Non-Targeted Effects of Synchrotron Radiation: Lessons from Experiments at the Australian and European Synchrotrons
Abstract
:Featured Application
Abstract
1. Introduction
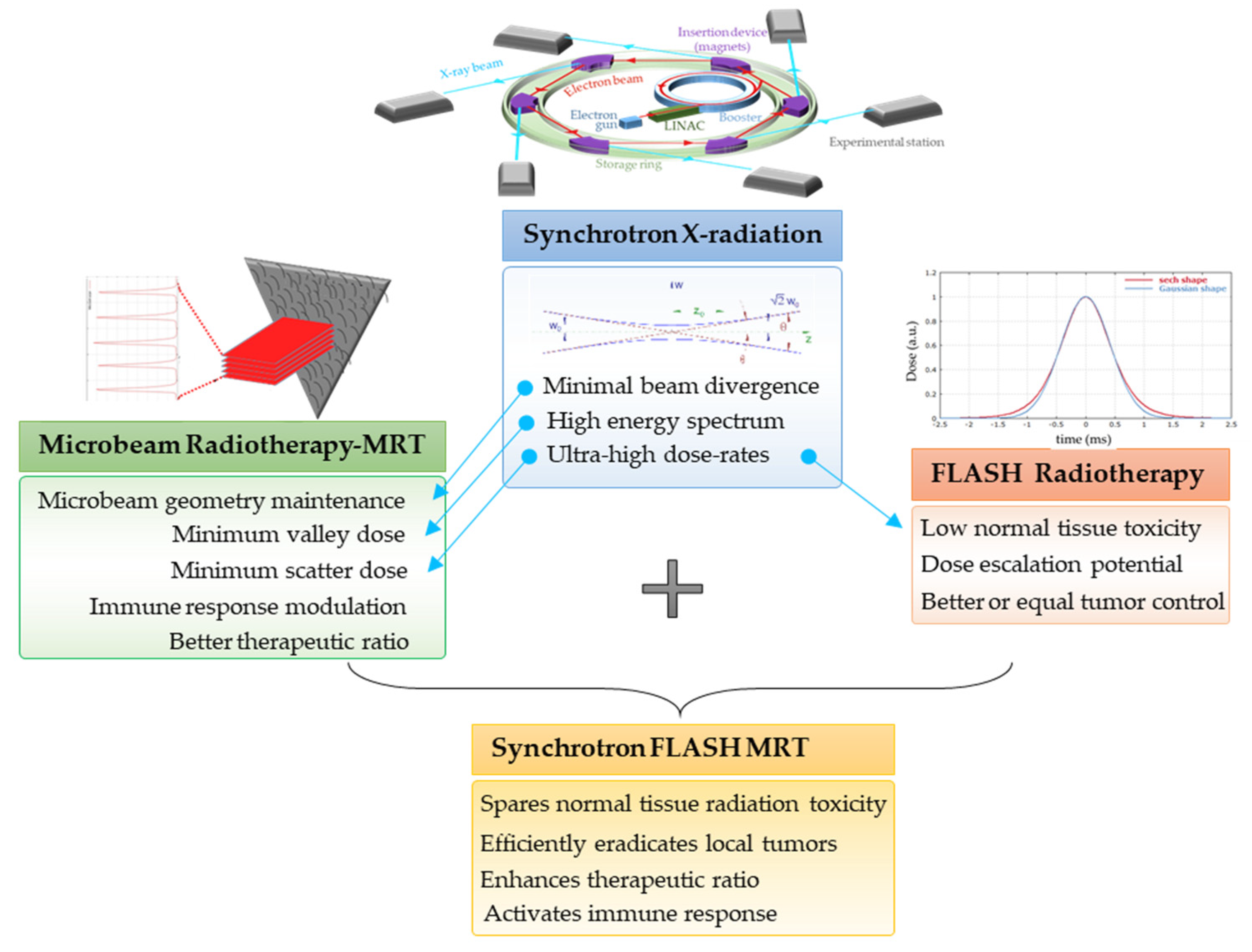
2. Characteristics of Synchrotron X-rays at AS and ESRF
- The high brightness, or brilliance, which describes synchrotron radiation power. Brilliance measures the source quality and implicates the number of photons produced per second. The higher the brilliance value, the stronger the emitted beam.
- The low divergence of the synchrotron beam enables the irradiation of the target with collimated parallel microbeams, in contrast to conventional RT. The low divergence results from the fact that the target is several meters away from the permanent magnet wiggler which generates the X-ray beam. At the AS, the distance between the wiggler and the target is about 32 m, while at the ESRF it is 40 m.
- The beam current, which is the basic quantity of the beam. In this regard, the ESRF’s characteristics are superior to those of the AS. The ESRF has a significantly longer periphery of 844 m, while the AS has 200 m. The brilliance values are 8 × 1020 and 4.6 × 1018 photons/(s × 0.1% bandwidth × mrad2) for the ESRF and AS, respectively [7]. Both have a beam current of 200 mA; however, the maximum electron energy is 6 GeV for ESRF and 3 GeV for AS.
- The intense flux (dose rate) of photons allows samples to be irradiated very quickly in the range of seconds and milliseconds. Dose rates in the AS range from 30–1000 Gy/s, while at the ESRF can be up to 16,000 Gy/s.
- The energy of the synchrotron beam is in the KeV range, which has the advantage of allowing for low secondary electron (Compton) scattering. The energy range at both the AS and ESRF is “tunable”, meaning, the energy spectrum can be filtered to remove low energy photons and use a poly energetic X-ray beam typically between 30 and 120 KeV.
3. Synchrotron-Generated Novel RT Modalities
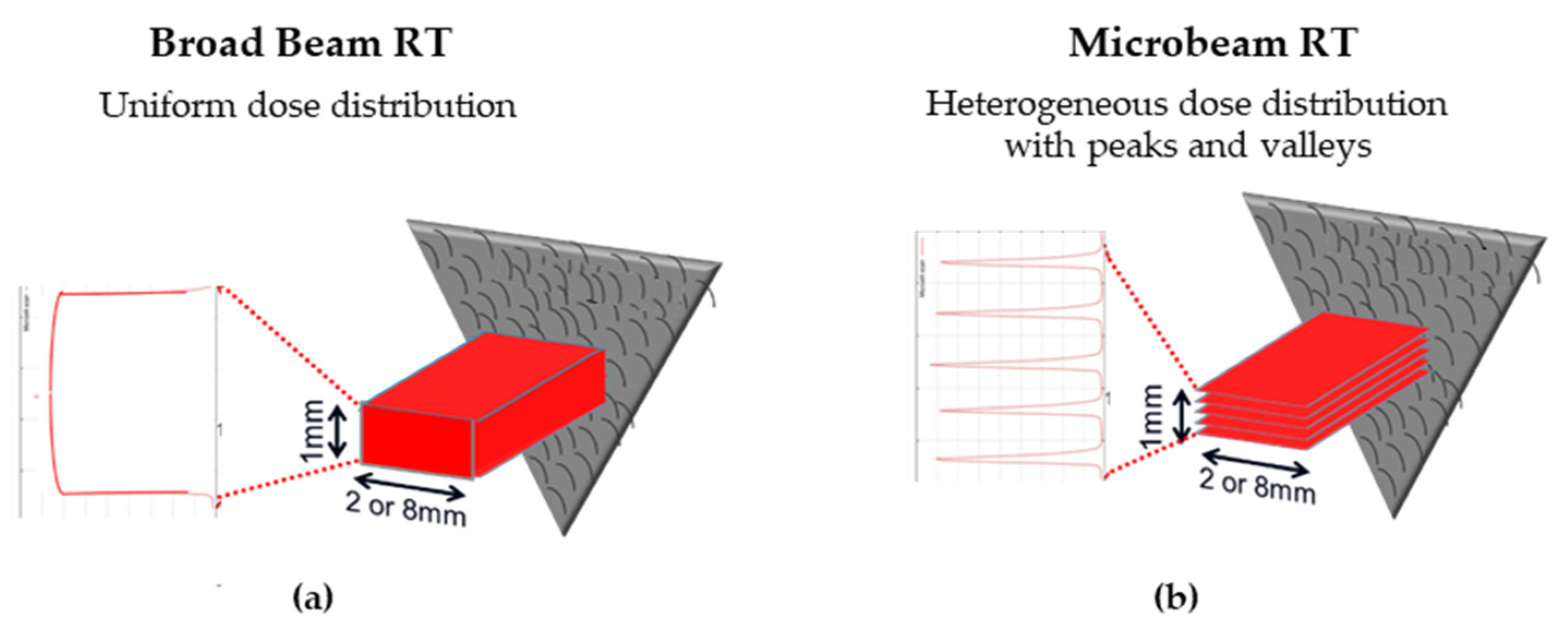
3.1. Microbeam Radiation Therapy (MRT)
- The main clinical research focus at the IMBL, AS, are RT and imaging. Imaging can be performed in an energy range of 15–150 KeV, while radiotherapy can be performed in an energy range of 30–120 KeV and with dose rates close to 1000 Gy/s [10]. The radiation source of MRT is a wiggler with a peak magnetic field of 4.2 T [11]. Starting from a storage ring current of 200 mA, a standard MRT spectral configuration can be delivered in 300 Gy/s by setting the wiggler to 3 T, which produces a spectrum with an average energy of 94 KeV and a peak energy of 87 KeV. Another MRT configuration can deliver 991.7 Gy/s by setting the wiggler to 4 T, with similar spectra as above (93 KeV on average) [12].
- The ESRF’s beamline ID17 is also intended for medical imaging and RT studies. The radiation used for MRT comes from a wiggler of 1.5 m in length and a magnetic field of 1.6 T at a gap of 24.8 mm. The X-ray beam produced by the wiggler then continues for 37 m until it is attenuated to produce one of three different spectral configurations: (i) the standard MRT configuration used for rodent experiments for many years has an average energy of 104.2 KeV and a peak energy of 87.7 keV, (ii) the preclinical MRT configuration developed for veterinary trials and has a mean energy of 119 KeV and a peak energy of 102.1 KeV, and/or (iii) the clinical MRT configuration for future clinical applications with a mean energy of 122.8 KeV and a peak energy of 108.2 KeV [3,13].
3.2. Ultra-High Dose Rate Radiotherapy (FLASH-RT)
3.3. MRT Delivered in a FLASH Mode
- MRT has been proven to be an efficient treatment strategy for several tumor types in animal models, including glioma, glioblastoma, mammary carcinoma, melanoma, squamous cell carcinoma and lung cancer [23].
- Ultra-high doses of MRT beams are likely to induce high DNA damage-generated ‘immunogenic cell death’, as has been suggested for FLASH irradiation [36].
- Similar to the activation of the immune system after heterogenous dose delivery with conventional-source SFRT [37], immune cells in the valleys are spared following MRT and can activate an anti-tumor immune response (manuscript under review). In addition, short-pulse FLASH mode is capable of protecting the majority of local and circulating immune cells [36], thus further contributing to the active recruitment of immune cells to the microbeam paths.
- MRT promotes an anti-tumor immune response that contributes to exceptional killing of the primary tumor [38]. We recently showed that fractionated MRT-induced immunomodulation is associated with a pronounced decrease in metastasis (manuscript under review). The ability of local MRT to trigger immune-mediated, systemic, non-targeted radiation effects can contribute significantly to the future clinical utility of this irradiation modality.
4. Radiation-Induced Bystander and Abscopal Effects
4.1. Studies of RIBE and RIAE and Their Mediators
4.2. First RIBE Studies at Synchrotrons
5. RIBE and RIAE Studies at the AS and ESRF Synchrotron Facilities
5.1. Studies at the AS
5.1.1. In Vitro RIBE Studies at the AS
5.1.2. In Vivo RIAE Studies at the AS
5.2. Studies at the ESRF
5.2.1. In-Vivo RIBE/RIAE Studies at the ESRF
5.2.2. Inter-Animal Communication of RIBE at the ESRF
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANSTO | Australian nuclear science and technology organization |
| AS | Australian synchrotron |
| BB | Broad beam |
| CCL2/MCP1 | Chemokine ligand 2/monocyte chemoattractant protein-1 |
| CCL22 | Chemokine ligand 22 |
| CCR2 | Chemokine ligand 2 receptor |
| CERN | European organization for nuclear research |
| CNS | central nervous system |
| COX2 | Cyclooxygenase-2 |
| CSF1R | Colony-stimulating factor-1receptor |
| CT | Computer tomography |
| DC | Dendritic cell |
| DLD | Dihydrolipoyl dehydrogenase |
| DSB | Double-strand break |
| DNA | Deoxyribonucleic acid |
| ESRF | European synchrotron radiation facility |
| FBA | Fructose bisphosphate aldolase |
| FLASH | Ultra-high dose rate radiotherapy |
| FT-IR | Fourier-transform infrared microscopy |
| Gamma (γ)-H2AX | phosporylated histone H2AX |
| GRID RT | Type of SFRT |
| Gy | Gray (unit of ionising radiation dose) |
| HRS | Hyper radiosensitivity |
| HSP-71 | Heat shock protein 71 |
| ID17 | ESRF biomedical beamline |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IMBL | Imaging and medical beamline |
| IR | Ionising radiation |
| IRR | Increased radioresistance |
| KeV, MeV, GeV | kiloelectron volts, megaelectron volts, gigaelectron volts (a unit of energy) |
| LHC | Large hadron collider |
| LINAC | Linear accelerator |
| MeJA | Methyl jasmonate |
| MeSA | Methyl salicylate |
| MDM2 | mouse double minute 2 homolog |
| MISTRAL | Full-field transmission X-ray microscopy beamline |
| MRT | Microbeam radiotherapy |
| NAD (P)H oxidase | nicotinamide adenine dinucleotide phosphate oxidase |
| NK | natural killer cells |
| NSG | NOD SCID gamma |
| NSLS | National synchrotron light source |
| OCDL | Oxidative clustered DNA lesion |
| PB | Pencil beam |
| PSICHE | Pressure, structure and imaging by contrast at high energy beamline |
| Ptch1 | Patched 1 |
| RIAE | Radiation induced abscopal effect |
| RIBE | Radiation induced bystander effect |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SCC | Squamous cell carcinoma |
| SFRT | spatially fractionated radiotherapy |
| SYRMEP | Synchrotron radiation for medical physics beamline |
| TAM | Tissue-associated macrophages |
| TGFβ | Tumour growth factor β |
| TGFβR1 | Tumour growth factor β receptor 1 |
| TIMP1 | Tissue inhibitor matrix metalloproteinase 1 |
| TNF-α | Tumor necrosis factor-α |
| TP53 | Tumor protein 53 |
| TPI | Triosephosphate isomerase |
| UVC | Ultraviolet C |
| VEGF | Vascular endothelial growth factor |
| WT | wild type |
| XRD-CT | X-ray diffraction tomography |
References
- Pełka, J. Synchrotron radiation in biology and medicine. Acta Phys. Pol. A 2008, 2, 309–329. [Google Scholar] [CrossRef]
- Stevenson, A.W.; Crosbie, J.C.; Hall, C.J.; Hausermann, D.; Livingstone, J.; Lye, J.E. Quantitative characterization of the X-ray beam at the Australian Synchrotron Imaging and Medical Beamline (IMBL). J. Synchrotron Radiat. 2017, 24, 110–141. [Google Scholar] [CrossRef] [PubMed]
- Pellicioli, P.; Donzelli, M.; Davis, J.A.; Esteve, F.; Hugtenburg, R.; Guatelli, S.; Petasecca, M.; Lerch, M.L.F.; Brauer-Krisch, E.; Krisch, M. Study of the X-ray radiation interaction with a multislit collimator for the creation of microbeams in radiation therapy. J. Synchrotron Radiat. 2021, 28, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Rai, A.; Sharma, S. Role of synchrotron radiation in cancer: A review on techniques and applications. Engl. J. Anal. Pharm. Res. 2018, 7, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [Green Version]
- Smyth, L.M.; Senthi, S.; Crosbie, J.C.; Rogers, P.A. The normal tissue effects of microbeam radiotherapy: What do we know, and what do we need to know to plan a human clinical trial? Int. J. Radiat. Biol. 2016, 92, 302–311. [Google Scholar] [CrossRef]
- Bharti, A.; Goyal, N. Fundamental of Synchrotron Radiations. In Synchrotron Radiation-Useful and Interesting Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Laissue, J.A.; Geiser, G.; Spanne, P.O.; Dilmanian, F.A.; Gebbers, J.O.; Geiser, M.; Wu, X.Y.; Makar, M.S.; Micca, P.L.; Nawrocky, M.M.; et al. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated X rays. Int. J. Cancer 1998, 78, 654–660. [Google Scholar] [CrossRef]
- Sprung, C.N.; Cholewa, M.; Usami, N.; Kobayashi, K.; Crosbie, J.C. DNA damage and repair kinetics after microbeam radiation therapy emulation in living cells using monoenergetic synchrotron X-ray microbeams. J. Synchrotron Radiat. 2011, 18, 630–636. [Google Scholar] [CrossRef]
- Hausermann, D.; Hall, C.; Maksimenko, A.; Campbell, C. The Imaging and Medical Beam Line at the Australian Synchrotron. AIP Conf. Proc. 2010, 1266, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, J.; Adam, J.-F.; Crosbie, J.C.; Hall, C.J.; Lye, J.E.; McKinlay, J.; Pelliccia, D.; Pouzoulet, F.; Prezado, Y.; Stevenson, A.W. Preclinical radiotherapy at the Australian Synchrotron’s Imaging and Medical Beamline: Instrumentation, dosimetry and a small-animal feasibility study. J. Synchrotron Radiat. 2017, 24, 854–865. [Google Scholar] [CrossRef]
- Trappetti, V.; Fernandez-Palomo, C.; Smyth, L.; Klein, M.; Haberthur, D.; Butler, D.; Barnes, M.; Shintani, N.; de Veer, M.; Laissue, J.A.; et al. Synchrotron Microbeam Radiotherapy for the treatment of lung carcinoma: A pre-clinical study. Int. J. Radiat. Oncol Biol. Phys. 2021. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Fournier, P.; Bartzsch, S.; Donzelli, M.; Cornelius, I.; Stevenson, A.W.; Requardt, H.; Brauer-Krisch, E. Energy spectra considerations for synchrotron radiotherapy trials on the ID17 bio-medical beamline at the European Synchrotron Radiation Facility. J. Synchrotron Radiat. 2015, 22, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.; Donzelli, M.; Pellicioli, P.; Brauer-Krisch, E.; Davis, J.A.; Lerch, M.L.F.; Rosenfeld, A.B.; Petasecca, M. First experimental measurement of the effect of cardio-synchronous brain motion on the dose distribution during microbeam radiation therapy. Med. Phys. 2020, 47, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Goncalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, R.M. FLASH radiotherapy: Ultra-high dose rates to spare healthy tissue. Int. J. Radiat. Biol. 2020, 96, 419–423. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupe, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra293. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Eling, L.; Bouchet, A.; Nemoz, C.; Djonov, V.; Balosso, J.; Laissue, J.; Bräuer-Krisch, E.; Adam, J.F.; Serduc, R. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiother. Oncol. 2019, 139, 56–61. [Google Scholar] [CrossRef]
- Smyth, L.M.L.; Donoghue, J.F.; Ventura, J.A.; Livingstone, J.; Bailey, T.; Day, L.R.J.; Crosbie, J.C.; Rogers, P.A.W. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 2018, 8, 12044. [Google Scholar] [CrossRef]
- Schultke, E.; Balosso, J.; Breslin, T.; Cavaletti, G.; Djonov, V.; Esteve, F.; Grotzer, M.; Hildebrandt, G.; Valdman, A.; Laissue, J. Microbeam radiation therapy—Grid therapy and beyond: A clinical perspective. Br. J. Radiol. 2017, 90, 20170073. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Fazzari, J.; Trappetti, V.; Smyth, L.; Janka, H.; Laissue, J.; Djonov, V. Animal Models in Microbeam Radiation Therapy: A Scoping Review. Cancers 2020, 12, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilmanian, F.A.; Qu, Y.; Feinendegen, L.E.; Pena, L.A.; Bacarian, T.; Henn, F.A.; Kalef-Ezra, J.; Liu, S.; Zhong, Z.; McDonald, J.W. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: Is a bystander effect involved? Exp. Hematol. 2007, 35, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Potez, M.; Fernandez-Palomo, C.; Bouchet, A.; Trappetti, V.; Donzelli, M.; Krisch, M.; Laissue, J.; Volarevic, V.; Djonov, V. Synchrotron Microbeam Radiation Therapy as a New Approach for the Treatment of Radioresistant Melanoma: Potential Underlying Mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1126–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Palomo, C.; Trappetti, V.; Potez, M.; Pellicioli, P.; Krisch, M.; Laissue, J.; Djonov, V. Complete Remission of Mouse Melanoma after Temporally Fractionated Microbeam Radiotherapy. Cancers 2020, 12, 2656. [Google Scholar] [CrossRef]
- Wright, M.D. Microbeam radiosurgery: An industrial perspective. Phys. Med. 2015, 31, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Griffin, R.J.; Ahmed, M.M.; Amendola, B.; Belyakov, O.; Bentzen, S.M.; Butterworth, K.T.; Chang, S.; Coleman, C.N.; Djonov, V.; Formenti, S.C.; et al. Understanding High-Dose, Ultra-High Dose-Rate and, Spatially Fractionated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 766–778. [Google Scholar] [CrossRef]
- Crosbie, J.C.; Anderson, R.L.; Rothkamm, K.; Restall, C.M.; Cann, L.; Ruwanpura, S.; Meachem, S.; Yagi, N.; Svalbe, I.; Lewis, R.A.; et al. Tumor cell response to synchrotron microbeam radiation therapy differs markedly from cells in normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 886–894. [Google Scholar] [CrossRef]
- Serduc, R.; Brauer-Krisch, E.; Bouchet, A.; Renaud, L.; Brochard, T.; Bravin, A.; Laissue, J.A.; Le Duc, G. First trial of spatial and temporal fractionations of the delivered dose using synchrotron microbeam radiation therapy. J. Synchrotron Radiat. 2009, 16, 587–590. [Google Scholar] [CrossRef]
- Uyama, A.; Kondoh, T.; Nariyama, N.; Umetani, K.; Fukumoto, M.; Shinohara, K.; Kohmura, E. A narrow microbeam is more effective for tumor growth suppression than a wide microbeam: An in vivo study using implanted human glioma cells. J. Synchrotron Radiat. 2011, 18, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Sabatasso, S.; Laissue, J.A.; Hlushchuk, R.; Graber, W.; Bravin, A.; Brauer-Krisch, E.; Corde, S.; Blattmann, H.; Gruber, G.; Djonov, V. Microbeam radiation-induced tissue damage depends on the stage of vascular maturation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1522–1532. [Google Scholar] [CrossRef]
- Bronnimann, D.; Bouchet, A.; Schneider, C.; Potez, M.; Serduc, R.; Brauer-Krisch, E.; Graber, W.; von Gunten, S.; Laissue, J.A.; Djonov, V. Synchrotron microbeam irradiation induces neutrophil infiltration, thrombocyte attachment and selective vascular damage in vivo. Sci. Rep. 2016, 6, 33601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchet, A.; Lemasson, B.; Christen, T.; Potez, M.; Rome, C.; Coquery, N.; Le Clec’h, C.; Moisan, A.; Brauer-Krisch, E.; Leduc, G.; et al. Synchrotron microbeam radiation therapy induces hypoxia in intracerebral gliosarcoma but not in the normal brain. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 108, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kanagavelu, S.; Gupta, S.; Wu, X.; Philip, S.; Wattenberg, M.M.; Hodge, J.W.; Couto, M.D.; Chung, K.D.; Ahmed, M.M. In vivo effects of lattice radiation therapy on local and distant lung cancer: Potential role of immunomodulation. Radiat. Res. 2014, 182, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Trappetti, V.; Fazzari, J.M.; Fernandez-Palomo, C.; Scheidegger, M.; Volarevic, V.; Martin, O.A.; Djonov, V.G. Microbeam Radiotherapy-A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int. J. Mol. Sci. 2021, 22, 7755. [Google Scholar] [CrossRef]
- Hall, E.J. The bystander effect. Health Phys. 2003, 85, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997, 71, 421–427. [Google Scholar] [CrossRef]
- Azzam, E.I.; de Toledo, S.M.; Gooding, T.; Little, J.B. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998, 150, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- Mothersill, C.; Seymour, C. Radiation-induced non-targeted effects: Some open questions. Radiat. Prot. Dosim. 2015, 166, 125–130. [Google Scholar] [CrossRef]
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Prise, K.M.; Little, M.P. Non-targeted effects of ionising radiation—Implications for low dose risk. Mutat. Res. 2013, 752, 84–98. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.B.; McNeill, F.E.; Byun, S.H.; Prestwich, W.V.; Mothersill, C.; Seymour, C.; Armstrong, A.; Fernandez, C. Ultra-Violet Light Emission from HPV-G Cells Irradiated with Low Let Radiation From 90Y; Consequences for Radiation Induced Bystander Effects. Dose Response 2013, 11, 498–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, M.; McNeill, F.E.; Seymour, C.; Rainbow, A.J.; Mothersill, C.E. An observed effect of ultraviolet radiation emitted from beta-irradiated HaCaT cells upon non-beta-irradiated bystander cells. Radiat. Res. 2015, 183, 279–290. [Google Scholar] [CrossRef]
- Sprung, C.N.; Ivashkevich, A.; Forrester, H.B.; Redon, C.E.; Georgakilas, A.; Martin, O.A. Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Lett. 2015, 356, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. gammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Sokolov, M.V.; Smilenov, L.B.; Hall, E.J.; Panyutin, I.G.; Bonner, W.M.; Sedelnikova, O.A. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene 2005, 24, 7257–7265. [Google Scholar] [CrossRef] [Green Version]
- Sedelnikova, O.A.; Nakamura, A.; Kovalchuk, O.; Koturbash, I.; Mitchell, S.A.; Marino, S.A.; Brenner, D.J.; Bonner, W.M. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007, 67, 4295–4302. [Google Scholar] [CrossRef] [Green Version]
- Dickey, J.S.; Baird, B.J.; Redon, C.E.; Sokolov, M.V.; Sedelnikova, O.A.; Bonner, W.M. Intercellular communication of cellular stress monitored by gamma-H2AX induction. Carcinogenesis 2009, 30, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation-induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, V.N.; Zhou, H.; Ghandhi, S.A.; Karasic, T.B.; Yaghoubian, B.; Amundson, S.A.; Hei, T.K. Radiation-induced bystander signaling pathways in human fibroblasts: A role for interleukin-33 in the signal transmission. Cell Signal. 2010, 22, 1076–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickey, J.S.; Baird, B.J.; Redon, C.E.; Avdoshina, V.; Palchik, G.; Wu, J.; Kondratyev, A.; Bonner, W.M.; Martin, O.A. Susceptibility to bystander DNA damage is influenced by replication and transcriptional activity. Nucleic Acids Res. 2012, 40, 10274–10286. [Google Scholar] [CrossRef]
- Narayanan, P.K.; LaRue, K.E.; Goodwin, E.H.; Lehnert, B.E. Alpha particles induce the production of interleukin-8 by human cells. Radiat. Res. 1999, 152, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene 2003, 22, 7050–7057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA 2001, 98, 473–478. [Google Scholar] [CrossRef]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets 2004, 4, 53–64. [Google Scholar] [CrossRef]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar]
- Shao, C.; Furusawa, Y.; Aoki, M.; Matsumoto, H.; Ando, K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002, 78, 837–844. [Google Scholar] [CrossRef]
- Shao, C.; Stewart, V.; Folkard, M.; Michael, B.D.; Prise, K.M. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003, 63, 8437–8442. [Google Scholar]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Ohnishi, K.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Kano, E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001, 155, 387–396. [Google Scholar] [CrossRef]
- Zhou, H.; Ivanov, V.N.; Gillespie, J.; Geard, C.R.; Amundson, S.A.; Brenner, D.J.; Yu, Z.; Lieberman, H.B.; Hei, T.K. Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14641–14646. [Google Scholar] [CrossRef] [Green Version]
- Poon, R.C.; Agnihotri, N.; Seymour, C.; Mothersill, C. Bystander effects of ionizing radiation can be modulated by signaling amines. Env. Res. 2007, 105, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, J.; Mersov, A.; Smith, R.; Seymour, C.; Mothersill, C. Effect of 5-hydroxytryptamine (serotonin) receptor inhibitors on the radiation-induced bystander effect. Int. J. Radiat. Biol. 2012, 88, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Desplanques, M.; Jella, K.K.; Garcia, A.; McClean, B. The importance of serum serotonin levels in the measurement of radiation-induced bystander cell death in HaCaT cells. Int. J. Radiat. Biol. 2012, 88, 770–772. [Google Scholar] [CrossRef] [Green Version]
- Mothersill, C.; Saroya, R.; Smith, R.W.; Singh, H.; Seymour, C.B. Serum serotonin levels determine the magnitude and type of bystander effects in medium transfer experiments. Radiat. Res. 2010, 174, 119–123. [Google Scholar] [CrossRef]
- Saroya, R.; Smith, R.; Seymour, C.; Mothersill, C. Injection of resperpine into zebrafish, prevents fish to fish communication of radiation-induced bystander signals: Confirmation in vivo of a role for serotonin in the mechanism. Dose Response 2009, 8, 317–330. [Google Scholar] [CrossRef]
- Al-Mayah, A.H.; Irons, S.L.; Pink, R.C.; Carter, D.R.; Kadhim, M.A. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat. Res. 2012, 177, 539–545. [Google Scholar] [CrossRef]
- Jella, K.K.; Rani, S.; O’Driscoll, L.; McClean, B.; Byrne, H.J.; Lyng, F.M. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Xu, S.; Xu, S.; Wu, L.; Wu, Y.; Bian, P. Radiation-induced epigenetic bystander effects demonstrated in Arabidopsis thaliana. Radiat. Res. 2015, 183, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Raaphorst, G.P.; Mitchel, R.E. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2 cells. Radiat. Res. 1996, 146, 369–373. [Google Scholar] [CrossRef]
- Mothersill, C.; Seymour, C. Radiation-induced bystander effects: Evidence for an adaptive response to low dose exposures? Dose Response 2006, 4, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Takahashi, A.; Ohnishi, T. Radiation-induced adaptive responses and bystander effects. Biol. Sci. Space 2004, 18, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Rugo, R.E.; Hendricks, C.A.; Loree, J.; Thibault, B.; Kutanzi, K.; Pogribny, I.; Yanch, J.C.; Engelward, B.P.; Kovalchuk, O. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene 2006, 25, 4267–4275. [Google Scholar] [CrossRef] [Green Version]
- Koturbash, I.; Loree, J.; Kutanzi, K.; Koganow, C.; Pogribny, I.; Kovalchuk, O. In vivo bystander effect: Cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 554–562. [Google Scholar] [CrossRef]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; Di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450. [Google Scholar] [CrossRef] [Green Version]
- Dubrova, Y.E. Radiation-induced transgenerational instability. Oncogene 2003, 22, 7087–7093. [Google Scholar] [CrossRef] [Green Version]
- Tamminga, J.; Koturbash, I.; Baker, M.; Kutanzi, K.; Kathiria, P.; Pogribny, I.P.; Sutherland, R.J.; Kovalchuk, O. Paternal cranial irradiation induces distant bystander DNA damage in the germline and leads to epigenetic alterations in the offspring. Cell Cycle 2008, 7, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; MacManus, M.P.; Martin, R.F.; Martin, O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015, 356, 82–90. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.C.; Benjamin, K.T.; Formenti, S.C. Generating antitumor immunity by targeted radiation therapy: Role of dose and fractionation. Adv. Radiat. Oncol. 2018, 3, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surinov, B.P.; Isaeva, V.G.; Karpova, N.A. Post-radiation communicative induction of blood and immunity disorders. Patol. Fiziol. Eksp. Ter. 1998, 3, 7–10. [Google Scholar]
- Mothersill, C.; Bucking, C.; Smith, R.W.; Agnihotri, N.; Oneill, A.; Kilemade, M.; Seymour, C.B. Communication of radiation-induced stress or bystander signals between fish in vivo. Env. Sci. Technol. 2006, 40, 6859–6864. [Google Scholar] [CrossRef]
- Mothersill, C.; Smith, R.W.; Agnihotri, N.; Seymour, C.B. Characterization of a radiation-induced stress response communicated in vivo between zebrafish. Env. Sci. Technol. 2007, 41, 3382–3387. [Google Scholar] [CrossRef]
- Smith, R.W.; Wang, J.; Bucking, C.P.; Mothersill, C.E.; Seymour, C.B. Evidence for a protective response by the gill proteome of rainbow trout exposed to X-ray induced bystander signals. Proteomics 2007, 7, 4171–4180. [Google Scholar] [CrossRef]
- Mothersill, C.; Smith, R.W.; Saroya, R.; Denbeigh, J.; Rowe, B.; Banevicius, L.; Timmins, R.; Moccia, R.; Seymour, C.B. Irradiation of rainbow trout at early life stages results in legacy effects in adults. Int. J. Radiat. Biol. 2010, 86, 817–828. [Google Scholar] [CrossRef]
- Zhong, N.; Morris, G.M.; Bacarian, T.; Rosen, E.M.; Avraham Dilmanian, F. Response of rat skin to high-dose unidirectional X-ray microbeams: A histological study. Radiat. Res. 2003, 160, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, H.; Blattmann, H.; Bräuer-Krisch, E.; Bravin, A.; Di Michiel, M.; Gebbers, J.-O.; Hanson, A.; Lyubimova, N.; Slatkin, D.; Stepanek, J. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J. Neuro-Oncol. 2006, 78, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kashino, G.; Kondoh, T.; Nariyama, N.; Umetani, K.; Ohigashi, T.; Shinohara, K.; Kurihara, A.; Fukumoto, M.; Tanaka, H.; Maruhashi, A.; et al. Induction of DNA double-strand breaks and cellular migration through bystander effects in cells irradiated with the slit-type microplanar beam of the spring-8 synchrotron. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 229–236. [Google Scholar] [CrossRef]
- Tomita, M.; Maeda, M.; Maezawa, H.; Usami, N.; Kobayashi, K. Bystander cell killing in normal human fibroblasts is induced by synchrotron X-ray microbeams. Radiat. Res. 2010, 173, 380–385. [Google Scholar] [CrossRef]
- Maeda, M.; Tomita, M.; Usami, N.; Kobayashi, K. Bystander cell death is modified by sites of energy deposition within cells irradiated with a synchrotron X-ray microbeam. Radiat. Res. 2010, 174, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lobachevsky, P.; Ivashkevich, A.; Forrester, H.B.; Stevenson, A.W.; Hall, C.J.; Sprung, C.N.; Martin, O.A. Assessment and Implications of Scattered Microbeam and Broadbeam Synchrotron Radiation for Bystander Effect Studies. Radiat. Res. 2015, 184, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Lobachevsky, P.; Forrester, H.B.; Ivashkevich, A.; Mason, J.; Stevenson, A.W.; Hall, C.J.; Sprung, C.N.; Djonov, V.G.; Martin, O.A. Synchrotron X-ray Radiatio.on-Induced Bystander Effect: An Impact of the Scattered Radiation, Distance From the Irradiated Site and p53 Cell Status. Front. Oncol. 2021, 11, 685598. [Google Scholar] [CrossRef]
- Ventura, J.A.; Donoghue, J.F.; Nowell, C.J.; Cann, L.M.; Day, L.R.J.; Smyth, L.M.L.; Forrester, H.B.; Rogers, P.A.W.; Crosbie, J.C. The gammaH2AX DSB marker may not be a suitable biodosimeter to measure the biological MRT valley dose. Int. J. Radiat. Biol. 2021, 97, 642–656. [Google Scholar] [CrossRef]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Speidel, D. The role of DNA damage responses in p53 biology. Arch. Toxicol. 2015, 89, 501–517. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb Perspect Med. 2016, 6, a026070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, J.; Lobachevsky, P.N.; Palazzolo, J.S.; Forrester, H.; Haynes, N.M.; Ivashkevich, A.; Stevenson, A.W.; Hall, C.J.; Ntargaras, A.; Kotsaris, V.; et al. Localized Synchrotron Irradiation of Mouse Skin Induces Persistent Systemic Genotoxic and Immune Responses. Cancer Res. 2017, 77, 6389–6399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Kareva, I.G.; Naf, D.; Nowsheen, S.; Kryston, T.B.; Bonner, W.M.; Georgakilas, A.G.; Sedelnikova, O.A. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 17992–17997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobachevsky, P.N.; Ventura, J.; Giannakandropoulou, L.; Forrester, H.; Palazzolo, J.S.; Haynes, N.M.; Stevenson, A.W.; Hall, C.J.; Mason, J.; Pollakis, G.; et al. A Functional Immune System Is Required for the Systemic Genotoxic Effects of Localized Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1184–1193. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Conti, I.; Rollins, B.J. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin. Cancer Biol. 2004, 14, 149–154. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Kapoor, V.; Buchlis, G.; Cheng, G.; Sun, J.; Wang, L.C.; Singhal, S.; Snyder, L.A.; Albelda, S.M. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Q.; Fei, T.; Han, J.D.; Chen, Y.G. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood 2007, 109, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Martin, O.A.; Martin, R.F. Cancer Radiotherapy: Understanding the Price of Tumor Eradication. Front. Cell Dev. Biol. 2020, 8, 261. [Google Scholar] [CrossRef]
- Forrester, H.B.; Lobachevsky, P.N.; Stevenson, A.W.; Hall, C.J.; Martin, O.A.; Sprung, C.N. Abscopal Gene Expression in Response to Synchrotron Radiation Indicates a Role for Immunological and DNA Damage Response Genes. Radiat. Res. 2020, 194, 678–687. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palomo, C.; Schültke, E.; Smith, R.; Bräuer-Krisch, E.; Laissue, J.; Schroll, C.; Fazzari, J.; Seymour, C.; Mothersill, C. Bystander effects in tumor-free and tumor-bearing rat brains following irradiation by synchrotron X-rays. Int. J. Radiat. Biol. 2013, 89, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Wang, J.; Schültke, E.; Seymour, C.B.; Bräuer-Krisch, E.; Laissue, J.A.; Blattmann, H.; Mothersill, C.E. Proteomic changes in the rat brain induced by homogenous irradiation and by the bystander effect resulting from high energy synchrotron X-ray microbeams. Int. J. Radiat. Biol. 2013, 89, 118–127. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Schültke, E.; Bräuer-Krisch, E.; Laissue, J.A.; Blattmann, H.; Seymour, C.; Mothersill, C. Investigation of abscopal and bystander effects in immunocompromised mice after exposure to pencilbeam and microbeam synchrotron radiation. Health Phys. 2016, 111, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Palomo, C.; Mothersill, C.; Bräuer-Krisch, E.; Laissue, J.; Seymour, C.; Schültke, E. γ-H2AX as a marker for dose deposition in the brain of wistar rats after synchrotron microbeam radiation. PLoS ONE 2015, 10, e0119924. [Google Scholar]
- Surinov, B.P.; Isaeva, V.G.; Dukhova, N.N. Postirradiation volatile secretions of mice: Syngeneic and allogeneic immune and behavioral effects. Bull. Exp. Biol. Med. 2004, 138, 384–386. [Google Scholar] [CrossRef]
- Zalcman, S.; Kerr, L.; Anisman, H. Immunosuppression elicited by stressors and stressor-related odors. Brain Behav. Immun. 1991, 5, 262–273. [Google Scholar] [CrossRef]
- Yao, Y.; Danna, C.H.; Ausubel, F.M.; Kovalchuk, I. Perception of volatiles produced by UVC-irradiated plants alters the response to viral infection in naïve neighboring plants. Plant. Signal. Behav. 2012, 7, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Wang, J.; Seymour, C.; Fernandez-Palomo, C.; Fazzari, J.; Schültke, E.; Bräuer-Krisch, E.; Laissue, J.; Schroll, C.; Mothersill, C. Homogenous and microbeam X-ray radiation induces proteomic changes in the brains of irradiated rats and in the brains of nonirradiated cage mate rats. Dose-Response 2018, 16, 1559325817750068. [Google Scholar] [CrossRef] [Green Version]
- Dullin, C.; di Lillo, F.; Svetlove, A.; Albers, J.; Wagner, W.; Markus, A.; Sodini, N.; Dreossi, D.; Alves, F.; Tromba, G. Multiscale biomedical imaging at the SYRMEP beamline of Elettra—Closing the gap between preclinical research and patient applications. Phys. Open 2021, 6, 100050. [Google Scholar] [CrossRef]
- King, A.; Guignot, N.; Zerbino, P.; Boulard, E.; Desjardins, K.; Bordessoule, M.; Leclerq, N.; Le, S.; Renaud, G.; Cerato, M. Tomography and imaging at the PSICHE beam line of the SOLEIL synchrotron. Rev. Sci. Instrum. 2016, 87, 093704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saslow, W.M. Chapter 10—How Electric Currents Interact with Magnetic Fields. In Electricity, Magnetism, and Light; Saslow, W.M., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 419–459. [Google Scholar] [CrossRef]
- Nave, R. Cyclotron Frequency. Available online: http://hyperphysics.phy-astr.gsu.edu/hbase/magnetic/cyclot.html (accessed on 1 February 2022).
- L’Annunziata, M.F. 8.7. Synchrotron Radiation. In Radioactivity, 2nd ed.; L’Annunziata, M.F., Ed.; Elsevier: Boston, MA, USA, 2016; pp. 269–302. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Palomo, C.; Nikitaki, Z.; Djonov, V.; Georgakilas, A.G.; Martin, O.A. Non-Targeted Effects of Synchrotron Radiation: Lessons from Experiments at the Australian and European Synchrotrons. Appl. Sci. 2022, 12, 2079. https://doi.org/10.3390/app12042079
Fernandez-Palomo C, Nikitaki Z, Djonov V, Georgakilas AG, Martin OA. Non-Targeted Effects of Synchrotron Radiation: Lessons from Experiments at the Australian and European Synchrotrons. Applied Sciences. 2022; 12(4):2079. https://doi.org/10.3390/app12042079
Chicago/Turabian StyleFernandez-Palomo, Cristian, Zacharenia Nikitaki, Valentin Djonov, Alexandros G. Georgakilas, and Olga A. Martin. 2022. "Non-Targeted Effects of Synchrotron Radiation: Lessons from Experiments at the Australian and European Synchrotrons" Applied Sciences 12, no. 4: 2079. https://doi.org/10.3390/app12042079







