Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fermentation Process
2.3. Kefir Grain Biomass Determination
2.4. Microbiological Analysis
2.5. Microstructure Analysis
2.6. Confocal Laser Scanning Microscopy
2.7. Image Digital Analysis
2.8. Image Texture Analysis
- (a)
- Energy measures uniformity textural image and is an opposite parameter to entropy. The energy parameter is also known as uniformity, energy uniformity and angular second moment (ASM).
- (b)
- Contrast is a measure of local variations in grayscale value pixels of an image. Contrast is also known as variance or inertia.
- (c)
- Inverse difference moment (IDM), also known as homogeneity parameter, which is a measure similar to energy; it also represents the homogeneity of the local image.
- (d)
- Entropy measures the disorder or randomness of images, can be used to characterize the texture of the image and is indicative of complexity within the image. Therefore, complex images will have high values of entropy.
2.9. Statistical Analysis
3. Results and Discussions
3.1. Fermentation Process Measurement
3.2. Biomass Increase
3.3. Texture Image Analysis
3.4. Confocal Laser Scanning Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeynep, B.; Guzel, S.; Gokırmakl, C.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Nielsen, B.; Gürakan, G.C.; Ünlü, G. Kefir: A multifaceted fermented dairy product. Probiotics Antimicrob. Proteins 2014, 6, 123–135. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.; Grzeskowiak, Å.M.; Reis, S.A.; Conceição, L.L.; Carmo, G.P. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sharma, H.; Melekoglu, E.; Ozogul, F. Recent developments in dairy kefir-derived lactic acid bacteria and their health benefits. Food Biosci. 2022, 46, 101592. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Microbial Interactions in Kefir: A natural probiotic drink. Biotech. Lac. Acid. Bact. Novel. Appl. 2010, 327. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.E.Y.; Wyffels, J.T.; Seydim, A.C.; Greene, A.K. Turkish kefir and kefir grains: Microbial enumeration and electron microscopic observation. Int. J. Dairy Technol. 2005, 58, 25–29. [Google Scholar] [CrossRef]
- Yamin, H.; Elmali, M.; Karadagoglu, G.; Cetinkaya, A. Observations of kefir grains and their structure from different geographical regions: Turkey and Germany. Ataturk Univ. Vet Bil. Derg. 2006, 1, 11–15. [Google Scholar]
- Guzel-Seydim, Z.; Kok-Tas, T.; Ertekin-Filiz, B.; Seydim, A.C. Effect of different growth conditions on biomass increase in kefir grains. Int. J. Dairy Sci. 2011, 94, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. Int. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Atalar, I. Functional kefir production from high pressure homogenized hazelnut milk. LWT-Food Sci. Technol. 2019, 107, 256–263. [Google Scholar] [CrossRef]
- Karim, A.; Aider, M. Comprehensive utilisation of electro-activated whey-based media in cell growth, metabolite production and aroma compounds synthesis using a starter culture originated from kefir grain. Int. Dairy J. 2022, 126, 105276. [Google Scholar] [CrossRef]
- Harta, O.; Iconomopoulou, M.; Bekatorou, A.; Nigam, P.; Kontominas, M.; Koutinas, A.A. Effect of various carbohydrate substrates on the production of kefir grains for use as a novel baking starter. Food Chem. 2004, 88, 237–242. [Google Scholar] [CrossRef]
- Zajšek, K.; Goršek, A.; Kolar, M. Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem. 2013, 139, 970–977. [Google Scholar] [CrossRef]
- Apar, D.K.; Demirhan, E.; Özel, B.; Özbek, B. Kefir grain biomass production: Influence of different culturing conditions and examination of growth kinetic models. J. Food Process Eng. 2017, 40, e12332. [Google Scholar] [CrossRef]
- Costa, G.M.; Paula, M.M.; Barão, C.E.; Klososki, S.J.; Bonafé, E.G.; Visentainer, J.V.; Pimentel, T.C. Yoghurt added with Lactobacillus casei and sweetened with natural sweeteners and/or prebiotics: Implications on quality parameters and probiotic survival. Int. Dairy J. 2019, 97, 139–148. [Google Scholar] [CrossRef]
- López, M.G.; Urías-Silvas, J.E. Agave fructans as prebiotics. Recent Adv. Fruct. Res. 2007, 37, 1–14. [Google Scholar]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemical studies on the biopolymer inulin: A critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009, 91, 687–699. [Google Scholar] [CrossRef]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as texture modifier in dairy products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Mancilla-Margalli, N.A.; López, M.G. Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT-Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Chacón-Villalobos, A. Nutritional facts concerning goat milk (Capra hircus) and its variations during the agroindustrial process. Agron. Mesoamericana 2005, 16, 239–252. [Google Scholar]
- Arrizon, J.; Hernández-Moedano, A.; Oner, E.T.; González-Avila, M. In Vitro Prebiotic Activity of Fructans with Different Fructosly Linkage for Symbiotics Elaboration. Int. J. Probiotics Prebiotics 2014, 9, 69–76. [Google Scholar]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular structures of fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Aguirre, A.L.; Camacho-Ruiz, R.M.; Arriaga-Alba, M.; Padilla-Camberos, E.; Kirchmayr, M.R.; Blasco, J.L.; González-Avila, M. Effects of Agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal Lactobacilli/Bifidobacteria in obese mice. Food Funct. 2013, 4, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, T.; Ye, T.; Yang, X.; Xue, Y.; Shen, Y.; Zhang, Q.; Zheng, X. Effect of lactic acid bacteria and yeast on the structure and fermentation properties of Tibetan kefir grains. Int. Dairy J. 2021, 114, 104943. [Google Scholar] [CrossRef]
- Dong, J.; Liu, B.; Jiang, T.; Liu, Y.; Chen, L. The biofilm hypothesis: The formation mechanism of Tibetan kefir grains. Int. J. Dairy Technol. 2017, 71, 44–50. [Google Scholar] [CrossRef]
- Almeida, A.P.; Pinto, E.P.; Pinheiro, P.G.; Da, H.D.; Matos, R.S. Distribution of microorganisms on surface of Kefir biofilms associated with Açaí extract. Sci. Amaz. 2019, 8, 10–18. [Google Scholar]
- Ferraro, M.A.N.; Pinto, E.P.; Matos, R.S. Study of the superficial distribution of microorganisms in kefir biofilms prepared with Cupuaçu juice. J. Bioenergy Food Sci. 2020, 7, 2732019. [Google Scholar] [CrossRef]
- Quevedo, R.; Mendoza, F.; Aguilera, J.M.; Chanona, J.; Gutiérrez-López, G. Determination of senescent spotting in banana (Musa cavendish) using fractal texture Fourier image. J. Food Process Eng. 2008, 84, 509–515. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural features for image classification. IEEE Trans. Syst. Man. Cybern. Syst. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Du, C.J.; Sun, D.W. Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci. Technol. 2004, 15, 230–249. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mery, D.; Mendoza, F.; Aguilera, J. Classification of potato chips using pattern recognition. J. Food Sci. 2004, 69, E1–E5. [Google Scholar] [CrossRef]
- Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Camacho-Díaz, B.H.; Campos-Mendiola, R.; Martínez-Velarde, R.; Vargas-Solano, S.V.; López-Bonilla, A. Modular System and Process to Obtain Different Agave Fructan Products. Patent No. 380041, 1 December 2015. [Google Scholar]
- Barãoa, C.E.; Klososkia, S.J.; Pinheiroa, K.H.; Marcolinoa, V.A.; Juniorb, O.V.; da Cruzc, A.G.; Pimentela, T.C. Growth kinetics of kefir biomass: Influence of the incubation temperature in milk. Chem. Eng. 2019, 75, 499–504. [Google Scholar] [CrossRef]
- De Sainz, I.; Redondo-Solano, M.; Solano, G.; Ramírez, L. Effect of different kefir grains on the attributes of kefir produced with milk from Costa Rica. Int. J. Dairy Sci. 2020, 103, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Yuan, S.Y.; Hsiao, H.; Hsieh, C.M. Algorithms to estimating fractal dimension of textured images. In Proceedings of the 2001 IEEE International Conference on Acoustics, Speech, and Signal Processing, Salt Lake City, UT, USA, 7–11 May 2001; Volume 3, pp. 1541–1544. [Google Scholar]
- Fongaro, L.; Kvaal, K. Surface texture characterization of an Italian pasta by means of univariate and multivariate feature extraction from their texture images. Int. Food Res. J. 2013, 51, 693–705. [Google Scholar] [CrossRef]
- Fernandez, L.; Castillero, C.; Aguilera, J.M. An application of image analysis to dehydration of apple discs. J. Food Eng. 2005, 67, 185–193. [Google Scholar] [CrossRef]
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Colour and image texture analysis in classification of commercial potato chips. Int. Food Res. J. 2007, 40, 1146–1154. [Google Scholar] [CrossRef]
- Bulat, T.; Topcu, A. Influences of oxidation-reduction potential on kefir: Microbial counts, organic acids, volatile compounds and sensory properties. LWT-Food Sci. Technol. 2021, 144, 111195. [Google Scholar] [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Kaptan, B.; Kayısoglu, S.; Oksuz, O. Mathematical modeling of pH variation as a function of temperature and time in kefir production. A. J. Food Sci. Nutr. Res. 2015, 2, 57. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; Wiley Eastern Publication: New Delhi, India, 1974. [Google Scholar]
- Farnworth, E.R.; Mainville, I. Kefir: A fermented milk product. In Handbook of Fermented Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 89–127. [Google Scholar]
- Pogačić, T.; Šinko, S.; Zamberlin, Š.; Samaržija, D. Microbiota of kefir grains. Mljekarstvo 2013, 63, 3–14. [Google Scholar]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.F.; Moure, M.C.; Quiñoy, F.; Esposito, F.; Simonelli, N.; Medrano, M.; León-Peláez, A. Water kefir, a fermented beverage containing probiotic microorganisms: From ancient and artisanal manufacture to industrialized and regulated commercialization. Future Food 2022, 5, 100123. [Google Scholar] [CrossRef]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Pop, C.; Apostu, S.; Salanţă, L.; Rotar, A.M.; Sindic, M.; Mabon, N.; Socaciu, C. Influence of different growth conditions on the kefir grains production used in the kefiran synthesis. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. Int. 2014, 71, 147–153. [Google Scholar] [CrossRef][Green Version]
- Suwannakham, S.; Yang, S.T. Enhanced propionic acid fermentation by Propionibacterium acidi propionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol. Bioeng. 2005, 91, 325–337. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Wang, Y.; Liu, L.; Li, P. Screening, identification and statistic optimization of a novel exopolysaccharide producing Lactobacillus paracasei HCT. Afr. J. Microbiol. Res. 2010, 4, 783–795. [Google Scholar]
- Pingitore, E.V.; Pessione, A.; Fontana, C.; Mazzoli, R.; Pessione, E. Comparative proteomic analyses for elucidating metabolic changes during EPS production under different fermentation temperatures by Lactobacillus plantarum Q823. Int. J. Food Microbiol. 2016, 238, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef]
- Gao, X.; Li, B. Chemical and microbiological characteristics of kefir grains and their fermented dairy products: A review. Cogent Food Agric. 2016, 2, 1272152. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Camacho-Díaz, B.H.; Acosta-García, M.C.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. Effect of salt and sugar osmotic stress on the viability and morphology of Saccharomyces boulardii. Int. J. Environ. Agric. Biotechnol. 2016, 1, 593–602. [Google Scholar] [CrossRef]
- Olagnero, G.; Abad, A.; Bendersky, S.; Genevois, C.; Granzella, L.; Montonati, M. Alimentos funcionales: Fibra, prebióticos, probióticos y simbióticos. Diaeta 2007, 25, 20–33. [Google Scholar]
- Wang, N.; Nobel, P.S. Phloem transport of fructans in the crassulacean acid metabolism species Agave deserti. Plant Physiol. 1998, 116, 709–714. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Hernando, I.; Sotelo-Díaz, I.; Quintanilla-Carvajal, M.X.; Quiles, A. Use of image analysis to evaluate the effect of high hydrostatic pressure and pasteurization as preservation treatments on the microstructure of red sweet pepper. Innov. Food Sci. Emerg. Technol. 2015, 27, 69–78. [Google Scholar] [CrossRef]
- Valenzuela-Lagarda, J.L.; García-Armenta, E.; Pacheco-Aguilar, R.; Gutiérrez-Dorado, R.; Mazorra-Manzano, M.Á.; Lugo-Sánchez, M.E.; Muy-Rangel, M.D. Relationships between morphometrical properties and the texture of an extrusion-expanded snack made from squid mantle (Dosidicus gigas). J. Texture Stud. 2018, 49, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.S.; Gonçalves, E.; Pinto, E.P.; Lopes, G.A.; Ferreira, N.S.; Resende, C.X. Nanoscale morphology, structure and fractal study of kefir microbial films grown in natura. Polímeros 2020, 30, e2020033. [Google Scholar] [CrossRef]
- Ordoñez, J.A.; Rodrigues, M.I.C.; Alvarez, L.F.; Sanz, M.L.G.; Miguillon, G. Componentes dos Alimentos e Processos-Tecnología de Alimento; Editora Artmed: Porto Alegre, Brazil, 2005. [Google Scholar]
- Matos, R.S.; Pinheiro, B.S.; Souza, I.S.; de Castro, R.R.P.; Ramos, G.Q.; Pinto, E.P.; da Fonseca Filho, H.D. 3D micromorphology evaluation of kefir microbial films loaded with extract of Amazon rainforest fruit Cupuaçu. Micron 2020, 142, 102996. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.N.; Calderón-Domínguez, G.; Chanona-Pérez, J.; Gutiérrez-López, G.F.; León, A.E.; Ribotta, P.D. Evaluation of the mechanical damage on wheat starch granules by SEM, ESEM, AFM and texture image analysis. Carbohydr. Polym. 2013, 98, 1449–1457. [Google Scholar] [CrossRef]
- Oliveira, A.F.D.; Santos, C.B.; Ferreira, A.M.; Bezerra, R.M.; Zamora, R.R.M.; Cruz, R.A.S.; Carvalho, J.C.T. A Viability study for the production of biofilms and in silico predictions of major compounds in kefir. J. Comput. Theor. Nanosci. 2017, 14, 2915–2926. [Google Scholar] [CrossRef]
- Yang, X.; Beyenal, H.; Harkin, G.; Lewandowski, Z. Quantifying biofilm structure using image analysis. J. Microbiol. Methods 2000, 39, 109–119. [Google Scholar] [CrossRef]
- Park, B.; Chen, Y.R. AE—Automation and emerging technologies: Co-occurrence matrix texture features of multi-spectral images on poultry carcasses. J. Agric. Eng. Res. 2001, 78, 127–139. [Google Scholar] [CrossRef]
- Alves, V.; Scapini, T.; Camargo, A.F.; Bonatto, C.; Stefanski, F.S.; de Jesus, E.; Techi-Diniz, L.G.; Canhadas-Bertan, L.; Resende-Maldonado, R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. LWT Food Sci. Technol. 2021, 145, 111364. [Google Scholar] [CrossRef]
- Ali, S. Kinetics of invertase production by Saccharomyces cerevisiae in batch culture Pakistan. J. Bot. 2007, 39, 907–912. [Google Scholar]
- Costa dos Santos, D.C.; Oliveira-Filho, J.G.; Araujo-Santana, C.; Guimarães-Silva, F.; Pereira-Takeuchi, K.P.; Buranelo-Egea, M. Optimization of soymilk fermentation with kefir and the addition of inulin: Physicochemical, sensory and technological characteristics. LWT Food Sci. Technol. 2019, 104, 30–37. [Google Scholar] [CrossRef]
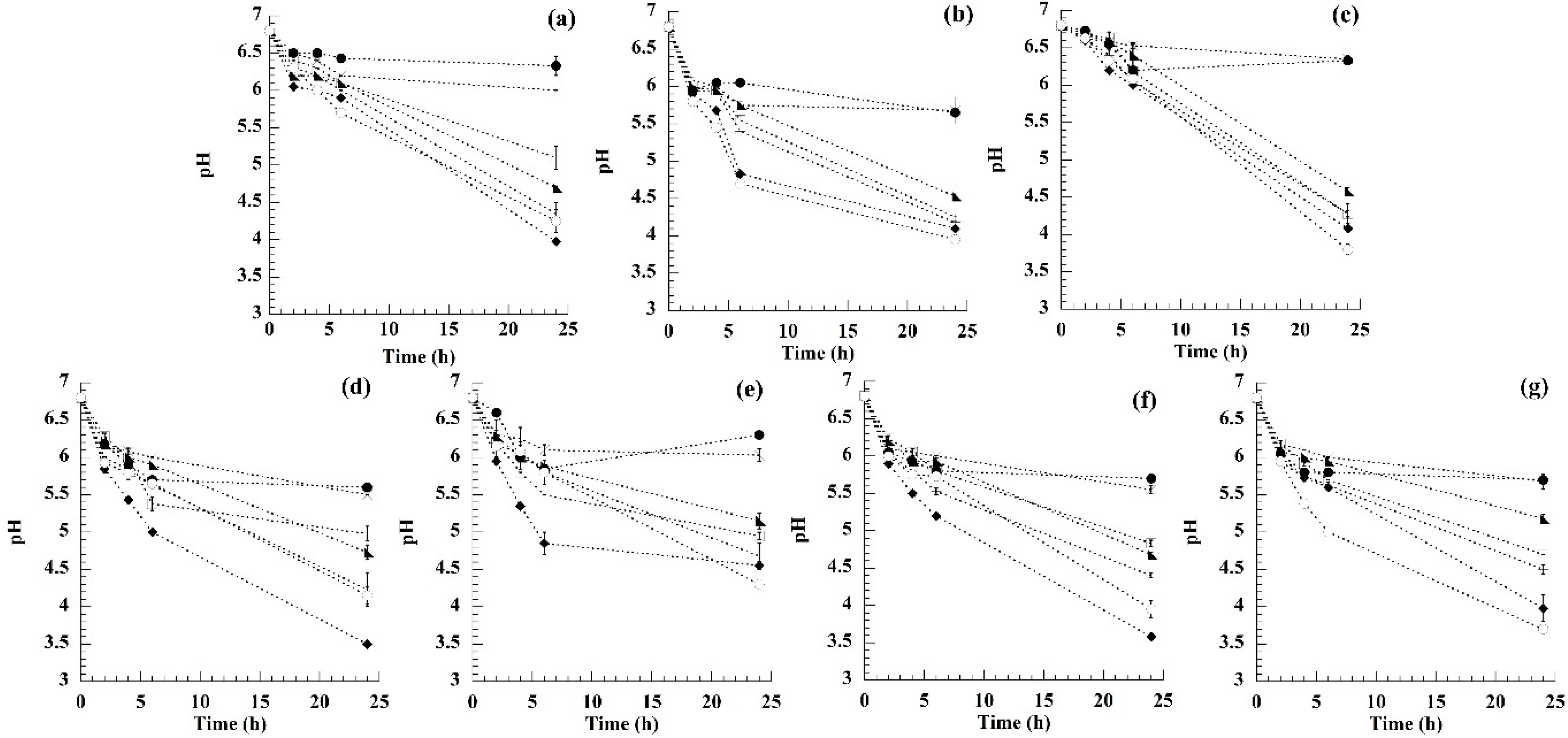
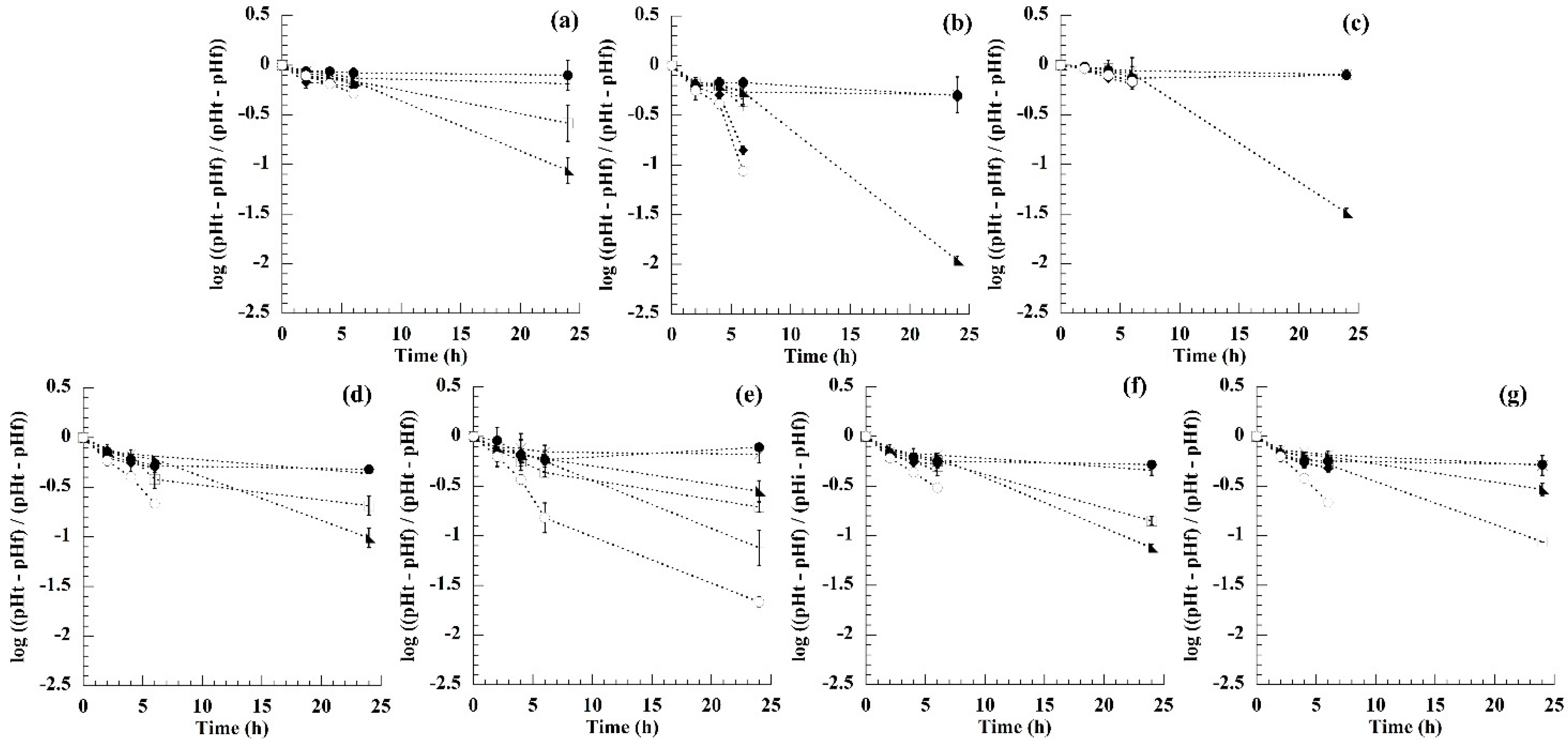

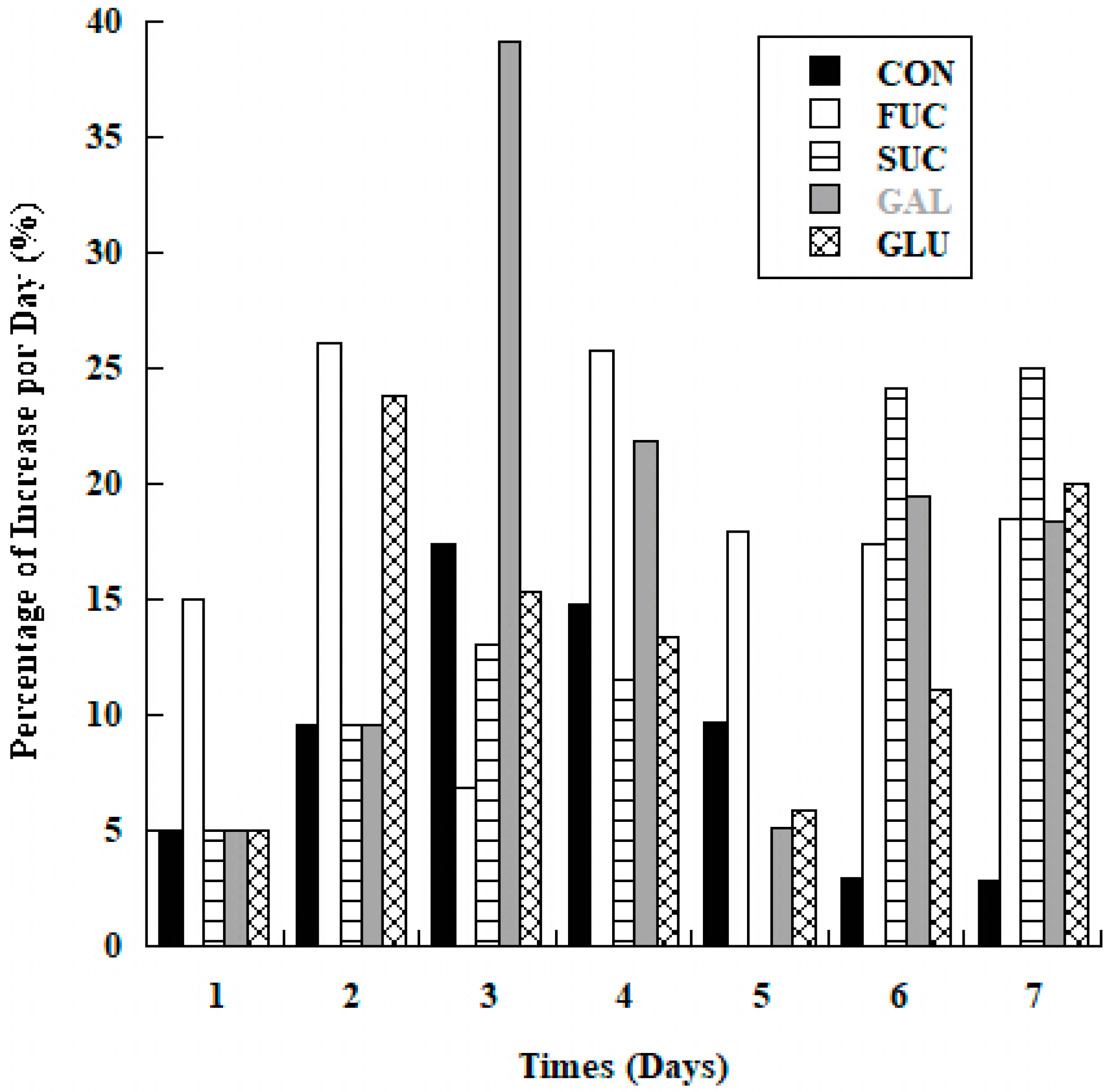

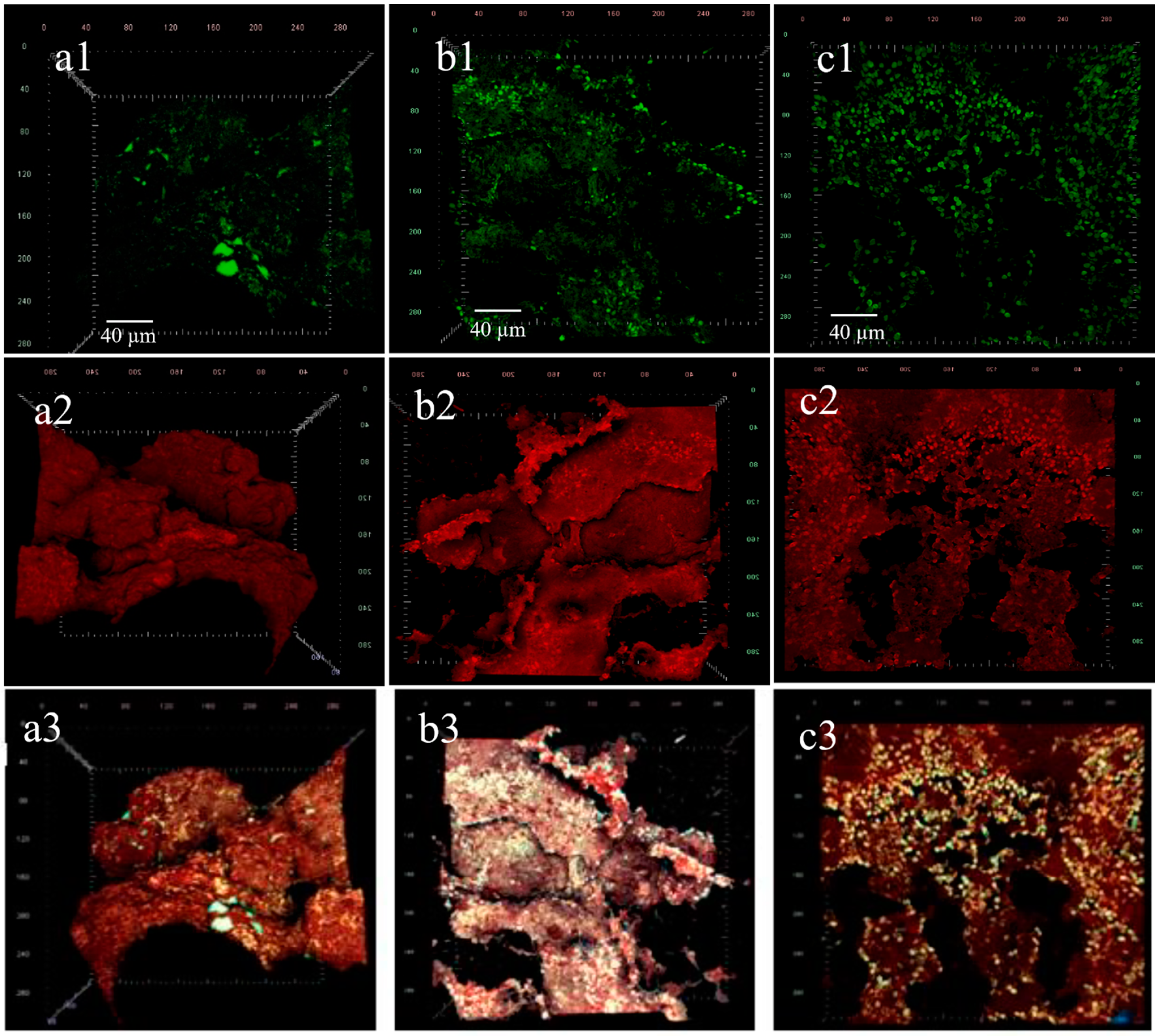
| Substrate | R2 | R2 | R2 | R2 | R2 | R2 | R2 |
|---|---|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | |
| Control | 0.565 | 0.768 | 0.983 | 0.997 | * | * | * |
| Fructans | 0.609 | 0.504 | 0.983 | * | * | * | * |
| Galactose | 0.358 | 0.827 | 0.965 | * | * | * | * |
| Fructose | 0.398 | 0.729 | 0.938 | 0.998 | * | * | * |
| Glucose | 0.543 | 0.852 | 0.994 | 0.866 | * | * | * |
| Lactose | 0.036 | 0.494 | 0.958 | 0.919 | 0.995 | * | 0.937 |
| Sucrose | 0.466 | 0.784 | 0.991 | 0.996 | * | * | * |
| Substrate | ASM | Contrast | IDM | Entropy | FD |
|---|---|---|---|---|---|
| Fructans | 2.838 × 10−4 ± 9.590 × 10−5 a | 336.929 ± 38.089 c | 1.019 × 10−1 ± 0.006 a | 8.541 ± 0.185 c | 2.380 ± 0.021 b |
| Galactose | 5.590 × 10−4 ± 1.723 × 10−4 b | 146.634 ± 9.143 b | 1.437 × 10−1 ± 0.003 b | 7.900 ± 0.264 a | 2.304 ± 0.029 a |
| Control | 3.577 × 10−4 ± 7.606 × 10−5 a | 81.942 ± 13.482 a | 1.520 × 10−1 ± 0.008 c | 8.279 ± 0.214 b | 2.327 ± 0.032 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Reyes, S.V.; Márquez-Morales, C.E.; Moreno-León, G.R.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Solorza-Feria, J.; García-Armenta, E.; Villalobos-Espinosa, J.C. Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Appl. Sci. 2022, 12, 2459. https://doi.org/10.3390/app12052459
Avila-Reyes SV, Márquez-Morales CE, Moreno-León GR, Jiménez-Aparicio AR, Arenas-Ocampo ML, Solorza-Feria J, García-Armenta E, Villalobos-Espinosa JC. Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Applied Sciences. 2022; 12(5):2459. https://doi.org/10.3390/app12052459
Chicago/Turabian StyleAvila-Reyes, Sandra V., Cruz E. Márquez-Morales, Germán R. Moreno-León, Antonio Ruperto Jiménez-Aparicio, Martha L. Arenas-Ocampo, Javier Solorza-Feria, Evangelina García-Armenta, and Julieta C. Villalobos-Espinosa. 2022. "Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains" Applied Sciences 12, no. 5: 2459. https://doi.org/10.3390/app12052459
APA StyleAvila-Reyes, S. V., Márquez-Morales, C. E., Moreno-León, G. R., Jiménez-Aparicio, A. R., Arenas-Ocampo, M. L., Solorza-Feria, J., García-Armenta, E., & Villalobos-Espinosa, J. C. (2022). Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Applied Sciences, 12(5), 2459. https://doi.org/10.3390/app12052459






