Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review
Abstract
:1. Introduction
2. Volatile Wood Composition
- Q. pyrenaica Wild
- Quercus faginea Lam
- Quercus humboldtii Bonpl
- Robinia pseudoacacia (Acacia)
- Castanea sativa (Chestnut)
- Prunus avium (Cherry)
- Fraxinus excelsior (European ash) and Fraxinus americana (American ash)
- Morus alba (mulberry)
3. Influence of Ageing with Alternative Woods on the Volatile Composition of Wine: Comparison with Traditional Oak Woods
3.1. Ageing in Barrels with Traditional and Alternative Woods
3.2. Aging of Wines with Chips and Staves from Traditional and Alternative Woods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, Y.; García, J.F.; Sun, D.W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine science: Principles and applications. In Oak and Cooperage; Academic Press: New York, NY, USA, 1994; pp. 299–311. [Google Scholar]
- Cadahía, E.; Muñoz, L.; De Simón, B.F.; García-Vallejo, M.C. Changes in low molecular weight phenolic compounds in Spanish, French, and American oak woods during natural seasoning and toasting. J. Agric. Food Chem. 2001, 49, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Alternative woods in enology: Characterization of tannin and low molecular weight phenol compounds with respect to traditional oak woods. A review. Molecules 2020, 25, 1474. [Google Scholar] [CrossRef] [Green Version]
- Fernández de Simón, B.; Cadahía, E.; Muiño, I.; del Álamo, M.; Nevares, I. Volatile composition of toasted oak chips and staves and of red wine aged with them. Am. J. Enol. Vitic. 2010, 61, 157–165. [Google Scholar]
- Martínez-Gil, A.M.; del Alamo-Sanza, M.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y.; Nevares, I. Volatile composition and sensory characteristics of Carménère wines macerating with Colombian (Quercus humboldtii) oak chips compared to wines macerated with American (Q. alba) and European (Q. petraea) oak chips. Food Chem. 2018, 266, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Caldeira, I.; Anjos, O.; Belchior, A.P.; Canas, S. Sensory impact of alternative ageing technology for the production of wine brandies. Cienc. Tec. Vitivinic. 2017, 32, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Cruz, S.; Canas, S.; Belchior, A.P. Effect of ageing system and time on the quality of wine brandy aged at industrial-scale. Cienc. Tec. Vitivinic. 2013, 27, 83–93. [Google Scholar]
- Fernández de Simón, B.; Cadahía, E. Utilización del Roble Español en el Envejecimiento de Vinos: Comparación con Roble Francés y Americano; Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria Ministerio de Educación y Ciencia: Madrid, Spain, 2004.
- Cadahía, E.; Fernández de Simón, B.; Poveda, P.; Sanz, M. Utilización de Quercus Pyrenaica Willd. de Castilla y León en el Envejecimiento de Vinos. Comparación con Roble Francés y Americano; Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria Ministerio de Educación y Ciencia: Madrid, Spain, 2008.
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Different Woods in Cooperage for Oenology: A Review. Beverages 2018, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Chira, K.; Teissedre, P.-L. Chemical and sensory evaluation of wine matured in oak barrel: Effect of oak species involved and toasting process. Eur. Food Res. Technol. 2015, 240, 533–547. [Google Scholar] [CrossRef]
- Cadahía, E.; Varea, S.; Muñoz, L.; Fernández de Simón, B.; García-Vallejo, M.C. Evolution of Ellagitannins in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Guchu, E.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; González-Viñas, M.A.; Cabezudo Ibáñez, M.D. Volatile composition and sensory characteristics of Chardonnay wines treated with American and Hungarian oak chips. Food Chem. 2006, 99, 350–359. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC–MS and GC–O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Ford, A. Variation of the Flavour and Extractives of European Oak Wood from Two French Forests. J. Sci. Food Agric. 1996, 70, 273–287. [Google Scholar] [CrossRef]
- Prida, A.; Puech, J.-L. Influence of Geographical Origin and Botanical Species on the Content of Extractives in American, French, and East European Oak Woods. J. Agric. Food Chem. 2006, 54, 8115–8126. [Google Scholar] [CrossRef]
- Sanz, M.; Fernández de Simón, B.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Hernández, M.T.; Estrella, I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta 2012, 732, 33–45. [Google Scholar] [CrossRef]
- Jordão, A.M.; Lozano, V.; Correia, A.C.; Ortega-Heras, M.; González-SanJosé, M.L. Comparative analysis of volatile and phenolic composition of alternative wood chips from cherry, acacia and oak for potential use in enology. BIO Web Conf. 2016, 7, 02012. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Leandro, M.C.; Spranger, M.I.; Belchior, A.P. Influence of botanical species and geographical origin on the content of low molecular weight phenolic compounds of woods used in Portuguese cooperage. Holzforschung 2000, 54, 255–261. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Fernández De Simón, B.; Hernández, T.; Estrella, I. Phenolic compounds in cherry (Prunus avium) heartwood with a view to their use in cooperage. J. Agric. Food Chem. 2010, 58, 4907–4914. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Esteruelas, E.; Muñoz, À.M.; Cadahía, E.; Sanz, M. Volatile compounds in acacia, chestnut, cherry, ash, and oak woods, with a view to their use in cooperage. J. Agric. Food Chem. 2009, 57, 3217–3227. [Google Scholar] [CrossRef]
- Culleré, L.; Fernández de Simón, B.; Cadahía, E.; Ferreira, V.; Hernández-Orte, P.; Cacho, J. Characterization by gas chromatography-olfactometry of the most odor-active compounds in extracts prepared from acacia, chestnut, cherry, ash and oak woods. LWT -Food Sci. Technol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Perez-Coello, M.S.; Díaz-Maroto, M.C. Volatile Compounds and Wine Aging. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 295–307. ISBN 9780387741161. [Google Scholar]
- Sánchez-Gómez, R.; del Alamo-Sanza, M.; Nevares, I. Volatile composition of oak wood from different customised oxygenation wine barrels: Effect on red wine. Food Chem. 2020, 329, 127181. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Boidron, J.N.; Pons, M. Maturation of red wines in oak barrels: Evolution of some volatile compounds and their aromatic impact. Sci. Aliment. 1990, 10, 565–587. [Google Scholar]
- Marco, J.; Artajona, J.; Larrechi, M.S.; Rius, F.X. Relationship Between Geographical Origin and Chemical Composition of Wood for Oak Barrels. Am. J. Enol. Vitic. 1994, 45, 192–200. [Google Scholar]
- De Simón, B.F.; Cadahía, E.; Sanz, M.; Poveda, P.; Perez-Magariño, S.; Ortega-Heras, M.; González-Huerta, C. Volatile compounds and sensorial characterization of wines from four spanish denominations of origin, aged in Spanish Rebollo (Quercus pyrenaica Willd.) oak wood barrels. J. Agric. Food Chem. 2008, 56, 9046–9055. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Towey, J.P. Oak lactone isomer ratio distinguishes between wine fermented in American and French oak barrels. J. Agric. Food Chem. 1994, 42, 1971–1974. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Ortega-Heras, M.; Pérez-Magariño, S.; González-Huerta, C. Volatile compounds of red wines macerated with Spanish, American, and French Oak chips. J. Agric. Food Chem. 2009, 57, 6383–6391. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography—Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red. J. Agric. Food Chem. 2004, 53, 1653–1660. [Google Scholar] [CrossRef]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile profile of Madeira wines submitted to traditional accelerated ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef]
- Herrero, P.; Sáenz-Navajas, M.P.; Avizcuri, J.M.; Culleré, L.; Balda, P.; Antón, E.C.; Ferreira, V.; Escudero, A. Study of Chardonnay and Sauvignon blanc wines from D.O.Ca Rioja (Spain) aged in different French oak wood barrels: Chemical and aroma quality aspects. Food Res. Int. 2016, 89, 227–236. [Google Scholar] [CrossRef]
- Caldeira, I.; de Sousa, R.B.; Belchior, A.P.; Climaco, M.C. A sensory and chemical approach to the aroma of wooden agend Lourinha wine brandy. Cienc. Tec. Vitivinic. 2008, 23, 97–110. [Google Scholar]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Henry, R.; Dubourdieu, D. Identifying new volatile compounds in toasted oak. J. Agric. Food Chem. 1999, 47, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Sefton, M.A.; Taylor, D.K.; Elsey, G.M. An odour detection threshold determination of all four possible stereoisomers of oak lactone in a white and a red wine. Aust. J. Grape Wine Res. 2006, 12, 115–118. [Google Scholar] [CrossRef]
- Juan, F.S.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Simón, B.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sefton, M.S.; Francis, I.L.; Pocock, K.F.; Williams, P.J. The influence of natural seasoning on the concentrations of eugenol, vanillin, and cis- and trans-β-methyl-γ-octalactone extracted from French and American oakwood. Sci. Aliment. 1993, 13, 629–643. [Google Scholar]
- Martínez-Gil, A.M.; del Alamo-Sanza, M.; Nevares, I.; Sánchez-Gómez, R.; Gallego, L. Effect of size, seasoning and toasting level of Quercus pyrenaica Willd. wood on wine phenolic composition during maturation process with micro- oxygenation. Food Res. Int. 2020, 128, 108703. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P.-L.L. Extraction of oak volatiles and ellagitannins compounds and sensory profile of wine aged with French wine woods subjected to different toasting methods: Behaviour during storage. Food Chem. 2013, 140, 168–177. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boidron, J.; Pons, M. Incidence du traitement thermique du bois de chêne sur sa composition chimique 2e partie: Évolution de certains composés en fonction de l’intensité de brûlage. J. Int. Sci. Vigne Vin 1989, 23, 223–250. [Google Scholar] [CrossRef]
- Chatonnet, P. Productos alternativos a la crianza en barrica. 3a parte: El tostado. In Proceedings of the IV Encuentro Enológico: Crianza en Barricas y Otras Alternativas; Fundación para la Cultura del Vino: Madrid, Spain, 2007; p. 5. [Google Scholar]
- Dumitriu, G.D.; López de Lerma, N.; Zamfir, C.I.; Cotea, V.V.; Peinado, R.A. Volatile and phenolic composition of red wines subjected to aging in oak cask of different toast degree during two periods of time. LWT -Food Sci. Technol. 2017, 86, 643–651. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of Oak-Derived Compounds on the Olfactory Perception of Barrel-Aged Wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar]
- Reazin, G.H. Chemical mechanisms of whiskey maturation. Am. J. Enol. Vitic. 1981, 32, 283–289. [Google Scholar]
- Otsuka, K.; Sato, K.; Yamashita, T. Structure of a precursor of beta methyl beta y gamma octalactone, an aging flavor compound of distilled liquors. J. Ferment. Technol. 1980, 58, 395–398. [Google Scholar]
- Díaz-Plaza, E.M.; Reyero, J.R.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Influence of oak wood on the aromatic composition and quality of wines with different tannin contents. J. Agric. Food Chem. 2002, 50, 2622–2626. [Google Scholar] [CrossRef] [PubMed]
- Cerdán, T.G.; Rodríguez Mozaz, S.; Ancín Azpilicueta, C. Volatile composition of aged wine in used barrels of French oak and of American oak. Food Res. Int. 2002, 35, 603–610. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Lencina, A.G.; Cano-López, M.; Pardo-Mínguez, F.; López-Roca, J.M.; Gómez-Plaza, E. The use of oak chips during the ageing of a red wine in stainless steel tanks or used barrels: Effect of the contact time and size of the oak chips on aroma compounds. Aust. J. Grape Wine Res. 2008, 14, 63–70. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Lorenzo, C.; Salinas, M.R.; Martínez, J.; Garde-Cerdán, T. Influence of oak barrel aging on the quality of red wines. In Oak: Ecology, Types and Management; Chuteira, C.A., Grão, A.B., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 59–86. [Google Scholar]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J. Use of Oak Fragments during the Aging of Red Wines. Effect on the Phenolic, Aromatic, and Sensory Composition of Wines as a Function of the Contact Time with the Wood. Beverages 2018, 4, 102. [Google Scholar] [CrossRef] [Green Version]
- Del Alamo-Sanza, M.; Nevares, I.; Martínez-Gil, A.; Rubio-Bretón, P.; Garde-Cerdán, T. Impact of long bottle aging (10 years) on volatile composition of red wines micro-oxygenated with oak alternatives. LWT -Food Sci. Technol. 2019, 101, 395–403. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-Huerta, C.; Herrera, P.; González-Sanjosé, M.L. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal. Chim. Acta 2004, 513, 341–350. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández de Simón, B.; Sanz, M.; Poveda, P.; Colio, J. Chemical and chromatic characteristics of Tempranillo, Cabernet Sauvignon and Merlot wines from DO Navarra aged in Spanish and French oak barrels. Food Chem. 2009, 115, 639–649. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández de Simón, B.; Vallejo, R.; Sanz, M.; Broto, M. Volatile compound evolution in Spanish oak wood (Quercus petraea and Quercus pyrenaica) during natural seasoning. Am. J. Enol. Vitic. 2007, 58, 163–172. [Google Scholar]
- Fernández de Simón, B.; Sanz, M.; Cadahía, E.; Poveda, P.; Broto, M. Chemical characterization of oak heartwood from Spanish forests of Quercus pyrenaica (Wild.). Ellagitannins, low molecular weight phenolic, and volatile compounds. J. Agric. Food Chem. 2006, 54, 8314–8321. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, I.J.; Martín-Alvarez, P.J.; Vila-Lameiro, P.; Díaz-Maroto, M.C. Influence of geographical location, site and silvicultural parameters, on volatile composition of Quercus pyrenaica Willd. wood used in wine aging. For. Ecol. Manag. 2011, 262, 124–130. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aromatic potential of Castanea sativa Mill. Compared to Quercus species to be used in cooperage. Food Chem. 2012, 130, 875–881. [Google Scholar] [CrossRef]
- Caldeira, I.; Clímaco, M.C.; Bruno De Sousa, R.; Belchior, A.P. Volatile composition of oak and chestnut woods used in brandy ageing: Modification induced by heat treatment. J. Food Eng. 2006, 76, 202–211. [Google Scholar] [CrossRef]
- Jordao, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Comparison of volatile composition of cooperage oak wood of different origins (Quercus pyrenaica vs. Quercus alba and Quercus petraea). Mitt. Klost. 2005, 55, 22–31. [Google Scholar]
- Cadahía, E.; Fernández de Simón, B.; Jalocha, J. Volatile compounds in Spanish, French, and American oak woods after natural seasoning and toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Fernández De Simõn, B.; Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M. Nontargeted GC-MS approach for volatile profile of toasting in cherry, chestnut, false acacia, and ash wood. J. Mass Spectrom. 2014, 49, 353–370. [Google Scholar] [CrossRef]
- Fernández De Simón, B.; Muiño, I.; Cadahía, E. Characterization of volatile constituents in commercial oak wood chips. J. Agric. Food Chem. 2010, 58, 9587–9596. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-Da-Silva, J.M.; Laureano, O.; Adams, A.; Demyttenaere, J.; Verhé, R.; De Kimpe, N. Volatile composition analysis by solid-phase microextraction applied to oak wood used in cooperage (Quercus pyrenaica and Quercus petraea): Effect of botanical species and toasting process. J. Wood Sci. 2006, 52, 514–521. [Google Scholar] [CrossRef]
- Doussot, F.; De Jéso, B.; Quideau, S.; Pardon, P. Extractives content in cooperage oak wood during natural seasoning and toasting; influence of tree species, geographic location, and single-tree effects. J. Agric. Food Chem. 2002, 50, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.L.; Prida, A.; Hayasaka, Y. Role of glycoconjugates of 3-methyl-4-hydroxyoctanoic acid in the evolution of oak lactone in wine during oak maturation. J. Agric. Food Chem. 2013, 61, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Cutzach, I.; Pons, M.; Dubourdieu, D. Monitoring toasting intensity of barrels by chromatographic analysis of volatile compounds from toasted oak wood. J. Agric. Food Chem. 1999, 47, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Dalla Vedova, A.; Cancian, D.; Panighel, A.; De Rosso, M. GC/MS-positive ion chemical ionization and MS/MS study of volatile benzene compounds in five different woods used in barrel making. J. Mass Spectrom. 2007, 42, 641–646. [Google Scholar] [CrossRef]
- Alañón, M.E.; Marchante, L.; Alarcón, M.; Díaz-Maroto, I.J.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Fingerprints of acacia aging treatments by barrels or chips based on volatile profile, sensorial properties, and multivariate analysis. J. Sci. Food Agric. 2018, 18, 5795–5806. [Google Scholar] [CrossRef]
- Alañón, M.E.; Schumacher, R.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Enological potential of chestnut wood for aging Tempranillo wines Part II: Phenolic compounds and chromatic characteristics. Food Res. Int. 2013, 51, 536–543. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Vedova, A.D.; Stella, L.; Flamini, R. Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Martínez, J.; Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.M. Volatile compounds and sensorial characterisation of red wine aged in cherry, chestnut, false acacia, ash and oak wood barrels. Food Chem. 2014, 147, 346–356. [Google Scholar] [CrossRef]
- Kozlovic, G.; Jeromel, A.; Maslov, L.; Pollnitz, A.; Orlić, S. Use of acacia barrique barrels—Influence on the quality of Malvazija from Istria wines. Food Chem. 2010, 120, 698–702. [Google Scholar] [CrossRef]
- Fernández De Simón, B.; Cadahía, E.; Jalocha, J. Volatile compounds in a Spanish red wine aged in barrels made of Spanish, French, and American oak wood. J. Agric. Food Chem. 2003, 51, 7671–7678. [Google Scholar] [CrossRef] [PubMed]
- Fernández De Simón, B.; Cadahía, E.; Hernández, T.; Estrella, I. Evolution of oak-related volatile compounds in a Spanish red wine during 2 years bottled, after aging in barrels made of Spanish, French and American oak wood. Anal. Chim. Acta 2006, 563, 198–203. [Google Scholar] [CrossRef]
- Santos, F.; Correia, A.C.; Ortega-Heras, M.; García-Lomillo, J.; González-SanJosé, M.L.; Jordão, A.M.; Ricardo-da-Silva, J.M. Acacia, cherry and oak wood chips used for a short aging period of rosé wines: Effects on general phenolic parameters, volatile composition and sensory profile. J. Sci. Food Agric. 2019, 99, 3588–3603. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Schumacher, R.; Castro-Vázquez, L.; Díaz-Maroto, I.J.; Díaz-Maroto, M.C.; Pérez-Coello, M. Enological potential of chestnut wood for aging Tempranillo wines part I: Volatile compounds and sensorial properties. Food Res. Int. 2013, 51, 325–334. [Google Scholar] [CrossRef]

| Derived from | Common Name | IUPAC Name | Molecule | Aroma Notes | Olfactory Threshold | |
|---|---|---|---|---|---|---|
| Volatile phenols | Lignin and polyphenols | Guaiacol | 2-Methoxyphenol |  | Smoke, sweet, medicine [31] | 9.5 µg/L [32] |
| 4-Ethylguaiacol (4-EG) | 4-Ethyl-2-methoxyphenol |  | Phenolic, smoked [31], leather [33] | 47 µg/L [27] | ||
| 4-Methylguaiacol (4-MG) | 4-Methyl-2-methoxyphenol |  | Spicy, phenolic, light green [16] | 20 µg/L [34] | ||
| 4-Vinylguaiacol (4-VG) | 4-Vinyl-2-methoxyphenol |  | Clove [31] | 40 µg/L [32] | ||
| Eugenol | 2-Methoxy-4-(prop-2-enyl) phenol, |  | Clove, honey, spicy [31], cinnamon [16] | 6 µg/L [32] | ||
| Isoeugenol | 1-Methoxy-4-(prop-2-enyl) phenol |  | Floral [32], clove, woody [16], | 6 µg/L [32] | ||
| Syringol | 2,6-Dimethoxyphenol |  | Smoke, burned, wood [35] | 570 µg/L [36] | ||
| Furanic compounds | Polysaccharides | Furfural | 2-Furancarboxaldehyde |  | Bread, almond, sweet [31] | 15 mg/L [31] |
| 5-Methylfurfural | 5-Methyl-2-furancarboxaldehyde |  | Almond, caramel, burnt sugar [31] | 16 mg/L [31] | ||
| Maltol | 3-Hydroxy-2-methyl-4H-pyran-4-one |  | Honey, toasty, caramel [37] | 5 mg/L [37] | ||
| 5-Hydroxymethylfurfural | 5-Hydroxymethyl-2-furaldehyde |  | Caramel [33] | 100 mg/L [34] | ||
| Lactones | Lipids | trans-β-Methyl-γ-octalactone | trans-4-Methyl-5-butyldihydro-2-(3H)-furanone |  | Coconut, woody, vanilla [31] | 140–370 µg/L [38] |
| cis-β-Methyl-γ-octalactone | cis-4-Methyl-5-butyldihydro-2-(3H)-furanone | Coconut, woody, vanilla [31] | 20–46 µg/L [38] | |||
| Phenolic aldehydes/Phenyl ketones | Lignin | Vanillin | 4-Hydroxy-3-methoxybenzaldehyde |  | Vanilla [31] | 1000 µg/L [32] |
| Syringaldehyde | 4-Hydroxy-3,5-dimethoxybenzaldehyde |  | Vanilla [31] | 50 mg/L [39] | ||
| Acetovanillone | 1-(4-hydroxy-3-methoxyphenyl)ethanone |  | Vanilla [31] | 1000 µg/L [39] | ||
| Butyrovanillone | 1-(4-Hydroxy-3-methoxyphenyl) butanone | 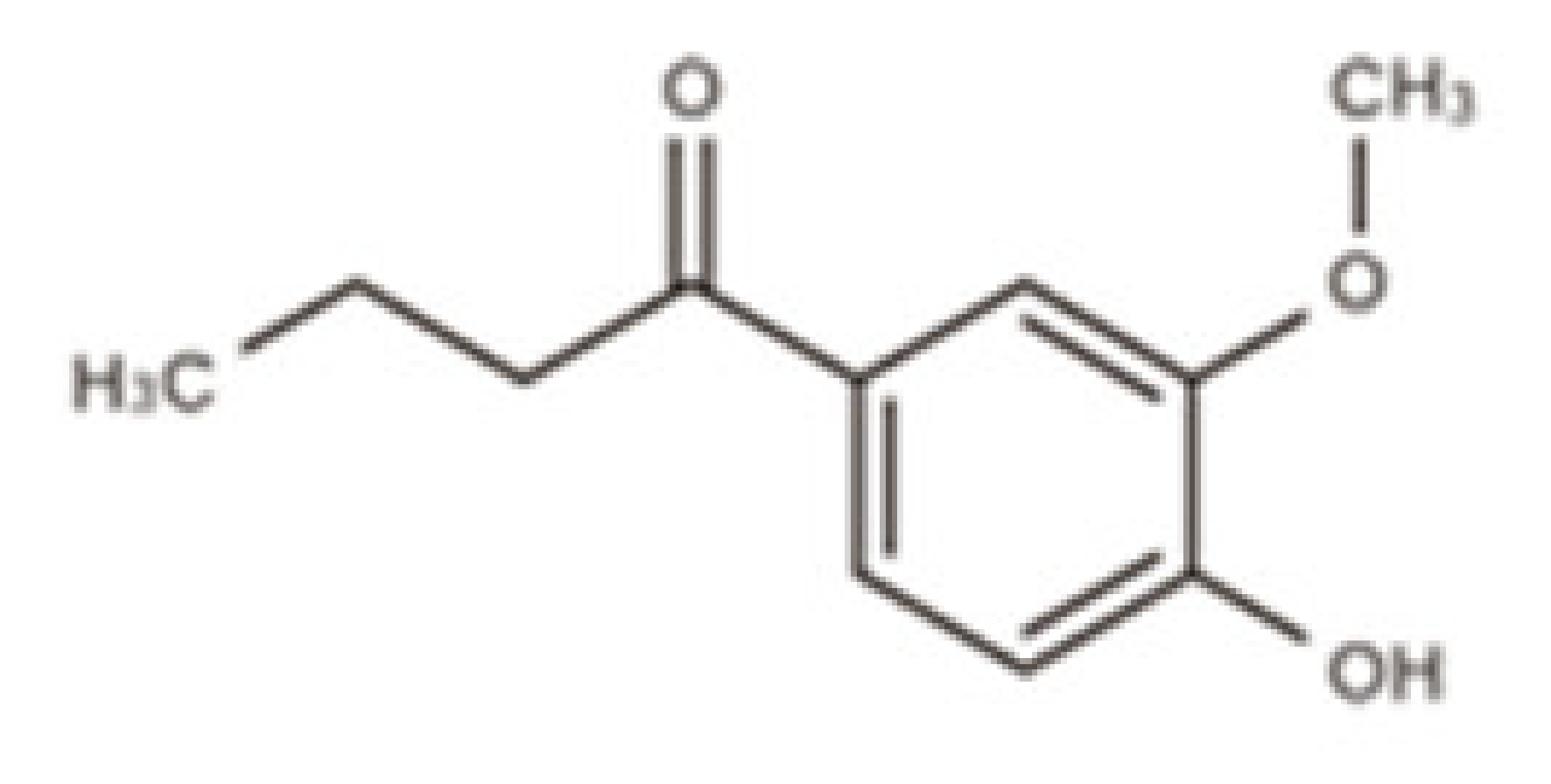 |
| Volatile Phenols | Furanic Compounds | Lactones | Phenolic Aldehydes/Phenyl Ketones | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Guaiacol | 4-EG | 4-MG | 4-VG | Eugenol | Isoeugenol | Syringol | Furfural | 5-MF | Maltol | 5-HMF | trans WL | cis WL | cis/trans Ratio | Vanillin | Syringaldehyde | Acetovanillone | Butyrovanillone |
| Alternative woods Q. pyrenaica | ||||||||||||||||||
| Gata/Peña de Francia [58] | 0.18 | 0.06 | 0.51 | 0.61 | 5.2 | 0.61 | 0.47 | 2.6 | 0.22 | 0.28 | 3.7 | 4.8 | 36 | 7.4 | 3.4 | 4.4 | 0.38 | 0.06 |
| Gata/Peña de Francia [11,59] | 0.21 | 0.07 | 0.76 | 0.67 | 5.7 | 0.71 | 0.51 | 2.4 | 0.25 | 0.27 | 3.8 | 6.4 | 59 | 12 | 3.6 | 5.1 | 0.50 | 0.08 |
| Gudarrama [11,59] | 0.16 | 0.02 | 0.36 | 0.64 | 2.3 | 0.28 | 0.17 | 2.4 | 0.19 | 0.24 | 1.7 | 0.84 | 15 | 17 | 3.8 | 5.4 | 0.26 | 0.04 |
| Cantábrica mountain range [11,59] | 0.24 | 0.14 | 0.52 | 0.72 | 1.5 | 0.26 | 0.23 | 1.4 | 0.33 | 0.23 | 2.7 | 7.3 | 27 | 7.5 | 2.4 | 4.8 | 0.24 | 0.04 |
| Sistema Ibérico [59] | 0.16 | 0.02 | 0.39 | 0.52 | 1.6 | 0.32 | 0.21 | 1.9 | 0.41 | 0.24 | 1.7 | 29 | 14 | 4.9 | ne | ne | 0.20 | 0.03 |
| Aliste-Margarita [11] | 0.12 | 0.07 | 0.08 | 0.54 | 0.73 | 0.24 | 0.34 | 0.9 | 0.22 | 0.17 | 3.2 | 2.8 | 12 | 3.8 | 1.5 | 3.5 | 0.21 | 0.02 |
| North of Sistema Ibérico [11] | 0.13 | 0.04 | 0.49 | 0.86 | 2.2 | 0.44 | 0.31 | 1.7 | 0.24 | 0.23 | 2.9 | 10 | 18 | 4.9 | 4.4 | 14 | 0.35 | 0.05 |
| Gredos and Sierra de Ávila [11] | 0.09 | 0.04 | 0.07 | 0.39 | 0.55 | 0.26 | 0.07 | 0.57 | 0.24 | 0.19 | 1.7 | 0.54 | 1.7 | 2.5 | 1.7 | 3.0 | 0.16 | 0.03 |
| Traditional Quercus | ||||||||||||||||||
| Q. petraea [58] | 0.09 | 0.01 | 0.06 | 0.39 | 2.1 | 0.18 | 0.07 | 1.2 | 0.22 | 0.24 | 0.63 | 18 | 37 | 3.9 | 2.2 | 2.7 | 0.16 | 0.56 |
| Volatile Phenols | Furanic Compounds | Lactones | Phenolic Aldehydes/Phenyl Ketones | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Guaiacol | 4-EG | 4-MG | 4-VG | Eugenol | Isoeugenol | Syringol | Furfural | 5-MF | Maltol | 5-HMF | trans WL | cis WL | cis/trans Ratio | Vanillin | Syringaldehyde | Acetovanillone | Butyrovanillone |
| Seasoned in oven (0% IH) | ||||||||||||||||||
| Alternative woods | ||||||||||||||||||
| Q. pyrenaica (Lugo) [60] | 1.0 | ns | ns | 1.2 | 3.8 | 2.6 | 1.2 | ns | ns | ns | ns | 5.9 | 21 | 3.6 | 21 | 36 | 1.2 | 4.0 |
| Q. pyrenaica (Pontevedra) [60] | 0.9 | ns | ns | 1.5 | 3.2 | 1.6 | 1.0 | ns | ns | ns | ns | 4.7 | 25 | 5.3 | 25 | 43 | 1.2 | 4.3 |
| Q. pyrenaica (Ourense) [60] | 0.6 | ns | ns | 0.9 | 1.6 | 2.3 | 0.5 | ns | ns | ns | ns | 3.6 | 18 | 4.9 | 19 | 33 | 1.0 | 3.7 |
| Q. pyrenaica (Portugal) [61] | 2.6 | ns | 0.8 | 3.1 | 2.4 | 0.3 | 0.9 | 0.6 | 0.4 | ns | 2.5 | 9.3 | 39 | 4.2 | 19 | 12 | 2.9 | 5.3 |
| Castanea sativa (Portugal) [61] | 5.2 | ns | 1.5 | 3.0 | 2.5 | 1.2 | 1.5 | 3.6 | 2.3 | ns | 6.9 | nd | nd | nd | 81 | 22 | 4.8 | 10 |
| Traditional Quercus [61] | ||||||||||||||||||
| Q. petraea | 4.5 | ns | 1.1 | 3.4 | 1.1 | 0.3 | 0.9 | 12 | 3.6 | ns | 6.2 | 2.1 | 6.1 | 2.9 | 46 | 26 | 3.4 | 6.9 |
| Q. robur | 4.3 | ns | 0.9 | 4.6 | 1.4 | 0.2 | 0.6 | 17 | 5.0 | ns | 27 | 2.9 | 3.2 | 0.5 | 6.4 | 24 | 0.5 | 2.2 |
| Q. alba | 0.9 | ns | 0.2 | 3.4 | 3.4 | 0.4 | 0.9 | 5.8 | 0.4 | ns | 0.9 | 1.6 | 39 | 12 | 70 | 23 | 2.7 | 7.4 |
| Seasoned in open air (nonspecific) | ||||||||||||||||||
| Alternative woods [62] | ||||||||||||||||||
| Q. pyrenaica (Portugal) | 0.4 | ns | ns | ns | 0.9 | ns | 0.4 | 1.9 | 0.0 | ns | 1.7 | 2.3 | 6.7 | 2.9 | 4.8 | ns | 1.9 | ns |
| Q. pyrenaica (Portugal) | 1.3 | ns | ns | ns | 2.7 | ns | 0.4 | 13 | 0.3 | ns | 2.4 | 2.7 | 7.2 | 2.7 | 4.8 | ns | 0.0 | ns |
| Q. pyrenaica (Portugal) | 0.3 | ns | ns | ns | 1.1 | ns | 0.0 | 4.6 | 0.0 | ns | 2.7 | 2.5 | 5.3 | 2.2 | 5.5 | ns | 0.0 | ns |
| Q. alba, stellata, lyrata, bicolor | 1.5 | ns | ns | ns | 4.5 | ns | 0.0 | 9.8 | 0.6 | ns | 0.8 | 2.7 | 35 | 13 | 5.8 | ns | 0.9 | ns |
| Castanea sativa (Portugal) | 0.4 | ns | ns | ns | 0.7 | ns | 0.5 | 2.3 | 0.3 | ns | 0.9 | 0.2 | 0.3 | 1.5 | 2.9 | ns | 1.3 | ns |
| Traditional Quercus [62] | ||||||||||||||||||
| Q. petraea | 1.2 | ns | ns | ns | 1.2 | ns | 0.4 | 14 | 0.8 | ns | 1.0 | 1.9 | 4.9 | 2.6 | 8.3 | ns | 0.4 | ns |
| Q. robur | 0.1 | ns | ns | ns | 1.0 | ns | 0.0 | 4.5 | 0.3 | ns | 0.3 | 2.9 | 7.2 | 2.5 | 1.2 | ns | 0.0 | ns |
| Alternative woodsQ. pyrenaica | Seasoned in open air (12 months) | |||||||||||||||||
| Gata/Peña de Francia [58] | 0.2 | 0.1 | 0.4 | 1.1 | 5.7 | 0.5 | 0.4 | 3.5 | 0.9 | 0.4 | 4.2 | 3.5 | 50 | 27 | 5.3 | 6.5 | 0.5 | 0.1 |
| Alternative woodsQ. pyrenaica | Seasoned in open air (18 months) | |||||||||||||||||
| Gata/Peña de Francia [58] | 0.2 | 0.0 | 1.1 | 1.2 | 3.9 | 0.3 | 0.2 | 4.9 | 1.6 | 0.4 | 3.4 | 4.6 | 73 | 24 | 4.5 | 5.0 | 0.3 | 0.1 |
| Seasoned in open air (24 months) | ||||||||||||||||||
| Alternative woods | ||||||||||||||||||
| Q. pyrenaica (Salamanca) [23] | 0.4 | 0.0 | 0.4 | 0.9 | 5.5 | 1.4 | 1.1 | 11 | 0.2 | 1.4 | 13 | 34 | 32 | 0.9 | 12 | 48 | 0.6 | 3.1 |
| Q. pyrenaica (Gata/Peña de Francia) [58] | 0.5 | 0.1 | 0.3 | 2.2 | 7.3 | 0.7 | 1.7 | 3.2 | 1.0 | 0.3 | 4.9 | 5.3 | 68 | 20 | 4.9 | 4.2 | 0.7 | 0.1 |
| Q. pyrenaica (Gerês forest of Portugal) [63] | nd | ns | nd | ns | nd | nd | ns | 3.9 | ns | ns | 0.9 | nd | 10 | - | 2.5 | 15 | ns | ns |
| Q. pyrenaica (Guarda forest of Portugal) [63] | nd | ns | nd | ns | nd | nd | ns | 4.5 | ns | ns | 1.3 | 8.3 | 5.3 | 0.6 | 1.6 | 17 | ns | ns |
| Robinia pseudoacacia [23] | 0.4 | 0.1 | 0.0 | 0.4 | 0.1 | 1.0 | 0.8 | 0.5 | 0.0 | 0.9 | 0.2 | nd | nd | - | 1.7 | 6.0 | 0.2 | 0.7 |
| Robinia pseudoacacia [65] | 0.9 | ns | 0.1 | ns | 0.2 | 3.4 | 1.9 | 0.9 | 0.1 | 1.4 | 0.5 | ni | ni | - | 3.5 | 10 | 0.3 | 1.0 |
| Castanea sativa [23] | 0.1 | 0.0 | 0.1 | 0.3 | 2.0 | 2.2 | 0.1 | 5.5 | 0.1 | 1.1 | 14 | nd | nd | - | 24 | 53 | 0.5 | 2.0 |
| Castanea sativa [65] | 0.2 | ns | 0.2 | ns | 4.5 | 2.4 | 0.3 | 6.7 | 0.2 | 2.0 | 21 | ni | ni | - | 17 | 38 | 0.4 | 1.9 |
| Prunus avium [23] | 0.2 | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 | 0.4 | 0.5 | 0.0 | 0.4 | 0.2 | nd | nd | - | 1.1 | 2.6 | 0.2 | 0.4 |
| Prunus avium [65] | 0.5 | ns | 0.1 | ns | 0.1 | 0.6 | 1.5 | 0.7 | 0.1 | 0.5 | 0.5 | ni | ni | - | 2.4 | 6.9 | 0.3 | 1.0 |
| Fraxinus excelsior (Europe) [23] | 0.1 | nd | 0.1 | 0.4 | 0.2 | 1.3 | 0.2 | 0.8 | 0.0 | 0.2 | 0.9 | nd | nd | - | 7.3 | 45 | 0.2 | 0.6 |
| Fraxinus excelsior (Europe) [65] | 0.1 | ns | 0.2 | ns | 0.6 | 7.7 | 0.6 | 0.5 | 0.1 | 0.5 | 3.3 | ni | ni | - | 10 | 20 | 0.4 | 1.3 |
| Fraxinus americana (American) [23] | 0.1 | 0.02 | 0.8 | 0.6 | 0.4 | 2.4 | 0.2 | 1.2 | 0.0 | 0.8 | 1.6 | nd | nd | - | 15 | 51 | 0.4 | 2.0 |
| Fraxinus americana (American) [65] | 0.2 | ns | 1.2 | ns | 0.9 | 2.1 | 0.9 | 1.3 | 0.1 | 0.9 | 2.0 | ni | ni | - | 14 | 37 | 0.9 | 2.4 |
| Traditional Quercus | ||||||||||||||||||
| Q. petraea [23,58,63] | nd-1.3 | nd-0.0 | nd-0.6 | 0.2–1.0 | 1.3–6.5 | 0.7–4.3 | 0.1–0.2 | 3.4–7 | 0.1–0.2 | 0.2–0.8 | 0.3–4 | 5–15 | 14–56 | 1.7–9 | 2–12 | 2.7–47 | 0.4 | 1.4–1.9 |
| Q. alba [23,63] | 0.0–3.3 | nd | 0.1–1.5 | 0.18 | 1.4–5.9 | 1.1–1.8 | 0.1 | 1.2–4.7 | 0.2 | 0.48 | 0.4–6.3 | 2.5–5 | 22–26 | 5.2–8.9 | 6.8–7.5 | 16–20 | 0.4 | 1.5 |
| Seasoned in open air (24–36 months) | ||||||||||||||||||
| Alternative woodsnorthern Italy [71] | ||||||||||||||||||
| Robinia pseudoacacia | nd | ns | ns | nd | nd | ns | nd | ns | ns | ns | ns | ns | ns | ns | 1.7 | 10 | ns | ns |
| Castanea sativa | nd | ns | ns | nd | 0.7 | ns | nd | ns | ns | ns | ns | ns | ns | ns | 5.2 | 4.2 | ns | ns |
| Prunus avium | nd | ns | ns | nd | nd | ns | 0.1 | ns | ns | ns | ns | ns | ns | ns | 0.1 | 0.4 | ns | ns |
| Mulberry | 0.0 | ns | ns | nd | nd | ns | nd | ns | ns | ns | ns | ns | ns | ns | 0.1 | 0.5 | ns | ns |
| Traditional Quercus [71] | ||||||||||||||||||
| France | nd | ns | ns | 0.1 | 2.0 | ns | 0.0 | ns | ns | ns | ns | ns | ns | ns | 2.0 | 9.3 | ns | ns |
| Seasoned in open air (36 months) | ||||||||||||||||||
| Alternative woods [64] | ||||||||||||||||||
| Q. pyrenaica (Alava) | 0.1 | 0.0 | 0.5 | 2.0 | 2.3 | 0.4 | 0.3 | 20 | 1.5 | 0.3 | 3.9 | 4.6 | 18 | 3.7 | 11 | 15. | 0.7 | 2.4 |
| Q. faginea (Alava) | 0.3 | 0.2 | 0.5 | 1.1 | 2.0 | 0.4 | 1.6 | 18 | 1.8 | 0.4 | 1.5 | 1.7 | 16 | 11 | 11 | 10 | 1.5 | 6.6 |
| Traditional Quercus [64] | ||||||||||||||||||
| Q. petraea | 0.1 | 0.1 | 0.1–0.4 | 1.0–1.1 | 0.6–3.2 | 0.2–0.3 | 0.2–0.3 | 10–20 | 1.5–1.7 | 0.2–0.4 | 0.5–2.6 | 0.1–7.8 | 0.4–12 | 3.5–5.0 | 14–19 | 17–19 | 0.9–1.2 | 1.9–2.3 |
| Q. robur | 0.1 | 0.1 | 0.2–0.7 | 1.0–1.3 | 1.1–1.6 | 0.3 | 0.2 | 8.9–11 | 1.1–2.0 | 0.3–0.4 | 0.8–2.7 | 3.4–4.0 | 2.8–23 | 4.0–7.0 | 9.3–16 | 14–18 | 0.7–1.0 | 1.7–2.5 |
| Q. alba | 0.1 | 0.0 | 0.7 | 0.5 | 5.7 | 0.3 | 0.2 | 4.0 | 0.3 | 0.2 | 1.8 | 3.5 | 33 | 16 | 7.9 | 16 | 0.5 | 1.6 |
| Volatile Phenols | Furanic Compounds | Lactones | Phenolic Aldehydes/Phenyl Ketones | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Seasoning Time (m: months) | Guaiacol | 4-EG | 4-MG | 4-VG | Eugenol | Isoeugenol | Syringol | Furfural | 5-MF | Maltol | 5-HMF | trans WL | cis WL | cis/trans Ratio | Vanillin | Syringaldehyde | Acetovanillone | Butyrovanillone |
| Heat treatment: light | |||||||||||||||||||
| Alternative woodsas staves and chips [66] | |||||||||||||||||||
| Q. pyrenaica | 24 m open air | 0.4 | 0.0 | 0.5 | 0.6 | 5.1 | 0.1 | 1.3 | 368 | 44 | 5.2 | 198 | 8.2 | 47 | 5.7 | 28 | 83 | 1.5 | ns |
| Traditional Quercusas staves and chips [66] | |||||||||||||||||||
| French oak | 0.3 | 0.1 | 1.0 | 0.3 | 1.2 | 0.1 | 1.4 | 78 | 17 | 5.2 | 37 | 12 | 11 | 1.0 | 120 | 196 | 3.3 | ns | |
| American oak | 0.4 | 0.1 | 0.7 | 0.2 | 1.6 | 0.1 | 1.3 | 41 | 6.7 | 2.0 | 15 | 3.4 | 25 | 7.4 | 27 | 57 | 0.9 | ns | |
| Heat treatment: 190 °C for 10 min | |||||||||||||||||||
| Alternative woodsQ. pyrenaica from Navarra [40] | |||||||||||||||||||
| staves | 24 m open air | 0.7 | ns | 0.6 | ns | 6.6 | 2.3 | 1.5 | 548 | 49 | 1.2 | 196 | 4.2 | 34 | 8.1 | 29 | 101 | 1.5 | ns |
| Accelerated | 0.2 | ns | 0.4 | ns | 2.2 | 0.9 | 0.6 | 462 | 51 | 3.3 | 247 | 2.5 | 69 | 27 | 19 | 84 | 1.0 | ns | |
| chips | 24 m open air | 0.2 | ns | 1.0 | ns | 3.9 | 1.7 | 0.6 | 71 | 11 | 11 | 84 | 8.3 | 36 | 4.3 | 42 | 74 | 1.4 | ns |
| Accelerated | 0.2 | ns | 0.7 | ns | 6.0 | 0.5 | 0.7 | 79 | 13 | 10 | 76 | 4.5 | 20 | 4.4 | 33 | 74 | 1.1 | ns | |
| Heat treatment: 160–170 °C for 20 min | |||||||||||||||||||
| Alternative woods Q. pyrenaica of Portugal [63] | |||||||||||||||||||
| Gerês forest | 24 m open air | 1.6 | ns | 2.5 | ns | 0.6 | 0.5 | ns | 2177 | 328 | ns | 3344 | 4.8 | 14 | 2.9 | 23 | 82 | ns | ns |
| Guarda forest | 2.5 | ns | 2.5 | ns | 1.4 | 0.7 | ns | 2670 | 465 | ns | 5078 | 7 | 3.6 | 0.5 | 11 | 88 | ns | ns | |
| Traditional Quercus [63] | |||||||||||||||||||
| Q. petraea | 2.9–3.4 | ns | nd-1.6 | ns | 1.7–4 | 0.5–0.8 | ns | 723–723 | 199–258 | ns | 1204–1722 | 5.4–11 | 14 –19 | 1.6–2.6 | 6.9–8.3 | 49–60 | ns | ns | |
| Q. alba | 3.7–7.3 | ns | 2.6–5.1 | ns | 6.2–12 | 1.3–1.9 | ns | 358–960 | 91–487 | ns | 1678–3221 | 6.4–7.4 | 27–46 | 4.1–6.2 | 7.7–11 | 24–35 | ns | ns | |
| Heat treatment: 160–170 °C for 35 min | |||||||||||||||||||
| Alternative woods | |||||||||||||||||||
| Q. pyrenaica from Alava [64] | 36 m open ai | 0.4 | 0.3 | 1.5 | 1.9 | 1.9 | 1.3 | 2.6 | 170 | 30 | 1.6 | 56 | 3.4 | 14 | 4.4 | 210 | 280 | 4.6 | 16 |
| Q. faginea from Alava [64] | 0.4 | 0.3 | 1.5 | 2.5 | 2.4 | 1.0 | 2.6 | 96 | 30 | 1.5 | 58 | 1.1 | 3.3 | 3.6 | 258 | 243 | 5.3 | 16 | |
| Robinia pseudoacacia l [65] | 24 m open air | 0.5 | ni | 0.2 | ni | 0.4 | 3.8 | 1.3 | 21 | 7.5 | 6.5 | 6.7 | ni | ni | ni | 19 | 57 | 1.0 | 3.0 |
| Castanea sativa [65] | 0.5 | ni | 2.6 | ni | 3.2 | 2.1 | 1.4 | 431 | 29 | 4.3 | 67 | ni | ni | ni | 72 | 114 | 1.7 | 14 | |
| Prunus avium [65] | 0.9 | ni | 0.6 | ni | 0.7 | 1.3 | 2.1 | 88 | 14 | 3.5 | 42 | ni | ni | ni | 45 | 115 | 1.5 | 4.56 | |
| Fraxinus excelsior from Europe [65] | 6.5 | ni | 3.7 | ni | 1.6 | 8.3 | 7.5 | 27 | 15 | 19 | 51 | ni | ni | ni | 76 | 98 | 4.2 | 9.1 | |
| Fraxinus americana from American [65] | 6.5 | ni | 3.7 | ni | 1.6 | 8.3 | 7.5 | 27 | 15 | 19 | 51 | ni | ni | ni | 76 | 98 | 4.2 | 9.1 | |
| Traditional Quercus [64] | |||||||||||||||||||
| Q. Petraea | 36 m open air | 0.2–0.4 | 0.2–0.6 | 0.8–2.1 | 1.1–7.4 | 0.9–3.3 | 0.4–4.2 | 1.3–3.3 | 59–186 | 26–30 | 1.2–1.5 | 44–52 | 0.1–15 | 0.3–20 | 1.3–2.9 | 119–370 | 160–452 | 2.7–7.9 | 16–25 |
| Q. Robur | 0.2–0.5 | 0.2 | 0.9–1.0 | 1.5–1.9 | 1.0–1.4 | 0.3–1.8 | 1.2–3.0 | 103–340 | 26–81 | 1.4–1.6 | 41–112 | 0.3–4.1 | 0.3–5.0 | 1.6–4.5 | 130–172 | 162–216 | 3.0–3.9 | 13–18 | |
| Q. Alba | 1.2 | 0.5 | 1.5 | 0.5 | 5.3 | 3.8 | 2.4 | 346 | 85 | 3.4 | 103 | 4.4 | 44 | 19 | 40 | 102 | 4.3 | 22 | |
| Heat treatment: medium | |||||||||||||||||||
| Alternative woodsas staves and chips [66] | |||||||||||||||||||
| Q. pyrenaica | 24 m open air | 1.3 | 0.1 | 1.6 | 0.6 | 5.0 | 0.3 | 3.4 | 919 | 135 | 18 | 322 | 11 | 47 | 4.2 | 54 | 135 | 3.4 | ns |
| Traditional Quercusas staves and chips [66] | |||||||||||||||||||
| French oak | 1.7 | 0.3 | 4.4 | 1.2 | 1.4 | 0.3 | 7.3 | 357 | 42 | 16 | 58 | 9.7 | 12 | 1.3 | 172 | 443 | 12 | ns | |
| American oak | 2.7 | 0.4 | 3.0 | 1.6 | 2.5 | 0.4 | 8.4 | 681 | 95 | 14 | 75 | 6.6 | 31 | 4.7 | 120 | 34 | 7.2 | ns | |
| Heat treatment: 200 °C for 15 min | |||||||||||||||||||
| Alternative woodsQ. pyrenaica from Navarra [40] | |||||||||||||||||||
| staves | 24 m open air | 0.6 | ns | 0.5 | ns | 3.8 | 1.2 | 1.4 | 1133 | 165 | 8.7 | 400 | 3.4 | 23 | 6.8 | 43 | 147 | 2.8 | ns |
| Accelerated | 0.4 | ns | 0.6 | ns | 3.3 | 1.3 | 1.7 | 1536 | 249 | 16 | 567 | 7.5 | 87 | 12 | 31 | 115 | 1.9 | ns | |
| chips | 24 m open air | 0.4 | ns | 2.0 | ns | 3.3 | 1.2 | 1.2 | 118 | 22 | 26 | 122 | 11 | 44 | 4.1 | 79 | 123 | 3.4 | ns |
| Accelerated | 0.4 | ns | 1.7 | ns | 4.8 | 1.1 | 1.5 | 201 | 29 | 28 | 115 | 5.4 | 25 | 4.6 | 83 | 129 | 3.3 | ns | |
| Heat treatment: 180 °C for 45 min | |||||||||||||||||||
| Alternative woods | |||||||||||||||||||
| Q. pyrenaica from Salamanca [23] | 24 m open air | 4.0 | 0.4 | 7.2 | 0.4 | 2.1 | 0.8 | 6.9 | 494 | 56 | 37 | 29 | 9.8 | 30 | 3.1 | 114 | 250 | 9.5 | 17 |
| Robinia pseudoacacia [23] | 5.4 | 0.6 | 1.5 | 0.6 | 2.4 | 12 | 21 | 840 | 94 | 20 | 113 | nd | nd | - | 77 | 272 | 11 | 45 | |
| Robinia pseudoacacia [65] | 6.1 | ni | 1.7 | ni | 2.2 | 7.6 | 21 | 714 | 91 | 19 | 94 | ni | ni | - | 106 | 420 | 9.6 | 42 | |
| Castanea sativa [23] | 5.1 | 0.8 | 6.9 | 0.6 | 2.1 | 2.0 | 13 | 1505 | 76 | 18 | 103 | nd | nd | - | 142 | 311 | 18 | 159 | |
| Castanea sativa [65] | 5.3 | ni | 7.2 | ni | 2.3 | 2.0 | 14 | 1675 | 76 | 18 | 103 | ni | ni | - | 143 | 311 | 18 | 160 | |
| Prunus avium [23] | 1.7 | 0.4 | 1.9 | 0.2 | 1.5 | 0.6 | 5.0 | 23 | 31 | 14 | 48 | nd | nd | - | 68.3 | 455 | 9.4 | 19 | |
| Prunus avium [65] | 1.6 | ni | 1.9 | ni | 1.5 | 0.6 | 5.0 | 175 | 29 | 15 | 46 | ni | ni | - | 91.7 | 535 | 8.6 | 18 | |
| Fraxinus excelsior from Europe [23] | 6.0 | 0.3 | 2.3 | 0.5 | 2.2 | 11 | 20 | 59 | 11 | 31 | 60 | nd | nd | - | 119 | 334 | 13 | 14 | |
| Fraxinus excelsior from Europe [65] | 12 | ni | 3.4 | ni | 3.0 | 12 | 23 | 64 | 15 | 32 | 59 | ni | ni | - | 160 | 376 | 15 | 22 | |
| Fraxinus americana from American [23] | 14 | 0.4 | 2.6 | 1.2 | 3.2 | 17 | 21 | 82 | 20 | 31 | 90 | nd | nd | - | 162 | 311 | 19 | 31 | |
| Fraxinus americana from American [65] | 13 | ni | 2.6 | ni | 3.1 | 11 | 24 | 62 | 17 | 33 | 82 | ni | ni | - | 187 | 351 | 17 | 29 | |
| Traditional Quercus [23] | |||||||||||||||||||
| Q. Petraea | 2.4 | 0.4 | 7.2 | 0.2 | 1.8 | 0.8 | 5.5 | 430 | 35 | 18 | 23 | 15 | 21 | 1.5 | 117 | 221 | 9.1 | 15 | |
| Q. Alba | 4.9 | 0.4 | 5.9 | 0.2 | 1.3 | 1.3 | 9.7 | 395 | 38 | 21 | 21 | 3.4 | 32 | 9.5 | 102 | 226 | 9.0 | 113 | |
| Heat treatment: 210 °C for 20 min | |||||||||||||||||||
| Alternative woodsQ. pyrenaica from Navarra [40] | |||||||||||||||||||
| Staves | 24 m open air | 1.0 | ns | 1.5 | ns | 5.9 | 1.7 | 2.7 | 1415 | 185 | 16 | 275 | 8.7 | 92 | 11 | 55 | 184 | 4.8 | ns |
| Accelerated | 1.6 | ns | 2.3 | ns | 5.6 | 3.8 | 6.5 | 2820 | 301 | 25 | 540 | 8.2 | 106 | 13 | 39 | 186 | 3.9 | ns | |
| Chips | 24 m open air | 1.0 | ns | 4.3 | ns | 1.9 | 1.2 | 3.7 | 161 | 30 | 38 | 91 | 9.8 | 40 | 4.1 | 177 | 373 | 13 | ns |
| Accelerated | 1.2 | ns | 3.9 | ns | 3.6 | 1.8 | 4.6 | 345 | 49 | 41 | 118 | 4.9 | 21 | 4.3 | 125 | 338 | 9.1 | ns | |
| Heat treatment: 200 °C for 35 min | |||||||||||||||||||
| Alternative woodsQ. pyrenaica from Salamanca [6] | |||||||||||||||||||
| staves | 36 m natural seasoning | 2.7 | 0.2 | 2.4 | 0.2 | 6.8 | 0.5 | 4.1 | 1636 | 193 | 20 | 182 | 8.3 | 32 | 3.9 | 40 | 79 | 2.2 | ns |
| chips | 3.8 | 0.4 | 6.9 | 0.3 | 2.1 | 0.2 | 6.8 | 458 | 54 | 37 | 29 | 9.9 | 32 | 3.2 | 113 | 229 | 8.9 | ns | |
| Traditional Quercus [6] | |||||||||||||||||||
| Q. petraea | 2.3–2.5 | 0.3–0.4 | 3.8–7.2 | 0.13–0.16 | 0.8–2.3 | 0.2–0.7 | 5.1–5.7 | 437–963 | 36–148 | 19 | 22–100 | 0.0–13 | 0.1–23 | 1.8–5.0 | 53–113 | 102–213 | 3.6–8.8 | ns | |
| Q. alba | 4.2–4.5 | 0.2–0.4 | 2.1–5.9 | 0.15–0.18 | 1.3–4.2 | 0.3–0.9 | 7.1–9.5 | 372–1539 | 38–237 | 17–21 | 21–122 | 3.3–6.3 | 21–32 | 3.3–9.7 | 42–99 | 86–229 | 2.3–8.4 | ns | |
| Heat treatment: 210 °C for 45 min | |||||||||||||||||||
| Alternative woods [7] | |||||||||||||||||||
| Q. humboldtii | semi-accelerated | 3.7 | ns | ns | 0.7 | 3.3 | 7.2 | 10 | 534 | 45 | ns | 463 | 0.0 | 0.3 | 5.0 | 22 | ns | ns | ns |
| Traditional Quercus [7] | 30 m natural seasoning | ||||||||||||||||||
| Q. petraea | 0.4–0.6 | ns | ns | 0.5 | 1.6–5.6 | 1.7–2.1 | 1.4–1.9 | 924–1150 | 23–28 | ns | 579–1192 | 2.1–20 | 13 | 0.7–6.2 | 23 | ns | ns | ns | |
| Q. alba | 0.9 | ns | ns | 0.5 | 2.1 | 1.5 | 3.4 | 1363 | 17 | ns | 804 | 3.5 | 27 | 7.8 | 57 | ns | ns | ns | |
| Heat treatment: medium plus | |||||||||||||||||||
| Alternative woodsas staves and chips [66] | |||||||||||||||||||
| Q. pyrenaica | 24 m open air | 2.6 | 0.3 | 2.6 | 1.4 | 6.1 | 1.1 | 11 | 1140 | 169 | 28 | 494 | 20 | 76 | 3.9 | 80 | 206 | 8.9 | ns |
| Traditional Quercusas staves and chips [66] | |||||||||||||||||||
| French oak | 2.3 | 0.4 | 3.6 | 0.6 | 1.7 | 0.3 | 8.1 | 301 | 38 | 16 | 54 | 6.1 | 6.1 | 1.0 | 145 | 731 | 9.3 | ns | |
| American oak | 4.1 | 0.6 | 4.6 | 1.0 | 2.7 | 0.5 | 12.3 | 825 | 71 | 18 | 66 | 6.6 | 17 | 2.6 | 143 | 632 | 10 | ns | |
| Heat treatment: 250–260 °C for 27 min | |||||||||||||||||||
| Alternative woodsQ. pyrenaica from Portugal [63] | |||||||||||||||||||
| Gerês forest | 24 m natural seasoning | 1.9 | ns | 1.7 | ns | nd | 0.9 | ns | 1635 | 173 | ns | 2977 | 7.2 | 9.6 | 1.3 | 34 | 69 | ns | ns |
| Guarda forest | 1.6 | ns | 0.8 | ns | 0.5 | 0.8 | ns | 2155 | 329 | ns | 2307 | 5.0 | 2.9 | 0.6 | 22 | 89 | ns | ns | |
| Traditional Quercus [63] | |||||||||||||||||||
| Q. petraea | 1.7–2.6 | ns | nd-0.6 | ns | 1.6–2.2 | 0.5–0.6 | ns | 118–613 | 244–250 | ns | 654–981 | 5.3–12 | 7.4–18 | 1.4 | 3.0–6.3 | 85–118 | ns | ns | |
| Q. alba | 3.3–5.3 | ns | 2.8–4.6 | ns | 5.1–7.9 | 0.8 | ns | 353–788 | 167–363 | ns | 782–922 | 4.2–7.4 | 16–24 | 3.2–3.8 | 7.5–8.8 | 32–21 | ns | ns | |
| Heat treatment: heavy | |||||||||||||||||||
| Alternative woodsas staves and chips [66] | |||||||||||||||||||
| Q. pyrenaica | 24 m open air | 3.1 | 0.4 | 3.0 | 0.8 | 4.4 | 0.6 | 9.8 | 1126 | 130 | 31 | 214 | 8.7 | 52 | 6.0 | 73 | 241 | 8.2 | ns |
| Traditional Quercusas staves and chips [66] | |||||||||||||||||||
| French oak | 1.7 | 0.4 | 3.5 | 0.7 | 0.9 | 0.1 | 11 | 170 | 25 | 16 | 44 | 4.4 | 7.6 | 1.7 | 262 | 721 | 15 | ns | |
| American oak | 2.1 | 0.4 | 1.6 | 0.4 | 1.0 | 0.1 | 16 | 61 | 11 | 12 | 30 | 2.8 | 15 | 5.3 | 244 | 768 | 14 | ns | |
| Volatile Phenols | Furanic Compounds | Lactones | Phenolic Aldehydes/ Phenyl Ketones | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat Treatment | Time (months) | Wine | Species | Guaiacol | 4-EG | 4-MG | 4-VG | Eugenol | Isoeugenol | Syringol | Furfural | 5-MF | Maltol | 5-HMF | trans WL | cis WL | cis/trans Ratio | Vanillin | Syringaldehyde | Acetovanillone | Butyrovanillone |
| Alternative woods | |||||||||||||||||||||
| 100 °C for 60 min | 4 | White [72] | Robinia pseudoacacia | 0.8 | 0.7 | 0.3 | 3.0 | 0.6 | ns | 1.5 | 9.2 | 0.1 | ns | 0.8 | ns | ns | ns | 1.2 | 1.0 | 35 | ns |
| nontoasted | 6 | Red [73] | Castanea sativa | 35 | 134 | 24.5 | 41 | 139 | 63 | 285 | 9.7 | 64 | 2.4 | 3.3 | ns | ns | ns | 157 | 108 | 136 | 57 |
| 160–170 °C for 45–50 min | 6 | Red [73] | Castanea sativa | 35 | 124 | 30 | 40 | 162 | 92 | 242 | 15.7 | 184 | 3.3 | 6.2 | ns | ns | ns | 241 | 253 | 155 | 58 |
| Alternative woods | |||||||||||||||||||||
| °C offire for 40 min | 9 | Red [63,74] | Robinia pseudoacacia | ns | 3250 | ns | ns | 21 | ns | ns | 30 | 30 | ns | ns | ns | ns | ns | 310 | ns | ns | ns |
| Castanea sativa | ns | 1840 | ns | ns | 26 | ns | ns | 70 | 40 | ns | ns | ns | ns | ns | 430 | ns | ns | ns | |||
| Prunus avium | ns | 2790 | ns | ns | 7 | ns | ns | nd | nd | ns | ns | ns | ns | ns | 120 | ns | ns | ns | |||
| Morus alba | ns | 1840 | ns | ns | 6 | ns | ns | nq | nq | ns | ns | ns | ns | ns | 80 | ns | ns | ns | |||
| Traditional Quercus | |||||||||||||||||||||
| Q. petraea | ns | 2900 | ns | ns | 18 | ns | ns | 600 | 320 | ns | ns | ns | ns | ns | 360 | ns | ns | ns | |||
| Alternative woods | |||||||||||||||||||||
| 165 °C for 35 min | 12 | Red [29] | Q. pyrenaica | 31–76 | 5.3–45 | 17–37 | 93–315 | 81–116 | 42–111 | 241–736 | 134–3575 | 94–2477 | 111–282 | 757–3533 | 184–231 | 718–1096 | 3.9–5.1 | 342–574 | 553–1748 | 121–223 | 897–1164 |
| Traditional Quercus | |||||||||||||||||||||
| Q. petraea | 25–40 | 5.6–105 | 17–48 | 68–409 | 23–44 | 27–120 | 194–631 | 110–1543 | 51–869 | 92–163 | 986–1611 | 125–735 | 396–1174 | 1.6–3.2 | 357–854 | 735–2244 | 102–309 | 417–981 | |||
| Q. alba | 27–55 | 5.0–77 | 16–66 | 109–269 | 50–63 | 30–82 | 185–724 | 187–5256 | 42–1543 | 159–197 | 713–2723 | 141–236 | 992–1313 | 5.6–7.0 | 437–806 | 1378–2274 | 128–286 | 488–995 | |||
| Alternative woods | |||||||||||||||||||||
| 185 °C for 45 min | 12 | Red [75] | Robinia pseudoacacia | 60 | 20 | 14 | 26 | 19 | 54 | 217 | 238 | 450 | 300 | 248 | nd | nd | 233 | 768 | 61 | 105 | |
| Castanea sativa | 59 | 49 | 52 | 24 | 118 | 36 | 153 | 509 | 241 | 125 | 689 | 21 | 31 | 1.5 | 456 | 1189 | 92 | 226 | |||
| Prunus avium | 43 | 73 | 25 | 23 | 11 | 19 | 169 | 101 | 32 | 133 | 145 | nd | nd | nd | 304 | 1877 | 75 | 132 | |||
| Fraxinus excelsior | 75 | 92 | 35 | 31 | 13 | 37 | 198 | 66 | 58 | 354 | 339 | nd | nd | nd | 696 | 1090 | 111 | 150 | |||
| Traditional Quercus | |||||||||||||||||||||
| Q. petraea | 44 | 24 | 32 | 21 | 101 | 53 | 178 | 40 | 842 | 142 | 703 | 99 | 577 | 408 | 1305 | 62 | 193 | ||||
| Alternative woodsRobinia pseudoacacia | |||||||||||||||||||||
| Medium | 12 | White [76] | from Medjimurje | 31 | 2.6 | ns | ns | 8.0 | 33 | ns | 1236 | 251 | ns | ns | 0.3 | 0.5 | 0.03 | 0.3 | ns | ns | |
| from Istria | 2.7 | 0.7 | ns | ns | 2.5 | 8.6 | ns | 83 | 4.3 | ns | ns | 0.2 | 0.4 | 0.02 | 0.3 | ns | ns | ||||
| Traditional Quercus | |||||||||||||||||||||
| Q. petraea | 4.9–9.2 | 0.6–1.3 | ns | ns | 4.4–6.6 | 3.6–7.9 | ns | 740–1796 | 93–173 | ns | ns | 0.5–39 | 11–43 | 0.02–0.05 | 0.1 | ns | ns | ||||
| Alternative woods | |||||||||||||||||||||
| Medium | 21 | Red [77,78] | Q. pyrenaica | 20 | ns | ns | ns | 42 | ns | ns | 49 | 15 | 78 | 49 | 67 | 229 | 3.4 | 78 | 75 | ns | ns |
| Q. faginea | 22 | ns | ns | ns | 41 | ns | ns | 44 | 13 | 72 | 26 | 103 | 352 | 3.4 | 74 | 64 | ns | ns | |||
| Traditional Quercus | |||||||||||||||||||||
| Q. petraea | 24–27 | ns | ns | ns | 35–48 | ns | ns | 90–93 | 26–28 | 118–135 | 49–75 | 28–150 | 66–347 | 2.3–2.4 | 80–91 | 135–150 | ns | ns | |||
| Q. robur | 23–28 | ns | ns | ns | 37–54 | ns | ns | 60–67 | 13–26 | 98–117 | 20–56 | 101–185 | 331–407 | 1.8–4.1 | 59–84 | 72–136 | ns | ns | |||
| Q. alba | 43 | ns | ns | ns | 89 | ns | ns | 124 | 96 | 172 | 85 | 92 | 788 | 8.8 | 145 | 168 | ns | ns | |||
| Volatile Phenols | Furanic Compounds | Lactones | Phenolic Aldehydes/Phenyl Ketones | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat Treatment | Dosage | Contact Time Days | Wine Type | Species | Guaiacol | 4-EG | 4-MG | 4-VG | Eugenol | Isoeugenol | Syringol | Furfural | 5-MF | Maltol | 5-HMF | trans WL | cis WL | cis/trans Ratio | Vanillin | Syringaldehyde | Acetovanillone | Butyrovanillone |
| Chips | ||||||||||||||||||||||
| Alternative woods | ||||||||||||||||||||||
| Medium | 1 g/L | 20 | Rosé [79] | Robinia pseudoacacia | ns | ns | ns | 1295 | 2 | ns | ns | 1746 | 329 | ns | ns | nq | 3 | nq | 2 | nq | ns | ns |
| 1.5 g/L | Robinia pseudoacacia | ns | ns | ns | 1734 | 3 | ns | ns | 2233 | 413 | ns | ns | nq | 20 | nq | 16 | 72 | ns | ns | |||
| 1 g/L | Prunus avium | ns | ns | ns | 1485 | 2 | ns | ns | 1350 | 281 | ns | ns | nq | 1 | nq | 39 | 74 | ns | ns | |||
| 1.5 g/L | Prunus avium | ns | ns | ns | 1704 | 3 | ns | ns | 1557 | 400 | ns | ns | nq | 7 | nq | 39 | 117 | ns | ns | |||
| Traditional Quercus | ||||||||||||||||||||||
| 1 g/L | Q. petraea | ns | ns | ns | 1484 | 4 | ns | ns | 2403 | 1435 | ns | ns | nq | 13 | nq | 392 | 572 | ns | ns | |||
| 1.5 g/L | Q. petraea | ns | ns | ns | 1819 | 5 | ns | ns | 1722 | 1439 | ns | ns | 13 | 29 | 2.23 | 722 | 860 | ns | ns | |||
| 1 g/L | Q. alba | ns | ns | ns | 1347 | 4 | ns | ns | 1788 | 1265 | ns | ns | nq | 49 | nq | 309 | 489 | ns | ns | |||
| 1.5 g/L | Q. alba | ns | ns | ns | 1914 | 5 | ns | ns | 2715 | 1919 | ns | ns | nq | 70 | nq | 748 | 1110 | ns | ns | |||
| Alternative woods | ||||||||||||||||||||||
| Nontoasted | 7 g/L | 25 | Red [80] | Castanea sativa | 116 | nd | nd | 115 | 44 | 18 | 655 | 4.4 | nd | nd | nd | ns | ns | ns | 41 | 37 | 66 | 53 |
| 100 °C for 60 min | 4 g/L | 30 | White [72] | Robinia pseudoacacia | 0.8 | 0.9 | 0.5 | 27 | 0.6 | ns | 1.3 | 8.7 | 0.2 | ns | 4.9 | ns | ns | ns | 3 | 2.1 | 34 | ns |
| Alternative woods | ||||||||||||||||||||||
| Medium | 4 g/L | 60 | Red [31] | Q. pyrenaica | 12 | 0.2 | ns | 6.7 | 8 | 3.6 | 15 | 1660 | 464 | ns | ns | 124 | 92 | 0.7 | 246 | 694 | 42 | ns |
| Q. pyrenaica | 12 | 0.4 | ns | 8.4 | 8.2 | 4.2 | 16 | 2722 | 758 | ns | ns | 13 | 31 | 2.38 | 364 | 959 | 48 | ns | ||||
| Traditional Quercus | ||||||||||||||||||||||
| Q. petraea | 18 | 0.3 | ns | 9.1 | 33 | 7.4 | 21 | 1848 | 1005 | ns | ns | 37 | 86 | 2.3 | 220 | 505 | 41 | ns | ||||
| Q. petraea+ Q. robur | 12 | 0.5 | ns | 7.7 | 9.4 | 5.9 | 16 | 168 | 169 | ns | ns | 30 | 66 | 2.2 | 774 | 1449 | 56 | ns | ||||
| Q. alba | 18 | 0.9 | ns | 10 | 14 | 9.8 | 38 | 90 | 179 | ns | ns | 27 | 188 | 7.0 | 988 | 2541 | 82 | ns | ||||
| Alternative woods | ||||||||||||||||||||||
| 210 °C for 45 min | 3 g/L | 90 | Red [7] | Q. humboldtii | 26 | ns | ns | 14 | 14 | 34 | 58 | 511 | 386 | ns | 319 | 8.3 | 8.3 | 1.0 | 62 | ns | ns | ns |
| Traditional Quercus | ||||||||||||||||||||||
| Q. alba | 3.9 | ns | ns | 13 | 7.6 | 6.9 | 8.7 | 1422 | 181 | ns | 702 | 33 | 3267 | 9.9 | 149 | ns | ns | ns | ||||
| Q. petraea | 3.3–3.8 | ns | ns | 12–13 | 4–16 | 6 | 10 | 1485–2192 | 190–260 | ns | 693–958 | 43–99 | 43–99 | 256–360 | ns | ns | ns | |||||
| Alternative woodsQ. pyrenaica | ||||||||||||||||||||||
| 190 °C for 10 min | 225 L barrel surface | 120 | Red [40] | traditional seasoning | 6.9 | 0.2 | 5.1 | 7.9 | 24 | 8.6 | 29 | 61 | 66 | 87 | 683 | 74 | 363 | 4.9 | 120 | 61 | ns | ns |
| accelerated seasoning | 6.1 | 0.3 | 3.9 | 6.3 | 42 | 8.7 | 28 | 134 | 74 | 84 | 351 | 55 | 180 | 3.3 | 83 | 76 | ns | ns | ||||
| 200 °C for 15 min | traditional seasoning | 8.2 | 0.4 | 10 | 5.9 | 19 | 11 | 35 | 120 | 100 | 178 | 659 | 80 | 396 | 5.0 | 293 | 203 | ns | ns | |||
| accelerated seasoning | 7.7 | 0.5 | 10 | 6.5 | 34 | 17 | 35 | 337 | 181 | 205 | 692 | 56 | 189 | 3.4 | 228 | 186 | ns | ns | ||||
| 210 °C for 20 min | traditional seasoning | 11 | 0.9 | 20 | 7.1 | 13 | 15 | 56 | 148 | 143 | 273 | 468 | 85 | 413 | 4.9 | 706 | 794 | ns | ns | |||
| accelerated seasoning | 13 | 1.2 | 22 | 6.6 | 23 | 27 | 68 | 240 | 252 | 338 | 727 | 55 | 223 | 4.1 | 530 | 811 | ns | ns | ||||
| Alternative woods | ||||||||||||||||||||||
| 200 °C for 35 min | 225 L barrel surface | 180 | Red [6] | Q. pyrenaica | 22 | 3.3 | 38 | 53 | 22 | 55 | 181 | 437 | 104 | 232 | 182 | 87 | 351 | 4.0 | 819 | 2393 | ns | ns |
| Traditional Quercus | ||||||||||||||||||||||
| Q. alba | 27 | 2.8 | 38 | 36 | 20 | 44 | 201 | 374 | 89 | 149 | 173 | 67 | 229 | 3.4 | 797 | 2533 | ns | ns | ||||
| Q. petraea | 22 | 2.9 | 45 | 45 | 17 | 46 | 156 | 715 | 145 | 144 | 240 | 122 | 289 | 2.4 | 811 | 2530 | ns | ns | ||||
| Staves | ||||||||||||||||||||||
| Alternative woodsQ. pyrenaica | ||||||||||||||||||||||
| 190 °C for 10 min | 225 L barrel surface | 120 | Red [40] | traditional seasoning | 16 | 76 | 13 | 15 | 51 | 31 | 92 | 48 | 146 | 127 | 2491 | 73 | 662 | 9.1 | 225 | 523 | ns | ns |
| accelerated seasoning | 19 | 13 | 16 | 14 | 75 | 33 | 68 | 98 | 207 | 136 | 3982 | 109 | 1442 | 13 | 188 | 432 | ns | ns | ||||
| 200 °C for 15 min | traditional seasoning | 24 | 45 | 22 | 13 | 45 | 27 | 78 | 136 | 622 | 250 | 4296 | 84 | 703 | 8.3 | 415 | 965 | ns | ns | |||
| accelerated seasoning | 33 | 1.6 | 29 | 24 | 76 | 41 | 153 | 13667 | 2135 | 323 | 7822 | 140 | 2342 | 17 | 326 | 918 | ns | ns | ||||
| 210 °C for 20 min | traditional seasoning | 33 | 31 | 33 | 16 | 38 | 38 | 146 | 232 | 473 | 446 | 4224 | 90 | 314 | 3.5 | 584 | 1456 | ns | ns | |||
| accelerated seasoning | 42 | 1.9 | 36 | 11 | 68 | 45 | 150 | 990 | 993 | 452 | 6177 | 215 | 2445 | 11 | 504 | 1234 | ns | ns | ||||
| Alternative woods | ||||||||||||||||||||||
| 200 °C for 35 min | 225 L barrel surface | 180 | Red [6] | Q. pyrenaica | 43.4 | 2.84 | 41 | 54 | 54 | 72 | 192 | 14262 | 2163 | 377 | 2485 | 99 | 588 | 5.9 | 740 | 1912 | ns | ns |
| Traditional Quercus | ||||||||||||||||||||||
| Q. alba | 44.3 | 2.68 | 34 | 31 | 36 | 71 | 184 | 13582 | 1802 | 305 | 1602 | 64 | 466 | 7.24 | 613 | 1716 | ns | ns | ||||
| Q. petraea | 36.5 | 3.39 | 55 | 40 | 29 | 64 | 184 | 8981 | 2063 | 372 | 1846 | 12 | 26 | 2.11 | 938 | 2304 | ns | ns | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gil, A.M.; del Alamo-Sanza, M.; del Barrio-Galán, R.; Nevares, I. Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review. Appl. Sci. 2022, 12, 2101. https://doi.org/10.3390/app12042101
Martínez-Gil AM, del Alamo-Sanza M, del Barrio-Galán R, Nevares I. Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review. Applied Sciences. 2022; 12(4):2101. https://doi.org/10.3390/app12042101
Chicago/Turabian StyleMartínez-Gil, Ana María, Maria del Alamo-Sanza, Rubén del Barrio-Galán, and Ignacio Nevares. 2022. "Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review" Applied Sciences 12, no. 4: 2101. https://doi.org/10.3390/app12042101
APA StyleMartínez-Gil, A. M., del Alamo-Sanza, M., del Barrio-Galán, R., & Nevares, I. (2022). Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review. Applied Sciences, 12(4), 2101. https://doi.org/10.3390/app12042101









