Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic Survival, In Vitro Digestion and Viability in Apple Juice and Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Probiotic Bacteria
2.2. Preparation of Capsules Loaded with Lacticaseibacillus rhamnosus GG
2.3. Viability of Lacticaseibacillus rhamnosus GG
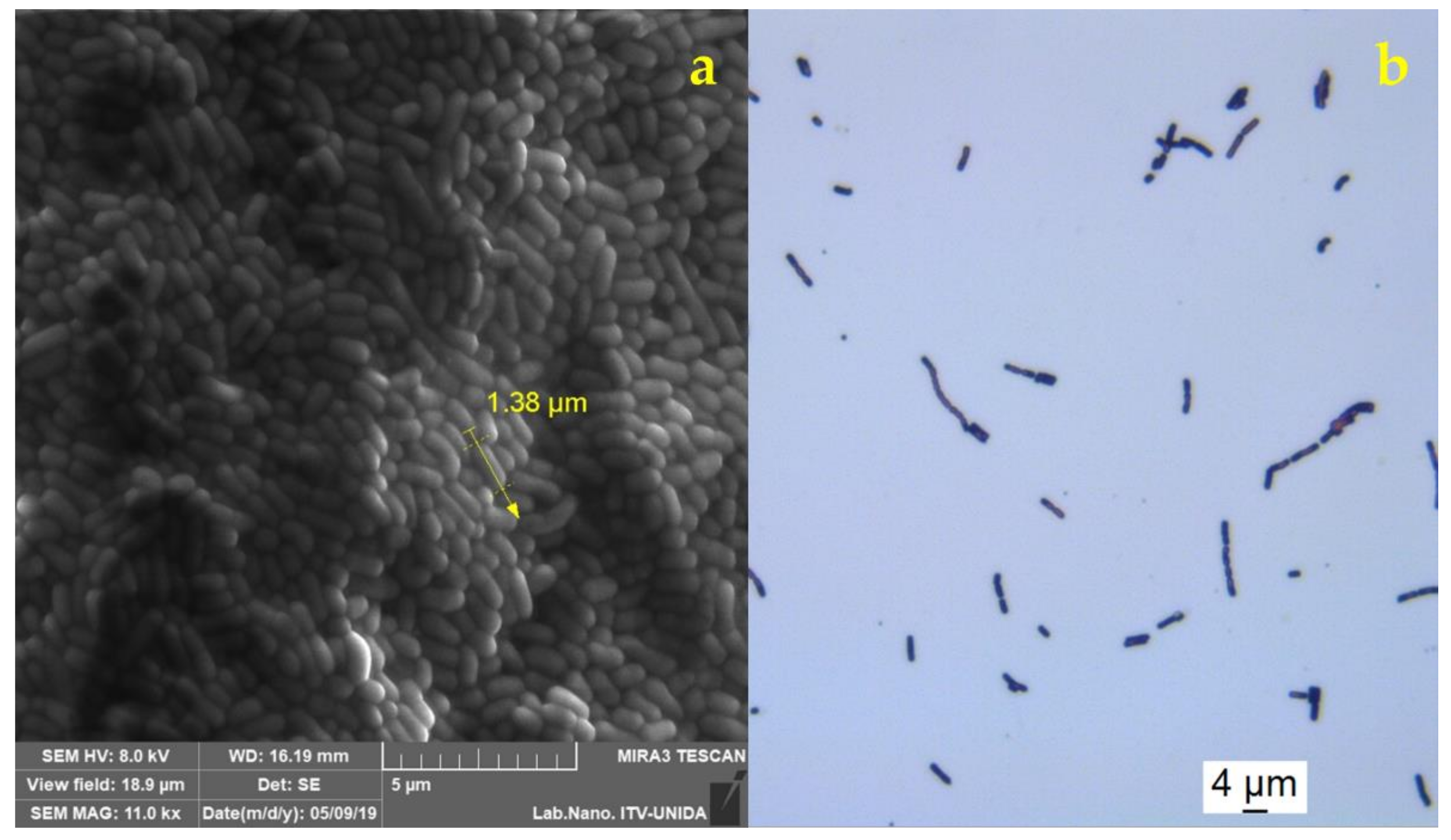
2.4. Morphological Analysis of Free and Encapsulated Lacticaseibacillus rhamnosus GG
2.5. Preparation of Food Matrices Inoculated with Lacticaseibacillus rhamnosus GG: Yogurt and Juice
2.6. Viability of Encapsulated and Free Lacticaseibacillus rhamnosus GG in Gastrointestinal Conditions In Vitro
2.7. Statistical Analysis
3. Results and Discussion
3.1. Viability of Encapsulated Lacticaseibacillus rhamnosus GG
3.2. Morphological Analysis of Encapsulated and Free Lacticaseibacillus rhamnosus GG
3.3. Viability of Encapsulated Lacticaseibacillus rhamnosus GG in Simulated Gastric Conditions
3.4. Viability of Lacticaseibacillus rhamnosus GG Encapsulated and Added to Food Matrices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 17 December 2021).
- Ji, Y.; Park, S.; Park, H.; Hwang, E.; Shin, H.; Pot, B.; Holzapfel, W.H. Modulation of Active Gut Microbiota by Lactobacillus rhamnosus GG in a Diet Induced Obesity Murine Model. Front. Microbiol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, K.; Ji, Y.; Park, S.; Holzapfel, W.; Hyun, C. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem. Biophys. Res. Commun. 2016, 473, 530–536. [Google Scholar] [CrossRef]
- Shin, J.; Nam, M.; Lee, H.; Lee, J.; Kim, H.; Chung, M.; Seo, J. Amelioration of obesity-related characteristics by a probiotic formulation in a high-fat diet-induced obese rat model. Eur. J. Nutr. 2017, 57, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Fact. 2014, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Lifeder. Lactobacillus rhamnosus: Características, Morfología. Available online: https://www.lifeder.com/lactobacillus-rhamnosus/ (accessed on 23 July 2020).
- Cheng, Y.; Liu, J. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- De Araújo, U.N.; Gutiérrez, R.L.; Ruiz, V.O.; Montoya, C.O. Técnicas para la microencapsulación de probióticos y el impacto en su funcionalidad: Una revisión. Aliment. Hoy 2015, 23, 112–126. [Google Scholar]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsøyen, E. Alginate as a Source of Dietary Fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araújo, E.M.; Lorenzoni, N.G.; Righi, N.B.; Smanioto, B.J.; Moraes, F.E.; Olivera, M.R.; Ragagnin, M.C. Improvement of the viability of encapsulated probiotics using whey proteins. LWT Food Sci. Technol. 2020, 117, 108601. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Effect of homogenization techniques on reducing the size of microcapsules and the survival of probiotic bacteria therein. J. Food Sci. 2009, 74, 231–236. [Google Scholar] [CrossRef]
- Seth, D.; Mishra, H.; Deka, S. Effect of microencapsulation using extrusion technique on viability of bacterial cells during spray drying of sweetened yoghurt. Int. J. Biol. Macromol. 2017, 103, 802–807. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Braschi, G.; Betoret, E.; Reinheimer, J.A.; Lanciotti, R. Microencapsulation of functional strains by high pressure homogenization for a potential use in fermented milk. Food Res. Int. 2017, 97, 250–257. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Álvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT Food Sci. Technol. 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- Zazzali, I.; Aguirre, C.; Pizones, R.; Santagapita, P.R.; Perullini, M. Effects of pH, extrusion tip size and storage protocol on the structural properties of Ca (II) alginate beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef]
- Dorożyński, P.; Kulinowski, P.; Mendyk, A.; Młynarczyk, A.; Jachowicz, R. Novel application of MRI technique combined with flow-through cell dissolution apparatus as supportive discriminatory test for evaluation of controlled release formulations. AAPS PharmSciTech. 2010, 11, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Perricone, M.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Viability of Lactobacillus reuteri in fruit juices. J. Funct. Foods 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Ortakci, F.; Sert, S. Stability of free and encapsulated Lactobacillus acidophilus ATCC 4356 in yoghurt and in an artificial human gastric digestion system. J. Dairy Sci. 2012, 95, 6918–6925. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García- Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Ribeiro, M.; Chaves, K.; Gebara, C.; Infante, F.; Grosso, C.; Gigante, M. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Zeashan, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Tufail, T.; Ahmed, A.; Muhammad, A.F. Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions. Food Sci. Nutr. 2020, 8, 2419–2426. [Google Scholar] [CrossRef]
- Sánchez, M.T.; Ruiz, M.A.; Lasserrot, A.; Hormigo, M.; Morales, M.E. An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study. Food Hydrocoll. 2017, 69, 67–75. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of homogenisation on bead size and survival of encapsulated probiotic bacteria. Food Res. Int. 2007, 40, 1261–1269. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Liu, H.; Lai, T.; Ma, J.; Wang, J.; Zhu, Y. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet. Microbiol. 2010, 141, 142–148. [Google Scholar] [CrossRef]
- Van Leusden, P.; den Hartog, G.J.M.; Bast, A.; Postema, M.; van der Linden, E.; Sagis, L.M.C. Strength of microbeads for the encapsulation of heat sensitive, hydrophobic components. Food Hydrocoll. 2016, 56, 318–324. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Alginate submicron beads prepared through w/o emulsification and gelation with CaCl2 nanoparticles. Food Hydrocoll. 2013, 31, 428–434. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Charalampopoulos, D. Encapsulation of Bifidobacterium longum in alginate-dairy matrices and survival in simulated gastrointestinal conditions, refrigeration, cow milk and goat milk. Food Biosci. 2018, 21, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Guedes, S.; Cezarino, E.; Michelon, M.; Kawazoe, S. Symbiotic microencapsulation to enhance Lactobacillus acidophilus survival. LWT Food Sci. Technol. 2018, 89, 503–509. [Google Scholar]
- Kumar, N.A.; Saquib, H.M. Alginates in Drug Delivery, 1st ed.; Academic Press: San Diego, CA, USA, 2020; pp. 1–416. [Google Scholar]
- Helgerud, T.; Gåserød, O.; Fjæreide, T.; Andersen, P.; Larsen, C. Alginates. In Food Stabilisers, Thickeners and Gelling Agents, 1st ed.; Imeson, A., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 50–69. [Google Scholar]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2018, 25, 120–129. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [Green Version]
- Cueto-Vigil, M.; Acuña-Monsalve, Y.; Valenzuela-Riaño, J. In Vitro evaluation of probiotic potential of lactic bacteria acid isolated from Coastal serum. Actu. Biol. 2010, 32, 129–138. [Google Scholar]
- Ding, W.K.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple Juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar]
- Van Leusden, P.; den Hartog, G.J.M.; Bast, A.; Postema, M.; van der Linden, E.; Sagis, L.M.C. Permeation of probe molecules into alginate microbeads: Effect of salt and processing. Food Hydrocoll. 2017, 73, 255–261. [Google Scholar] [CrossRef]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Yoha, K.S.; Nida, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Targeted delivery of probiotics: Perspectives on research and commercialization. Probiotics Antimicrob. Proteins 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]







| Method | log CFU/mL (N0) | log CFU/mL (N) | Viability (%) |
|---|---|---|---|
| AM | 10.55 ± 0.02 a | 8.08 ± 0.07 b | 76.59 |
| UT | 10.55 ± 0.03 a | 9.05 ± 0.13 b | 85.80 |
| Method | Maximum Feret Diameter (mm) | Minimum Feret Diameter (mm) | Circularity |
|---|---|---|---|
| AM | 2.68 ± 0.12 a | 2.33 ± 0.13 a | 0.94 ± 0.03 a |
| UT | 2.67 ± 0.05 a | 2.55 ± 0.06 b | 1 ± 0.00 b |
| Method | Initial (log CFU/mL) | Final (log CFU/mL) | Viability (%) |
|---|---|---|---|
| AM | 6.01 ± 0.60 a | 5.84 ± 0.80 a | 97.22 |
| UT | 5.71 ± 0.07 a | 5.72 ± 0.06 a | 100 |
| Free LGG | 7.04 ± 0.00 a | 6.21 ± 0.69 b | 88.28 |
| Method | log CFU/mL (N0) | log CFU/mL (N) | Viability (%) |
|---|---|---|---|
| AM | 5.74 ± 0.07 a | 4.94 ± 0.04 b | 86.16 |
| UT | 4.84 ± 0.21 a | 4.88 ± 0.14 a | 100.81 |
| Free LGG | 7.14 ± 0.07 a | 5.74 ± 0.05 b | 80.50 |
| Method | log CFU/mL (N0) | log CFU/mL (N) | Viability (%) |
|---|---|---|---|
| AM | 5.12 ± 0.11 a | 5.74 ± 0.26 a | 112.14 |
| UT | 5.45 ± 0.14 a | 5.56 ± 0.02 a | 102.04 |
| Free LGG | 7.04 ± 0.00 a | 6.52± 0.01 b | 92.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Chapol, O.O.; Varela-Pérez, A.; Castillo-Olmos, A.G.; García, H.S.; Singh, J.; García-Ramírez, P.J.; Viveros-Contreras, R.; Figueroa-Hernández, C.Y.; Cano-Sarmiento, C. Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic Survival, In Vitro Digestion and Viability in Apple Juice and Yogurt. Appl. Sci. 2022, 12, 2141. https://doi.org/10.3390/app12042141
Romero-Chapol OO, Varela-Pérez A, Castillo-Olmos AG, García HS, Singh J, García-Ramírez PJ, Viveros-Contreras R, Figueroa-Hernández CY, Cano-Sarmiento C. Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic Survival, In Vitro Digestion and Viability in Apple Juice and Yogurt. Applied Sciences. 2022; 12(4):2141. https://doi.org/10.3390/app12042141
Chicago/Turabian StyleRomero-Chapol, Oscar O., Abigail Varela-Pérez, Ana G. Castillo-Olmos, Hugo S. García, Jaspreet Singh, Pedro J. García-Ramírez, Rubí Viveros-Contreras, Claudia Y. Figueroa-Hernández, and Cynthia Cano-Sarmiento. 2022. "Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic Survival, In Vitro Digestion and Viability in Apple Juice and Yogurt" Applied Sciences 12, no. 4: 2141. https://doi.org/10.3390/app12042141










