1. Introduction
Complications from neurological disorders may leave patients with physical and/or mental impairments which affect their function in daily activities and quality of life. A consequence of these neurological disorders is often physical weakness, both in the upper and lower limbs. Physiotherapy in the acute stage can be less focused on upper-limb rehabilitation [
1] as the use of the lower limbs for mobility is considered of greater importance. Conventional therapy services are resource limited and can be a source of disappointment to participants [
2,
3]. This is a problem for the patients who are discharged from hospital wards and need to continue to undertake rehabilitation.
Since the early 1990s the use of rehabilitation robotics to aid and administer therapy to participants has been developed [
4,
5]. Robots can help a patient to complete a task and have been seen to motivate patients using computerised interfaces. Studies have shown that after robot training participants can improve arm function and ability in Activities of Daily Living (ADL) [
6,
7].
There is a wide range of neurological conditions, but the research described in this paper will focus on adults with stroke. In adulthood, stroke is one of the major causes of disability [
8,
9]. In the UK alone more than 100,000 people have a stroke each year (currently 1.3 million survivors in the UK) [
10] at an estimated cost that exceeds £26 billion per year [
11]. The success of rehabilitation can vary on the type of stroke. After hemispheric infraction (obstruction of blood to the brain) about 75% of survivors report weakness in their affected hand making it difficult to perform ADL [
12]. Rehabilitation plays a large part in the recovery of stroke participants. However, the type of rehabilitation and choice of intervention play an important role in terms of impact on participant outcomes. Conventional therapy generally involves one-on-one interaction between patient and therapist. The therapist assists and encourages the patient through a number of repetitive movements. The therapy aims at reduction of impairment and improvement of functions for ADL [
13].
Novel technologies which assist a person to undertake arm exercise can provide a means of supplementing physical treatments provided through conventional therapy. Increasing the intensity of practice is an important component of recovery, particularly for functionally useful movements [
13,
14]. There are several devices currently being developed with varying degrees of complexity. Many of these require the system to be used and supervised in a clinical or hospital setting. These devices are intended to be used for patients with moderate to severe arm weakness. However, patients with some good residual function could benefit from using devices which are less complex and allow independent use.
There have been a number of rehabilitation robots that have been developed over the last 30 years, and studies have shown that they have their place [
15]. But the devices found in research studies are not suitable for home-based rehabilitation [
16], and there are few commercial offerings available.
With the COVID-19 pandemic, the impact on the NHS and patients was devastating. Across Europe, over 50% of patients in the later stages of recovery were refused in-house therapy [
17]. Although rehabilitation from stroke is focused on many areas [
18], rehabilitation robotics could have played an important factor in home-based rehabilitation. However, the cost–benefit ratio is yet to be explored for robotic therapy en masse. Since 2019, the rehabilitation landscape has changed [
19], and this is an opportunity for robots to make a difference—if the price is right [
20].
In a recent large-scale study with 770 participants called RATLUS [
21], the key findings were that robot therapy is just as useful as conventional therapy but using expensive rehabilitation robotic devices is not a cost-effective solution. The current rehabilitation devices on the market require a therapist to be present, usually in a hospital setting, which reduces the cost-effectiveness of the technology [
22].
There are currently no low-cost commercial devices on the market that allow independent robotic rehabilitation in the home. This paper focuses on the potential for robotic rehabilitation in a home setting, and the potential practices for implementation. There have been a number of recent research studies within the home, particularly inspired by the COVID-19 pandemic, that show there is a place for home-based robotics [
23,
24], and the use of tele-rehabilitation, although it is in its early stages of research [
25,
26,
27].
It has been shown that clinical-based measurements are not accurate enough to pick up small changes but can be combined with kinematic measures for better measurement outcomes [
28,
29,
30]. This paper discusses a number of kinematic measurements that were taken alongside robotic therapy and compared to clinical scales.
Our intervention lasts eight weeks but it has been shown that improvement through rehabilitation can happen over as little as a couple weeks [
31]. The paper will discuss what would happen if we halved the time of the intervention, and if the rehabilitation benefit can be maintained.
2. Materials and Methods
This study was a single centre prospective design involving 16 people recovering from stroke. Participants were recruited from the local stroke services who were over 18 years old, had a diagnosis of ischaemic, or hemorrhagic, stroke at least one month prior, had residual strength of the upper limb, and had enough voluntary movement to initiate movement of the joystick. However, participants were not taken on to the study if they had pain in the arm affecting use of the system, had cognitive impairments affecting understanding and capacity to consent, or were medically unstable (e.g., uncontrolled epilepsy).
The clinical exploratory study evaluates the system in people’s homes across an eight-week period with functional clinical assessments at the start, the end, and after a four-week washout period from the end. The washout period allows assessment of any improvement after the robotic study has finished. Clinical assessment measures include the Fugl Meyer (FM) measure, which evaluates recovery after stoke and is a commonly used measure, the Action Research Arm Test (ARAT) which assesses upper limb function using observational methods, the Chedoke Arm and Hand Activity Inventory (CAHAI) which uses a number of functional tasks to assess recovery, the ABILHAND which is another measure of manual ability for upper limb impairment based on interview questions, and the Motor Assessment Scale (MAS) which is an activity observation scale and the Medical Research Council (MRC) scale for muscle strength. The clinical results for this study are fully presented by Sivan et al. [
32]. Robotic measures were calculated between start and end of the eight weeks, as the device was used by each participant.
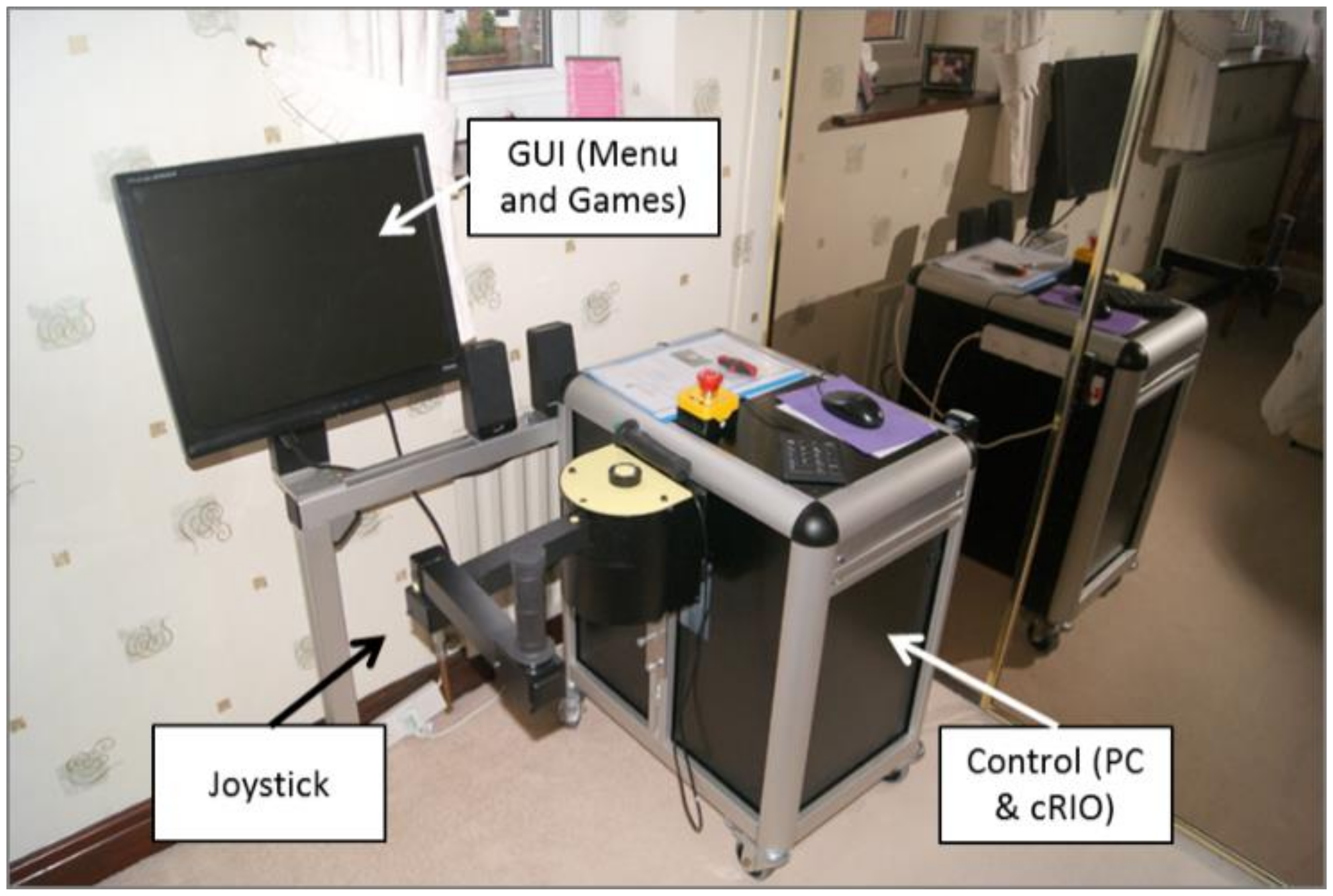
MyPAM (University of Leeds, Leeds, UK) is a bespoke rehabilitation device consisting of a 2D planar robot powered by two DC motors, controlled by National Instruments CompactRIO (National Instruments, Austin, TX, USA) linked to a PC which displays menus and games to the participant.
Figure 1 shows the original MyPAM device in a home setting. MyPAM was built using principles of user centred design and design philosophies such as Ulrich and Eppinger’s six-phase product-development process [
33] where usability, safety, and functionality are essential.
The key features of the MyPAM device are:
2D planar movement over a comfortable workspace, allowing the patient to be assisted in a safe area—the device was designed to be 2D to reduce complexity and reduce cost, but still provide appropriate movement therapy;
Doctor-/therapist-led initial assistance profiles based on clinical assessment to get the user started with the system;
An adaptive algorithm (based on user performance to alter the force profile through the user’s rehabilitation);
Interactive games to engage the user in the rehabilitation; and
All data is stored locally for later analysis, as presented in this paper.
Studies have relied on clinical measures to identify changes in participant improvement, but there are several suggestions that clinical measures are not sensitive enough to capture small changes over a short amount of time [
34]. However, as rehabilitation robotic devices are used movement analysis can be collected and analysed continuously. This raises several questions around capturing the impact of robotic therapy. Can robots be used to deliver therapy that is effective and useful for the user? Can robotic data be used for more or better measurement to inform the efficacy of the therapy being delivered?
The intervention involved 19 participants recruited who were recovering from stroke, however, two participants dropped out due to personal factors and one user didn’t use the system leaving 16 sets of data. Thirteen were male and three female, with an age range of 34 to 81 years old (mean = 56.8, median = 60.7, IQR = 12.04, and SD = 11.2). Seven of the participants have left-sided paralysis and the remaining nine right-sided.
Various assessment methods were evaluated including a “paint-the-screen” task to measure range of motion, a “wave-tracking” task where the participants follow a combination of sine waves as accurately as possible moving back and forth and a “point-to-point pentagram” task, each giving different kinematic measures. During initial evaluation it was shown that many assessment tasks took a lot of time. In order to minimise the time taken to assess, one task was taken forward, the point-to-point pentagram task,
Figure 2. This task also included movement in two directions whilst capturing fine and gross arm motor control. The assessment task is performed without assistance so the user must try to move their arm on their own to collect current movement ability.
Participants were encouraged to play as many sessions as they liked. Each session consisted of a warm-up game for 30 s. This allowed the participant to make sure they were seated comfortably and had a good grip on the joystick. Then a 1 min unassisted assessment exercise where the participant followed a pentagram, which creates standard movement components in a 2D plane. Each component movement is the same length, allowing for greater analysis and comparison of many small movements with users moving from point-to-point on the pentagram and the points appearing in succession for a five-point sequence. Larger movements incorporating gross arm motor control is needed between each point, and fine motor control is needed to hit each target.
The outcome measures available from the pentagram tasks are number of hits per minute, each component movement path length, path length time, and an expression of smoothness measured as normalised jerk [
35]. Path length is an expression of the actual distance travelled, a sum of motion from point to point (p1 to p2, respectively):
This is a useful measure to assess accuracy and movement compared to the intended path length. The path length time is the corresponding time it took to travel the path length, expressed in milliseconds:
Smoothness can be measured as a calculation of normalised jerk, where T = movement time, L = path length, and x = displacement, taken from:
A measure of movement ability of the participant at the start of a session can be used to monitor performance over time, and also within the rehabilitation system for display, activity generation, and assistive force through an adaptive algorithm. The assessment task needs to be engaging, easy to use, and accurate in terms of measuring movement.
From the different outcomes of the pentagram assessment tasks some measures can show more change than others. The hits-per-minute is a suitable measure to show, in a way that can be understood by patients and clinicians alike, how movement is progressing. This score includes a summary of the increase (or decrease) in how fast and smooth the participant can move over time.
The score from the assessment task was used as part of an adaptive algorithm, changing the assistance force profile during gameplay.
3. Results
Each participant has individual characteristics,
Table 1, which identify them into certain groups. Although age seemed to play little part in predicting improvement (younger people do not necessarily improve more than older people), there are lifestyle issues at different stages of life. Some of the younger participants had small children to look after and jobs to attend, whereas some older participants had no work commitments and more free time for sessions with the device.
Two categories can be distinguished from the time since stroke, where participants less than six months can be classed as subacute, and later than this as chronic. The distinction is important, as traditional therapy believes that the best recover is in the subacute phase [
36,
37]. The data suggests that both subacute and chronic participants improved, but with more improvement in hits and normalised jerk in subacute than the chronic group.
The starting level of impairment can show if those who are most affected can improve more or less than those who start with good motor function. The Fugl Meyer (FM) assessment of motor recovery after stroke is chosen as the measure as this is most used in clinics and rehabilitation studies to show the level of impairment [
38]. A low score indicates more impairment, up to a maximum score of 226. However, in this study, the FM score at the start showed little relevance in predicting who would improve.
Taking the assessment scores for each subject and breaking it down into snapshots at the start, middle, and end by taking a three-point average, progression can be seen across the eight weeks period. Furthermore, analysis can be performed on the snapshots to see if there are statistically significant differences between the start, middle, and end of the study,
Figure 3.
The Friedman two-way analysis of variance followed by the post hoc Wilcoxon analysis was performed between the start, middle, and end of the trial. Analysis between the start and the middle, the middle and then end, and the start and the end are checked for significance.
Table 2 shows that there is a significant difference across each test, and the increase in overall hits per minute by each participant shows there is a positive increase. That is to say that there is a significant difference for the group from start to middle, the middle to the end, and, hence, the start to the end. Looking at the change in hits, the first half of the study showed more improvement than the second half with mean of 11.3 ± 6.1 and 3.1 ± 3.5 for the second half.
The normalised jerk values can become very high for jerky movement. The large values are linked to large path length and path length time. The statistical analysis shows there is a significant drop in normalised jerk across the cohort,
Table 2. This is shown across all three tests, indicating that the participants movements become smoother across the study.
The measures of path length and path length time were also analysed, with p-values of 0.001 and 0.000, respectively. Both measures correlate to the inverse of the hits score. The more hits a user gets the shorter the path length and path length time.
The improvement in clinical scores all showed statistical improvement from the start of the intervention to the end. A more detailed discussion on the clinical scores is found in [
32] which shows the median gains for the scores were: FM +1, ARAT +3, CAHAI +5.5, ABILHAND +3, MRC +2, and MAS −1.5 points. The results were consistent after the washout assessment which implied that the participant’s improvements were maintained. Alongside statistical change the minimum clinically important difference (MCID) improvements in all measures were observed in three participants, at least one MCID improvement was seen in eight participants and four participants had no MCID improved change in any measure.
The results for the measures showed that the hits-per-minute score was the best measure to show improvement over time. As the hits improved, the path length dropped. The path length time continued to drop inversely as the hits increased. The normalised jerk was the least coupled to the hits per minute score. For this assessment task it is recommended that the hits per minute and normalised jerk be used as the primary outcomes.
The results for each participant were stored separately on the computer for analysis. Case studies of profiles show how the robotic assessment task was recorded individually,
Figure 4. Although there are improvements across the group, time since stroke shows that there is increased improvement for participants in the early stages. The amount of use of the system also shows increased improvement. Looking at these factors the following case studies show the difference between subacute (less than six months post stroke) and chronic participants, and low and high usage of the system as examples.
Participant ID 5 was an 81-year-old female, who was 4.3 months post stroke (subacute). She played the system for 7.6 h, one of the lowest usages of the subacute group. Participant ID 6 was a 63-year-old male, who was 5 months post stroke (subacute). He played the system for almost 25 h, the longest use of the system. Participant ID 14 was a 67-year-old male, who was 33 months post stroke (chronic). He played the system for 1.5 h. Participant ID 3 was a 61-year-old male, who was 26 months post stroke (chronic). He played the system for over 16 h. These participants were chosen to represent a cross-section of acute and chronic, and low and high usage. The case study data shows any gaps in usage across the days of the study. Participant ID 14 for example did not use the system for a period in the middle of the study, and the improvement is lower than other participants.
One element of consideration is the natural learning curve with the device as the patients start to use it. At the start the users may be getting used to the device and, hence, the improvement is not indicative of rehabilitation progress. To overcome this, when the MyPAM device was installed in the patients home the participants were given training and assistance to overcome the learning effect. Once the participants were happy and could use the device at ease, they were left to continue the trial. In addition, the warmup exercise at the start of each session was used for the participant to get in a comfortable position and ready for the assessment session. Additionally, the clinical improvement was seen to continue into the washout period, suggesting real benefit over learned performance.
4. Discussion
This study has shown increases in clinical and robotic outcomes, both showing statistically significant changes. Rehabilitation robots can be used in the delivery of therapy in aiding current therapy regimes. In this study we showed that robotic therapy can be delivered in people’s homes, safely and effectively. There is little evidence of home-based therapy, and this being one of the first. However, recent studies are tending towards home-based research. The benefits of participants being able to administer their own therapy which fits in their own time schedules allows for improved take-up and retention. Some of the main considerations for a home-based study revolve around the design of the device (size, style, ease of use, appeal, etc.) as well as the functionality of the software, control mechanisms, and assessment of movement ability.
This research attempted to assess and predict in advance which participant groups would benefit from robotic therapy. Once delivered to a participant’s home the point at which maximum benefit has been achieved with the technology will be assessed to see if an alternative treatment could be better applied, with the same device or otherwise. This is particularly important for the economics and viability of rehabilitation robotic devices, where cost-effective usage will influence their viability for health-care providers, businesses, and users alike.
On rehabilitation studies clinical measures are taken as a snapshot at a point in time. This does not take in to account that participants have good days and bad days with their affected limbs. The assessment itself, which can take up to a couple of hours, can cause fatigue. Some of the clinical assessments are questionnaire based and could be done over the phone or in the participant’s home, but some assessment requires a clinical setting due to their nature of the assessments apparatus or expertise of the assessor. All participants in this study came to our rehabilitation laboratory for evaluation.
Collection of the data for the clinical assessment requires a lot of resources—time of a trained professional, equipment, laboratory space, and money (refreshments and travel expenses, for example). Because of these resource implications and the practicality of the available time of the participants it is not possible to continually assess and gather feedback on progress using many clinical measures. This is where the rehabilitation devices could play a part in the collection of assessment data, and, if responsive enough, could complement, or even eliminate, the need for clinical measures.
The four measures explored on the assessment task were hits per minute, path length, path length time, and normalised jerk. It was discovered that the path length and path length time had close correlations to the hits-per-minute score; more hits equal shorter and quicker movement between the points. Normalised jerk was less coupled, showing that faster movement does not necessarily mean smoother movement and, hence, was the second focal measure.
These measures correlate with the clinical data which showed the same statistical improvement across the cohort on the clinical measures, which also showed clinical important change in some participants. The benefit of the robotic measure is that is can be collected continuously as the participant uses the device, and not just snapshots at the start and end of the intervention. This aids the optimum usage of the device, when to collect the device and when to monitor improvement.
In the assessment task the more a participant used the system the more they improved. Implying that as part of a rehabilitation study the motivation of the system is important to maintain usage and improve outcomes. In our system we used bespoke computer games, different levels, and tracking of scores to maintain interest. It was seen that everyone continued to improve in the second half of the study, but that the improvement was less than the first half.
The robotic data shows consistent improvement, with statistical significance found (p < 0.05) for all assessment measures across the cohort. This was further explored with the difference between the first half (four weeks) and second half of the intervention. The eight-week intervention was chosen after consultation with healthcare professionals. We chose to evaluate the data across two halves to see if a difference could be observed at four weeks.
Improvement was seen over the first four weeks and continued to improve statistically for the second half. This study ran for eight weeks, but statistical change was seen within the first four weeks. The benefit of the second four weeks vs the time on intervention can be optimised for maximum efficiency of the rehabilitation device. Interestingly, the normalised jerk seems to plateau as movement improves and there is less improvement in the second half of the study. If most of the improvement has been reached in a short time, then the benefit of leaving the device in the home is diminished. This will allow for more people to use the system and benefit from it. Devices that focus on different muscle groups of movements relevant to the patient’s recovery can then be swapped in for a further short period of intense therapy.
Notably there is a limit that can be reached on the pentagram assessment task, with healthy adults being able to get about a maximum of 60 hits per minute. Each participant will recover at different rates and the rate of improvement could be assessed to gauge the best course of action for each patient and their rehabilitation regime. The constant monitoring of the robotic data allows for this. However, this relies on the participants using the system enough to get the required data to make an assessment. NICE (The National Institute for Health and Care Excellence) guidelines suggest that rehabilitation should be undertaken for at least 45 min for at least five days a week [
39,
40].
Recovery over a short period of time is encouraging. One of the main complications of the robotic therapy is to keep it engaging. Engaging activities, computer games, profiles, and improvement graphs can be used to keep participants involved with the device and take some onus off therapy. The concept of flow becomes important in these aspects [
41,
42]. However, if enough sustained improvement can be administered in a shorter time system designs can be simpler, hence easier and cheaper to produce. Future designs of the system could include real time monitoring of the data and when the participant ceases to improve, or no longer uses the system can be removed from their home for something more appropriate (a modification to the device or alternative therapy strategies). Additionally, if a participant improves so much, they no longer need an assistive device then alternative technologies can be swapped in for passive or resistive devices, or even gaming systems which have been known to be used in rehabilitation.
Improvement was evident in both the subacute and chronic participants, implying that both groups can benefit from robotic therapy. Common theory is that most improvement is seen in the subacute phase of recovery, however, it can be seen that both acute and chronic participants improved.
Age showed a small difference to the outcome, with older people having better improvement on the assessment task than younger people. The lifestyles of the participants may have had an effect on the availability to use the system, with the younger participants having more family and work commitments. Elder participants tended not to work and spent more time at home. One anticipated barrier was the use of technology with an older generation. However, in our study this was not an issue. Our oldest member was an 82-year-old lady who had never used a computer before, but she was seen to have high usage on the study and her family reported benefits to improved vision, cognition, and enjoyment.
The initial Fugl Meyer assessment showed little correlation with the improvement on the assessment task. It was hoped that the measure could be used to identify a group that would recover the most using the system based on an initial clinical evaluation, however this showed no indication. Based on this small group of participants, the MyPAM system can be used by those with mild to severe disability and still show improvement. However, the inclusion criteria for this study made sure that there was at least some residual strength to grip the device handle and move the arm to hit the targets.
In order to reach sustained improvement each participant should perform therapy as often as possible. Data shows that the more a device is used the better the outcomes. This corresponds to conventional therapy thinking and has been shown in other studies [
43]. The minimum viable therapy will depend on each participant, as each stroke is different. The benefit of the continuous assessment with the device means that this is easier to detect and gives therapist more information on what rehabilitation methods to administer.
Because the robotic rehabilitation system is constantly monitoring and measuring participants, therapists can benefit from the data to show non-compliance where the participant is rarely or never using the system, is having minimal effect with no or little improvement, has reached a state where there is no assistance needed or communicate issues outside of the rehabilitation system. This leads on to linking systems to a central source for remote monitoring of patient usage and performance.
It can be seen that rehabilitation robotic devices can not only provide an avenue of therapy but can also be used to optimise the rehabilitation for each patient based on their performance with the assessment task over time. The assessment task has shown to pick up changes in all participants on the study, and the graphs provided are simple to understand and to see changes over time. There is added benefit of presenting the same data to the both the participant and the therapist alike.
The clinical measures have been presented alongside the robotic data. Many clinical measures were presented as part of this study, which came with a number of difficulties for both therapist and participant. Doing multiple clinical measures is difficult to administer. They are taken at snapshots which may not be fully representative of the participant’s current movement ability and improvement. This is not to say that clinical measures do not have a place, but that the evaluation can be optimised through analysis of the robotic data. If there is little improvement over a short time the device can be left longer and monitored over time. This can save resources for therapists and maximise robotic therapy for the participants.
The cost-to-benefit ratio is very important if robotic rehabilitation systems are going to be taken up by health-care professionals. In the UK, the National Health Service (NHS) requires more resource for therapy but does not have the funds to spend lots of money. If the benefits of the therapy can be optimised to show improvement in function and hence activities of daily living (ADL) at reasonable costs, then they are more likely to be taken up.
Also of interest is the translational impact on rehabilitation. Although not scientifically recorded there were anecdotal stories of improvement in other areas of rehabilitation from a number of participants including general improvement of movement other than the arm, improved visual function, and improved speech. This is an interesting area of research that could also be explored in these types of studies [
44,
45].
5. Conclusions
Robotic rehabilitation systems can be used to deliver decent therapy efficiently in patients’ homes. The study showed that devices could be delivered and used over an eight-week period by a number of different participants in different personal situations and different stages of recovery. The added benefit of having the device on hand and no set schedule for use allowed the participants to use the system when it was convenient to use for them. There were, however, different levels of usage. Some participants realised the benefits of committing to using the system, whereas others were distracted by their daily lives of their families and work commitments. The results showed that there was statistical improvement, which also correlated with clinical assessment data. The clinical measures showed improvement both statistically and reaching the thresholds for the minimal clinical important difference (MCID).
In order to maximise the effective delivery of clinical assessment that can be evaluated against robotic measures, the robotic systems can have some clinical questionnaires built in. There are only some measures that can be delivered in this way, and there may still be need for a therapist to evaluate on delivery and collection of the device. This is beneficial for the research stages of device development and clinical assessments could be reduced if there becomes a strong consistent correlation between robotic and clinical scales.
Presentation of the robotic data is taken continuously as the participant uses the system is easy to understand, which makes is a useful measure to show patients improvement. Because this is done little and often, it can be presented to the patient as a means of motivation to continue the rehabilitation regime.
It was seen that more therapy results in greater improvement and greater participant benefit. The more a participant uses the system the more likely they will improve. There are several factors that are important to optimise the efficacy of the system; monitoring of usage for non-compliance allows for encouragement from a therapist or removal of the device, usage and improvement statistics to encourage the user to use the system more and optimised use of technology as the user reaches a plateau of improvement. The more data collected from the systems the better a prediction of the intervention profile can be evaluated. The optimum time for testing of clinically important difference can be predicted, and the devices can be left in the home for a length of time that is relevant to each individual patient.
However, analysis showed the largest improvements are seen within the first weeks of intervention, with less improvement later in this study. For optimal use of the system and benefit to the patients, the device can be removed and given to another patient in need. Short intense periods of robotic rehabilitation on a movement or muscle group may give greater benefits than prolonged therapy regimes. With analysis on the improvement of a patient the rehabilitation robotic device can be optimised for usage vs. benefit. Resources can be removed and replaced at a time that will optimise the benefit to the patient, and also affect the business benefit of using devices where they have most impact. Based on this study we can see a significant change in robotic assessment after several weeks. Although it is unknown if this will create a clinically important difference, the numbers suggest it might. This study shows analysis at eight weeks and four weeks as a comparison, each patient has a different recovery pathway and should be analysed individually. With the use of robotic devices and the Internet of Things (IoT) approach, continuous remote monitoring can be used to assess the optimum time to remove devices. Something our research group is looking in to. Having simple devices that focus on a muscle group or movement can be swapped for another short time to give maximum benefit of the rehabilitation. If research studies are shortened then more people could be given the rehabilitation they need with the robotic devices.
The cost–benefit ratio of using robotic devices has been approached from two standpoints: making devices cheaper so more people can use them, and optimising rehabilitation through monitoring patient improvement. There seems a space for commercial robotic rehabilitation if these elements can be met.
In order to implement the actions in this study our recommendations are that kinematic analysis is considered for evaluation of stroke improvement alongside clinical measures, and that each user of robotic rehabilitation systems are evaluated for effectiveness, thus, optimising the use of rehabilitation robots. Secondarily, the exploration of low-cost devices was introduced and shown to have impact. This is an area for further research and evaluation, but the hope is that low-cost devices will have a greater impact within the UK NHS service. Furthermore, outside the UK there are possibilities to make an impact with low-cost, home-based, and unattended devices, such as in low-economic countries.
Since the study presented in this paper, the MyPAM device has gone through several design iterations based on stakeholder feedback. It is now set to go through further regulatory approval for use in a new trial that will test the efficacy of this paper, alongside remote health-care professional monitoring of the patient in real time.