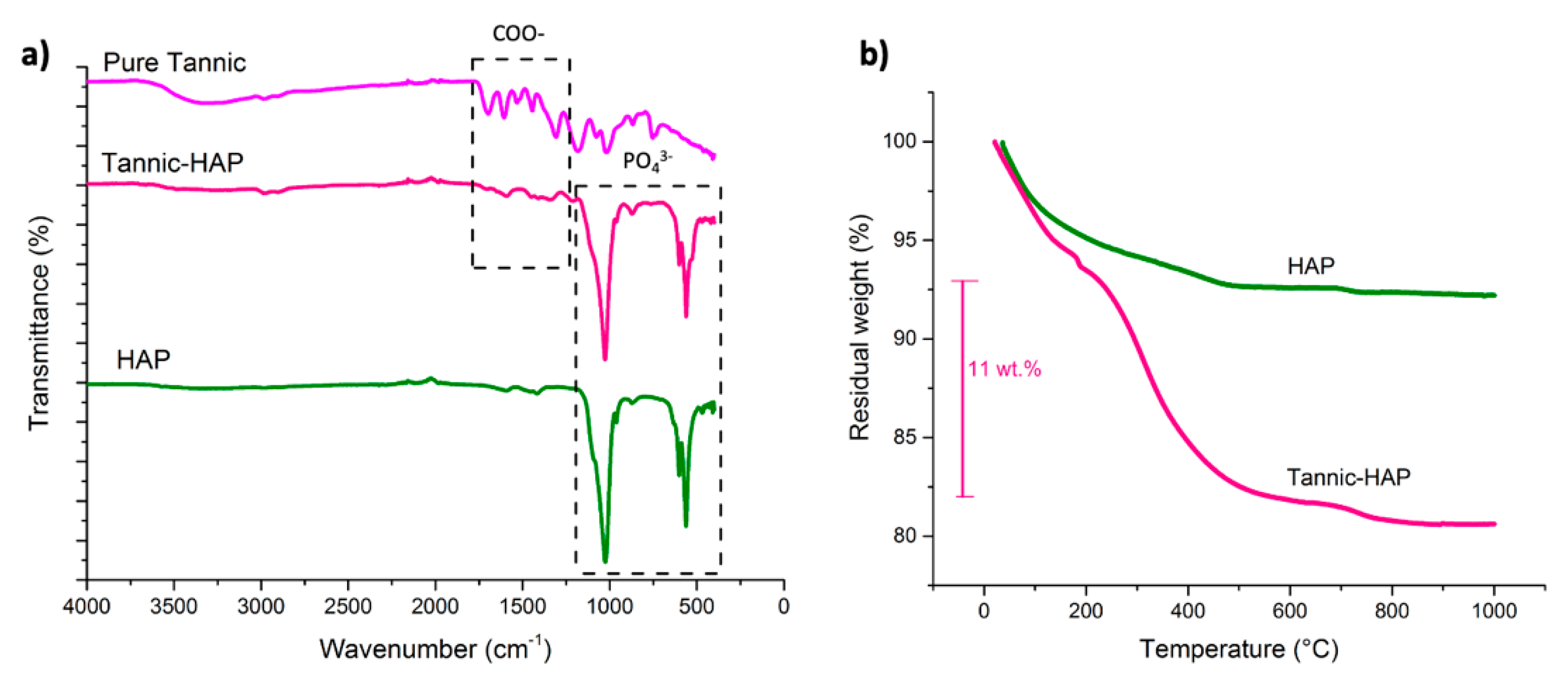
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Thermogravimetric Analysis (TGA)
3.5. Electrochemical Studies
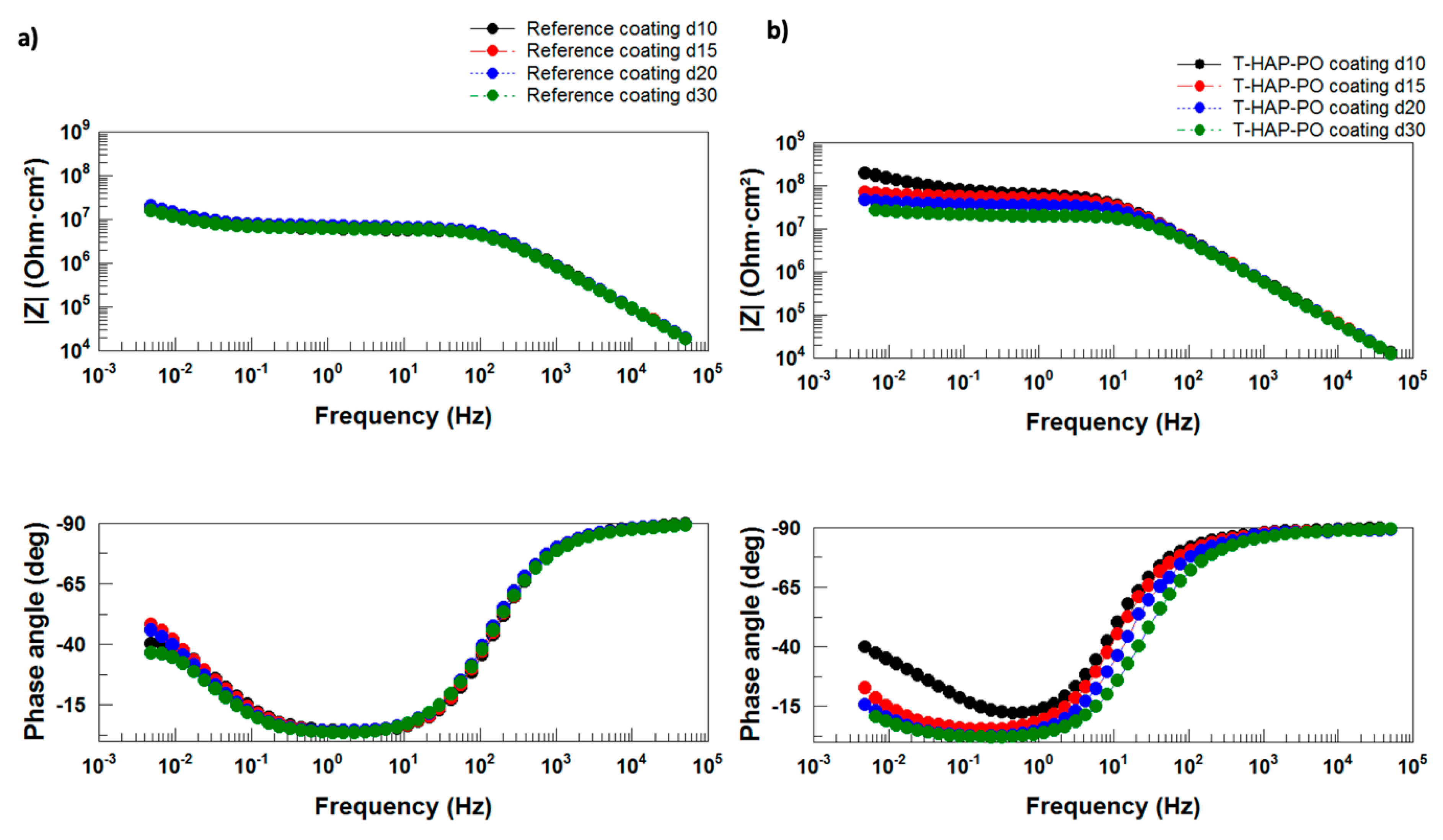
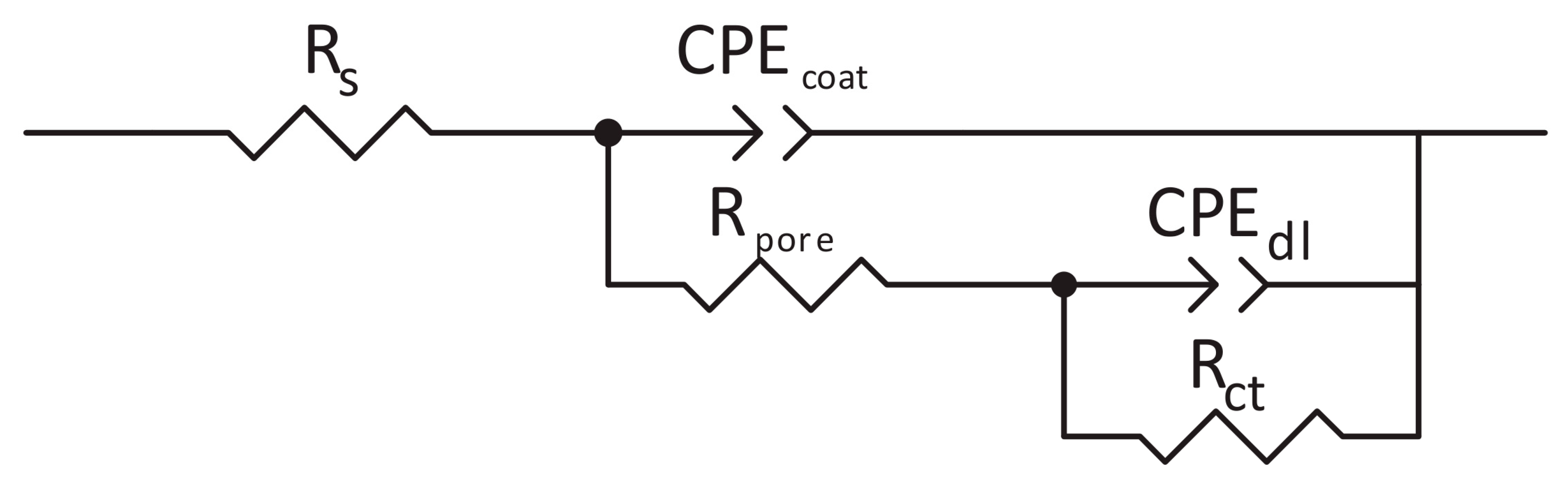
Electrochemical Impedance Spectroscopy
4. Results and Discussion
4.1. Characterization of the Hydroxyapatite Particles
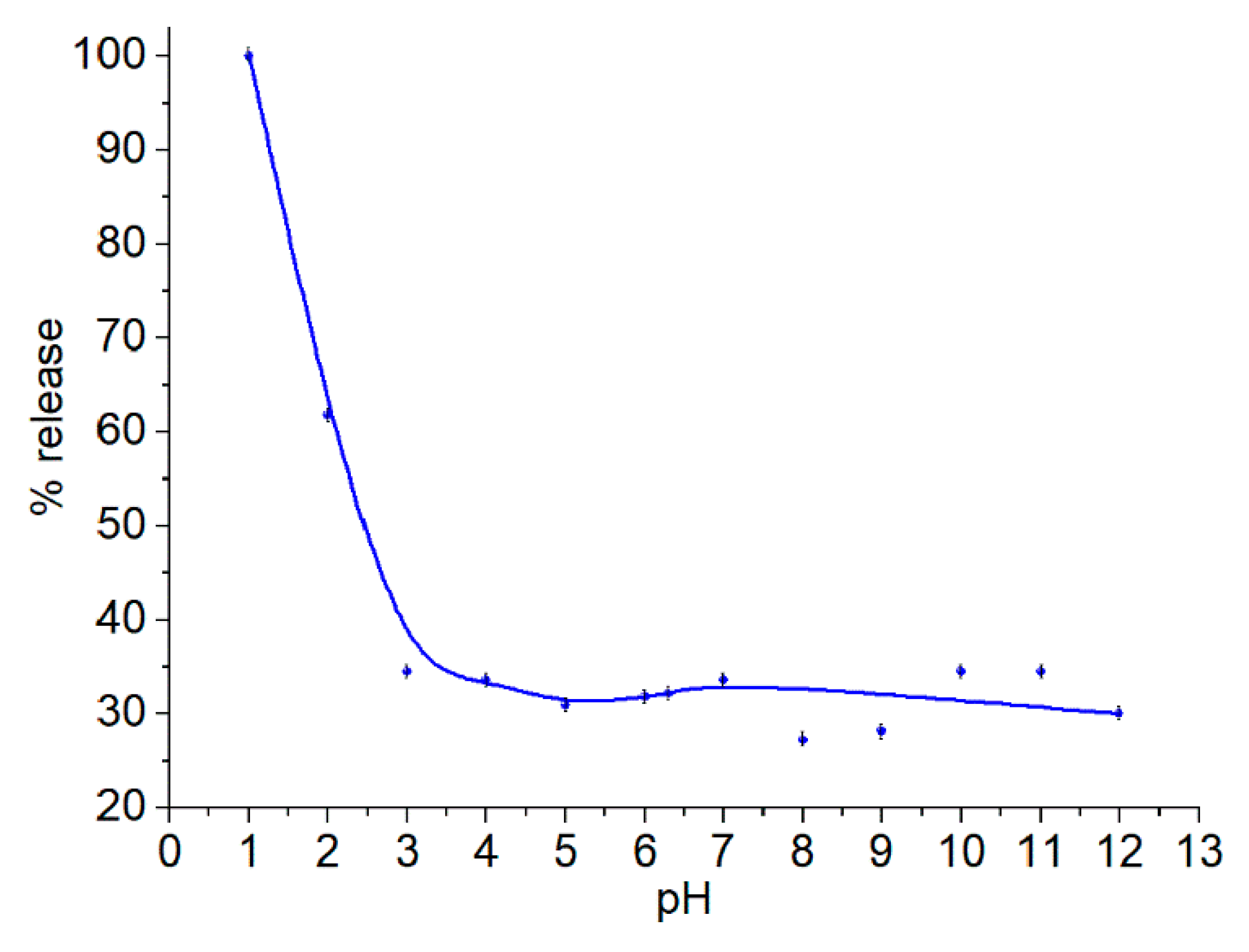
Release from the Particles
4.2. Corrosion Performance of Polyolefin Coated Steel Modified with Tannic Acid-HAP Particles
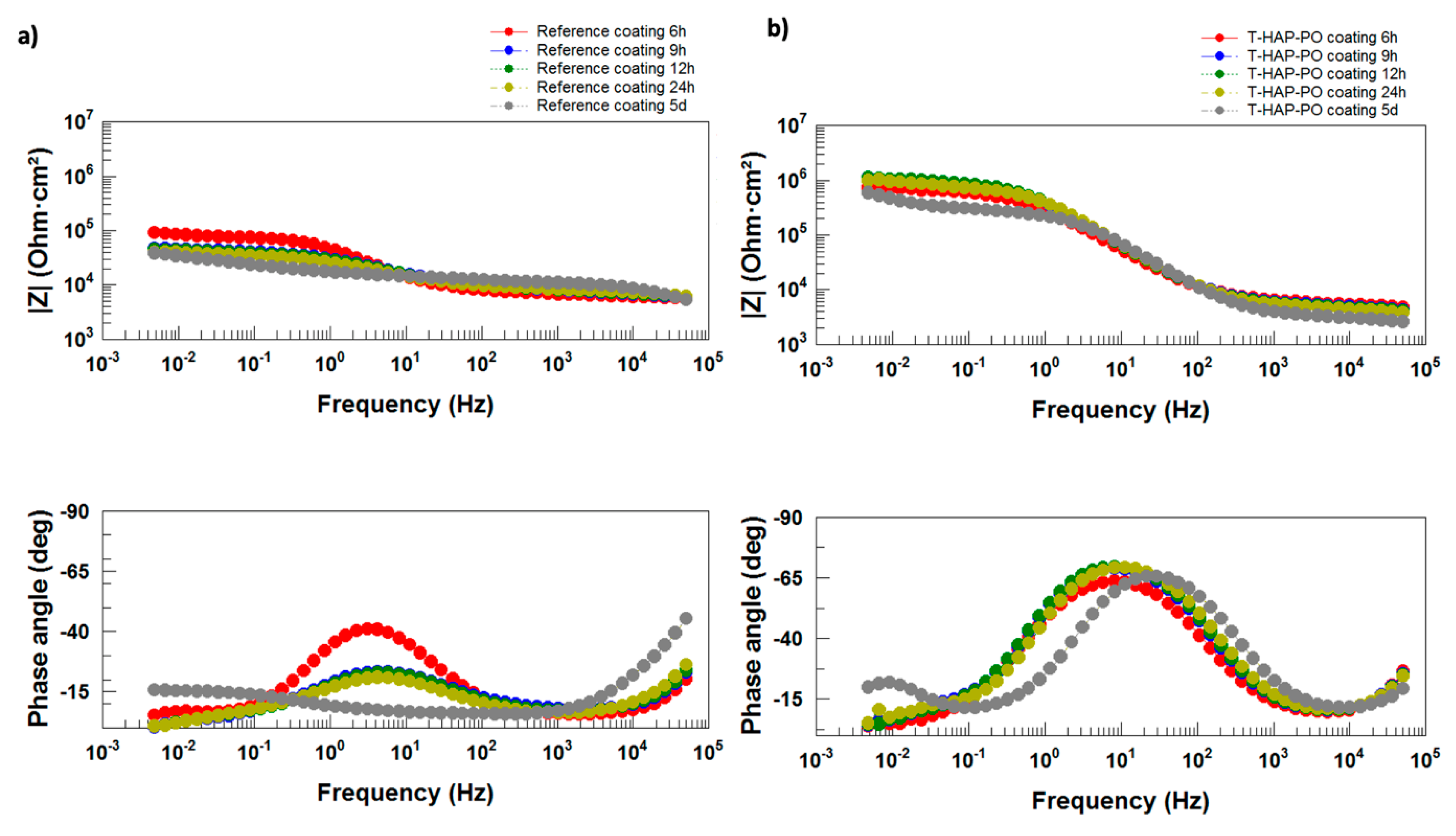
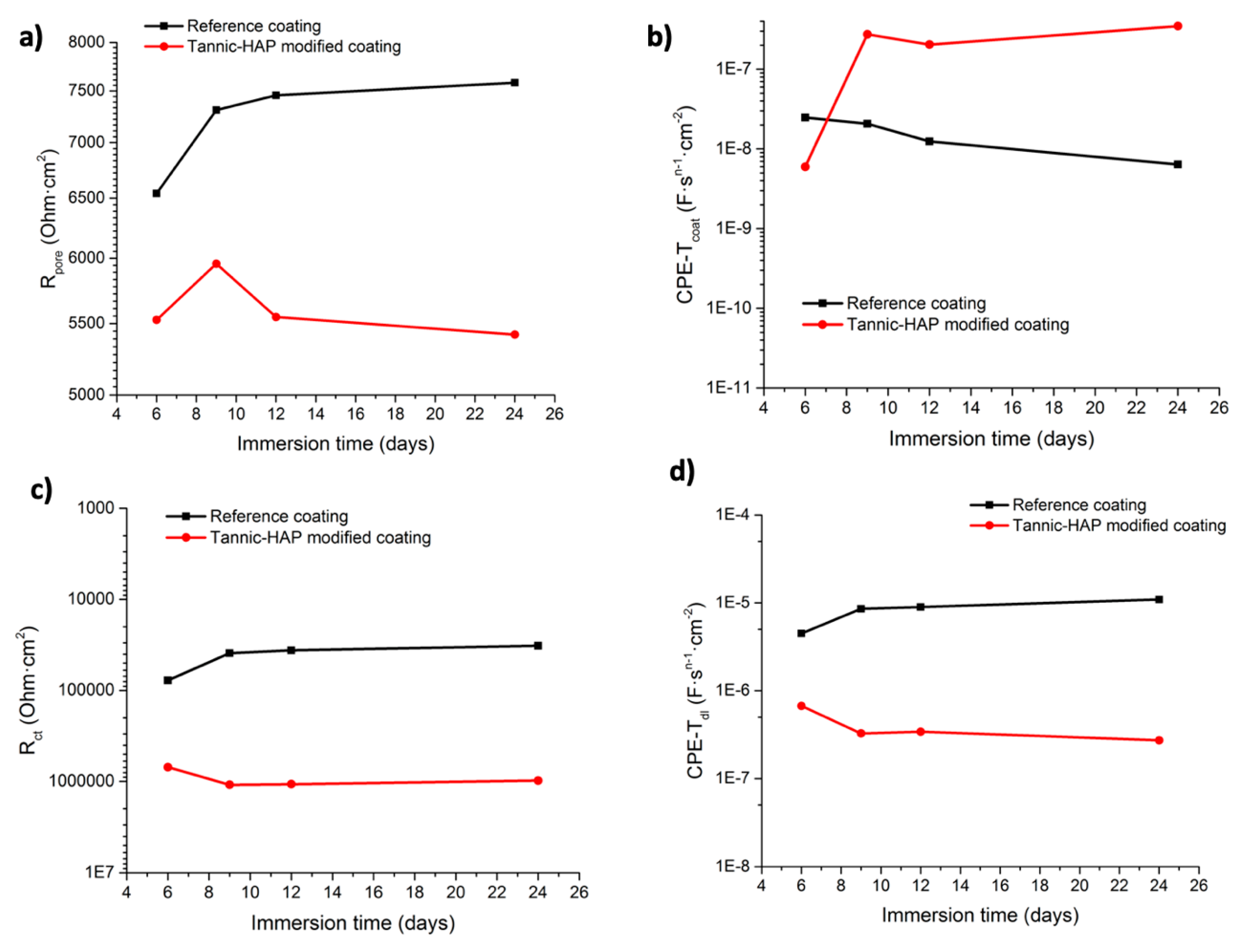
Electrochemical Impedance Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Prabakaran, M.; Durainatarajan, P.; Ramesh, S.; Periasamy, V. Enhanced corrosion inhibition behavior of carbon steel in aqueous solution by Phosphoserine-Zn2+ system. J. Adhes. Sci. Technol. 2016, 30, 1487–1509. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Samiento-Bustos, E.; Rodriguez, J.G.G.; Uruchurtu, J.; Dominguez-Patiño, G.; Salinas-Bravo, V.M. Effect of inorganic inhibitors on the corrosion behavior of 1018 carbon steel in the LiBr + ethylene glycol + H2O mixture. Corros. Sci. 2008, 50, 2296–2303. [Google Scholar] [CrossRef]
- Abd El Aal, E.E.; Abd El Wanees, S.; Farouk, A.; Abd El Haleem, S.M. Factors affecting the corrosion behaviour of aluminium in acid solutions. II. Inorganic additives as corrosion inhibitors for Al in HCl solutions. Corros. Sci. 2013, 68, 14–24. [Google Scholar] [CrossRef]
- Moutarlier, V.; Neveu, B.; Gigandet, M.P. Evolution of corrosion protection for sol–gel coatings doped with inorganic inhibitors. Surf. Coat. Technol. 2008, 202, 2052–2058. [Google Scholar] [CrossRef]
- Mu, G.; Li, X.; Qu, Q.; Zhou, J. Molybdate and tungstate as corrosion inhibitors for cold rolling steel in hydrochloric acid solution. Corros. Sci. 2006, 48, 445–459. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Young, T.; Frankel, G.S. Aluminum Alloy Corrosion Inhibition by Vanadates. J. Electrochem. Soc. 2006, 153, B533. [Google Scholar] [CrossRef]
- Madden, S.B.; Scully, J.R. Inhibition of AA2024-T351 Corrosion Using Permanganate. J. Electrochem. Soc. 2014, 161, C162. [Google Scholar] [CrossRef]
- Seddik, N.B.; Raissouni, I.; Draoui, K.; Aghzzaf, A.A.; Chraka, A.; Aznag, B.; Chaouket, F.; Bouchta, D. Anticorrosive performance of lanthanum ions intercalated Stevensite clay on brass in 3% NaCl medium. Mater. Today Proc. 2020, 22, 78–82. [Google Scholar] [CrossRef]
- Hu, T.; Shi, H.; Wei, T.; Liu, F.; Fan, S.; Han, E.H. Cerium tartrate as a corrosion inhibitor for AA 2024-T3. Corros. Sci. 2015, 95, 152–161. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Lakhlifi, T.; Traisnel, M.; Vezin, H.; Bentiss, F. New 1H-pyrrole-2,5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: Electrochemical, XPS and DFT studies. Corros. Sci. 2015, 90, 572–584. [Google Scholar] [CrossRef]
- Boughoues, Y.; Benamira, M.; Messaadia, L.; Ribouh, N. Adsorption and corrosion inhibition performance of some environmental friendly organic inhibitors for mild steel in HCl solution via experimental and theoretical study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124610. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, S.; Sinha, R.R.; Bahadur, I.; Ebenso, E.E. New pyrimidine derivatives as efficient organic inhibitors on mild steel corrosion in acidic medium: Electrochemical, SEM, EDX, AFM and DFT studies. J. Mol. Liq. 2015, 211, 135–145. [Google Scholar] [CrossRef]
- Obot, I.B.; Ankah, N.K.; Sorour, A.A.; Gasem, Z.M.; Haruna, K. 8-Hydroxyquinoline as an alternative green and sustainable acidizing oilfield corrosion inhibitor. Sustain. Mater. Technol. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Rihan, R.; Shawabkeh, R.; Al-Bakr, N. The Effect of Two Amine-Based Corrosion Inhibitors in Improving the Corrosion Resistance of Carbon Steel in Sea Water. J. Mater. Eng. Perform. 2013, 23, 693–699. [Google Scholar] [CrossRef]
- Quartarone, G.; Bonaldo, L.; Tortato, C. Inhibitive action of indole-5-carboxylic acid towards corrosion of mild steel in deaerated 0.5 M sulfuric acid solutions. Appl. Surf. Sci. 2006, 252, 8251–8257. [Google Scholar] [CrossRef]
- Cen, H.; Cao, J.; Chen, Z.; Guo, X. 2-Mercaptobenzothiazole as a corrosion inhibitor for carbon steel in supercritical CO2-H2O condition. Appl. Surf. Sci. 2019, 476, 422–434. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Montemor, M.F. “SMART” protective ability of water based epoxy coatings loaded with CaCO3 microbeads impregnated with corrosion inhibitors applied on AA2024 substrates. Electrochim. Acta 2012, 83, 439–447. [Google Scholar] [CrossRef]
- Dkhireche, N.; Galai, M.; Ouakki, M.; Rbaa, M.; Ech-chihbi, E.; Lakhrissi, B.; EbnTouhami, M. Electrochemical and theoretical study of newly quinoline derivatives as a corrosion inhibitors adsorption onmild steel in phosphoric acid media. Inorg. Chem. Commun. 2020, 121, 108222. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Cardoso, M.M.; Condeço, J.A.D.; Ferreira, H.E.C.S.; Montemor, M.d. pH-sensitive polymeric particles with increased inhibitor-loading capacity as smart additives for corrosion protective coatings for AA2024. Electrochim. Acta 2014, 145, 123–131. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Pei, C.; Hou, B. Experimental and theoretical investigation of the adsorption behaviour of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim. Acta 2007, 52, 6386–6394. [Google Scholar] [CrossRef]
- Taryba, M.; Lamaka, S.V.; Snihirova, D.; Ferreira, M.G.S.; Montemor, M.F.; Wijting, W.K.; Toews, S.; Grundmeier, G. The combined use of scanning vibrating electrode technique and micro-potentiometry to assess the self-repair processes in defects on “smart” coatings applied to galvanized steel. Electrochim. Acta 2011, 56, 4475–4488. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Michailidis, M.; Bilton, M.; Hobson, T.; Zheng, Z.; Shchukin, D. Tannic complexes coated nanocontainers for controlled release of corrosion inhibitors in self-healing coatings. Electrochim. Acta 2019, 297, 1035–1041. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Han, E.h.; Liang, X.; Wang, G.; Yi, Z.; Li, N. Corrosion resistance of tannic acid, d-limonene and nano-ZrO2 modified epoxy coatings in acid corrosion environments. J. Mater. Sci. Technol. 2021, 65, 137–150. [Google Scholar] [CrossRef]
- Raj, R.; Taryba, M.G.; Morozov, Y.; Kahraman, R.; Shakoor, R.A.; Montemor, M.F. On the synergistic corrosion inhibition and polymer healing effects of polyolefin coatings modified with Ce-loaded hydroxyapatite particles applied on steel. Electrochim. Acta 2021, 388, 138648. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of Cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mater. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Kandori, K.; Oketani, M.; Sakita, Y.; Wakamura, M. FTIR studies on photocatalytic activity of Ti(IV)-doped calcium hydroxyapatite particles. J. Mol. Catal. A Chem. 2012, 360, 54–60. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, J.; Liu, R.; Liu, X. Tannic acid stabilized silver nanoparticles for inkjet printing of conductive flexible electronics. RSC Adv. 2016, 6, 83720–83729. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, R.; Kahraman, R.; Shakoor, A.; Montemor, F.; Taryba, M. Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel. Appl. Sci. 2022, 12, 10263. https://doi.org/10.3390/app122010263
Raj R, Kahraman R, Shakoor A, Montemor F, Taryba M. Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel. Applied Sciences. 2022; 12(20):10263. https://doi.org/10.3390/app122010263
Chicago/Turabian StyleRaj, Roma, Ramazan Kahraman, Abdul Shakoor, Fatima Montemor, and Maryna Taryba. 2022. "Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel" Applied Sciences 12, no. 20: 10263. https://doi.org/10.3390/app122010263
APA StyleRaj, R., Kahraman, R., Shakoor, A., Montemor, F., & Taryba, M. (2022). Tannic Acid-Loaded Hydroxyapatite Carriers for Corrosion Protection of Polyolefin-Coated Carbon Steel. Applied Sciences, 12(20), 10263. https://doi.org/10.3390/app122010263






