Abstract
Vojta’s therapy is a widely used approach in both the prevention and therapy of musculoskeletal disorders. Changes in the musculoskeletal system have been described repeatedly, but the principles of the approach have not yet been clarified. The objective of our study was to evaluate changes of intracerebral activity using electromagnetic tomography (sLORETA) that arise during reflex locomotion stimulation of the breast trigger zone according to Vojta’s therapy. Seventeen healthy women took part in the experiment (aged 20–30 years old). EEG activity was recorded 5 min prior to the reflex locomotion stimulation, during stimulation, and 5 min after the stimulation. The obtained data were subsequently processed in the sLORETA program and statistically evaluated at the significance level p ≤ 0.05. The analysis found statistically significant differences in the frequency bands alpha-2, beta-1, and beta-2 between the condition prior to stimulation and the actual stimulation in BAs 6, 7, 23, 24, and 31 and between the resting condition prior to stimulation, and the condition after the stimulation was terminated in the frequency bands alpha-1, alpha-2, beta-1, and beta-2 in BAs 3, 4, 6, and 24. The results showed that reflex locomotion stimulation according to Vojta’s therapy modulates electrical activity in the brain areas responsible for movement planning and regulating and performing the movement.
1. Introduction
Reflex locomotion according to Vojta’s therapy is a neurophysiological rehabilitation method that employs general, motor (as well as non-motor) responses aroused with reflex locomotion stimulation applied to specific zones. The involuntary motor response usually occurs in the form of symmetrical, as well as asymmetrical movement patterns of the limbs, trunk, head, and neck [1].
Reflex locomotion employs specific reactions produced by the stimulation of certain body zones. These so-called trigger zones are stimulated with pressure during reflex locomotion stimulation. We can therefore expect that the CNS response will contain, in addition to the specific changes related to reflex locomotion stimulation, reactions to the tactile stimulus. Detailed descriptions of these reactions to the tactile stimulus have been provided in several studies. In fMRI studies, the authors describe activation changes in the Brodmann area (BA) 3b and further in BAs 1 and 2 in the somatosensory stimulation in facial areas [2,3]. Fabri et al. in 2005 [4] described the representation of the upper trunk part at the border between BAs 3a and 3b. The stimulation of the lower trunk part results in activity in BAs 3b, 1, and 2. Several articles have described the neuronal representation of the sensitive perception of fingers in BAs 3b, 3a, 1, and 2 [5,6,7,8]. Activation changes were observed in BAs 2, 3b, and 1 during stimulation in the palm of the hand [2,5]. Akselrod et al. in 2017 [9] elicited activation changes in the contralateral BAs 3b, 1, and 2 in all stimulated body parts with somatosensory lower extremity stimulation (thigh, big toe, calf, heal, hip, little toe). Another study used EEG to describe the activity reduction of the low-frequency bands alpha and beta over the contralateral region of the somatosensory cortex, that is BAs 1, 2, and 3, following a pleasant touch on the forearm [10]. Though the above-stated studies described the inter-individual differences in the representation of individual body parts in the sensory cortex, all reactions to somatosensory stimulation were bound only to the primary sensory cortex (BAs 1, 2, and 3).
So far, only a small number of studies have been published that have demonstrated changes in the CNS during reflex locomotion stimulation. Hok et al. (2017) [11] found reduced activity in fMRI in the bilateral pontocerebellar reticular formation and in the posterior cerebellar hemispheres and vermis and reduced activity in BAs 6, 7, and 2. Sanz-Esteban et al. (2018) [12] described activation changes in BAs 6, 21, 28, and 46, putamen, and cerebellum in an fMRI study and, later on, in an EEG study (2021) [13], changes in BAs 6, 8, 5, 7, 23, and 31.
The clinical effect of the reflex locomotion stimulation on the locomotion apparatus and neuroplasticity has been verified across several studies. Some studies have demonstrated the effect of the reflex locomotion stimulation on the brain’s neuroplasticity in individuals with developmental delay and hypotonia. The study of Ha and Sung (2021) [14] described changes in the neuronal pathways involved in motor functions in patients with developmental delay and hypotonia. They observed increased plasticity induced by the reflex locomotion stimulation in the corticospinal tract and non-corticospinal pathways after 8 mo of the reflex locomotion stimulation. Another study of Ha and Sung (2021) [15] demonstrated thickening of the deep neck flexors and increased neck stability after the reflex locomotion stimulation in individuals with hypotonia. Different studies have described the effect of the reflect locomotion stimulation on the neuromotor development in patients with periventricular leukomalacia. After 11 mo of the regular reflex locomotion stimulation, all probands showed clinically significant progress in development assessed by the Bayley Scales of Infant and Toddler Development and Denver II [16]. Epple et al. [17] demonstrated the effect of the reflex locomotion stimulation in a very early stroke rehabilitation. Compared to the traditional physiotherapy, individuals treated with the reflex locomotion stimulation showed, within 9 d of the stroke, greater statistically significant improvement in tests assessing impairment caused by stroke, trunk stability, and upper extremity functions in stroke. The study of the implementation of the reflex locomotion stimulation in patients with multiple sclerosis demonstrated that the patients treated with the reflex locomotion stimulation showed improved balance in everyday skills according to the Berg Balance scale, the 10 m walk, and the tandem test, compared to a standard therapeutic procedure [18].
The objective of the present study was to examine whether reflex locomotion stimulation results in changes of the source electrical activity in the cerebral cortex observable in sLORETA imaging.
2. Materials and Methods
2.1. Participants
Seventeen healthy women aged 20–30 (25 ± 6.4) were involved in the experiment, (Table 1). There was no determined criterion regarding sensitivity to evoking stimulation in reflex locomotion according to Vojta’s therapy or obtaining their own experience with this method. All subjects were first familiarized with the course of the experiment and the anonymous result processing as part of their informed consent. The study was approved by the Charles University FTVS Ethics Committee (ID 90/2019).

Table 1.
Basic characteristics of the participants.
2.2. Methods
The telemetric 32-channel ECG instrument Nicolet EEG wireless 32/64 Amplifier by Natus Neurology (USA) was used to record the signal. Registration of the scalp EEG was performed with an EEG cap (Electro-Cap) from elastic textiles with embedded registration electrodes. The cap carries a total of 19 electrodes (leads) (Fp1, Fp2, F7, F3, Fz, F8, T5, T3, C3, Cz, C4, T4, T6, P3, Pz, P4, O1, O2) located according to the international system 10–20. The sampling frequency was 512 Hz, with a band range of 0.5–70 Hz, and impedance resistance below 10 kΩ. The whole course of the experiment was also documented in a video recording.
2.3. Measurements and Data Collection
Registration and evaluation were carried out by an electroencephalographist and the stimulation of the breast zone by a therapist qualified in Vojta’s therapy. EEG was measured in all individuals with eyes closed in order to reduce ocular artifacts and to reduce visual afferentation. EEG activity was registered for 5 min at rest with closed eyes (PRE CE) prior to the actual reflex locomotion stimulation. Subsequently, the electrical activity was followed for 15 min during trigger zone stimulation with closed eyes (VOJTA CE). The experiment was terminated with the registration of the resting EEG recording with closed eyes until 5 min after the reflex locomotion stimulation of the trigger zone (POST CE 1). The subjects lied in the supine position over the course of the entire experiment. For the stimulation, the left-sided breast zone according to the Vojta reflex locomotion was always chosen in all subjects; it was always located in the intercostal space between the 7th and 8th ribs under the mammilla in the mammillary line. The stimulation of the trigger zone was performed in the starting supine position on the back with the head placed in the spine axis with a maximum alignment of the cervical spine with a 30° rotation to the stimulated breast zone, the upper extremities positioned freely along the body, and the lower extremities in flexion and underlain. There was no observation of an algesic reaction to reflex locomotion stimulation in the subjects.
In the present study, we used the method of standard low-resolution brain electromagnetic tomography (sLORETA) to evaluate changes in intracerebral electrical activity; the method makes it possible to compute the sources of this electrical activity in the cerebral tissue from the scalp EEG and depict them in the Talairach 3D image. The method determines current densities in a total of 6239 voxels with a spatial resolution of 5 mm [19,20,21,22].
2.4. Data Analysis
The registered EEG data were subsequently exported into the NeuroGuide program (Applied Neuroscience) where the electroencephalographist selected the thirty-second segment without artifacts from the last 3 min of each phase from each experiment phase of each subject (PRE CE; VOJTA CE; POST CE 1). These segments were exported into the sLORETA program, where they were mathematically processed, and individual parts of the experiment were mutually statistically compared (PRE CE X VOJTA CE and PRE CE X POST CE 1) using the paired t-test with logarithmic transformation of the data, with a smoothing parameter of 0.5, using a permutation method with 5000 randomizations at the significance level p ≤ 0.05, with correlation for repeated testing [23]. We then depicted the statistically significant difference at the significance level p ≤ 0.05 in the Talairach atlas and evaluated individual active Brodmann areas [22].
In the statistical module of the sLORETA program, we compared the resting recording with closed eyes prior to stimulation with the recording during stimulation of the trigger zone with closed eyes (PRE CE X VOJTA CE) and the resting recording with closed eyes prior to stimulation with the resting recording with closed eyes running until 5 min after stimulation (PRE CE X POST CE 1).
3. Results
3.1. Statistical Comparison of the Condition with Closed Eyes Prior to Stimulation to the Condition during Stimulation with Closed Eyes
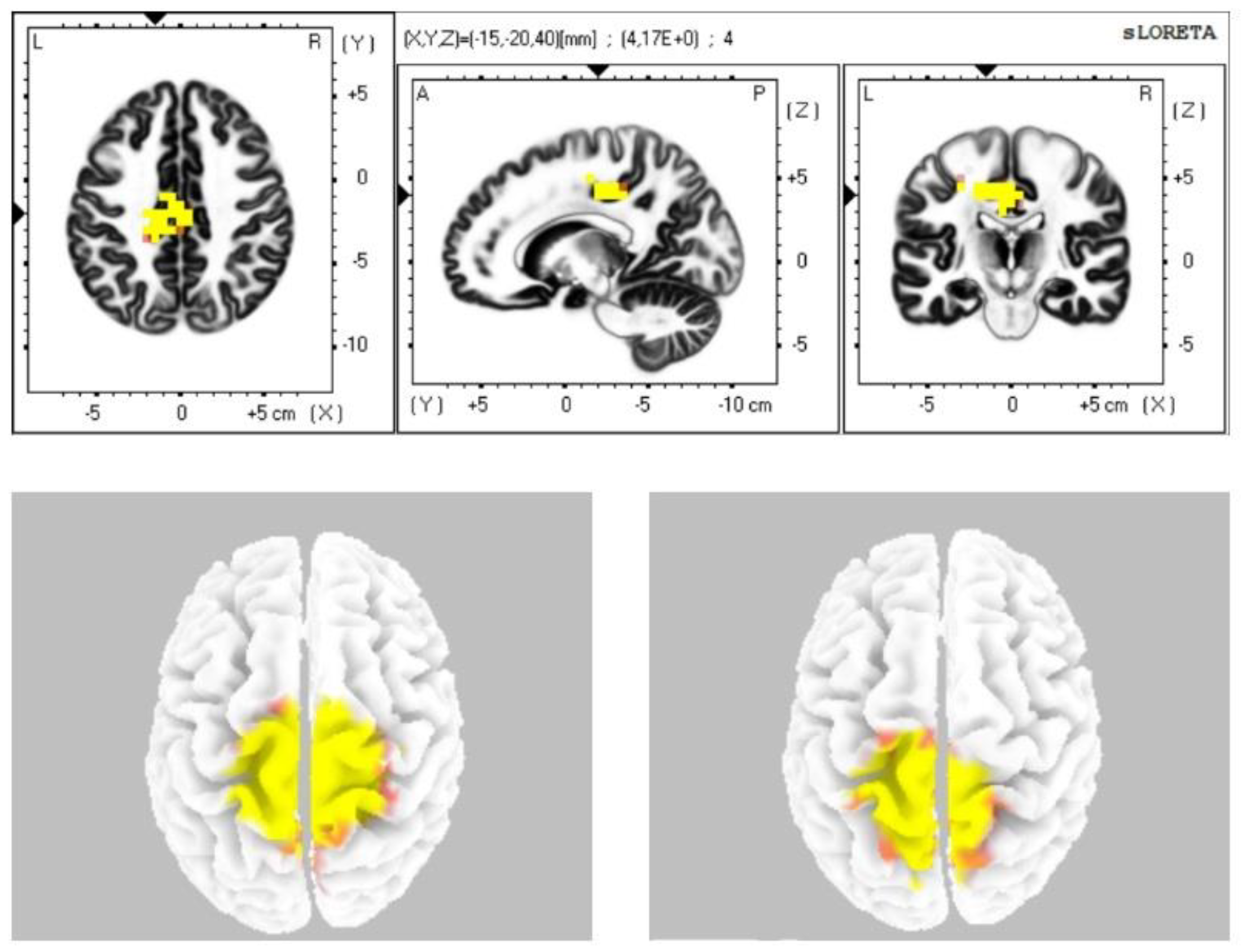
The statistical comparison of the data (Table 2) in the sLORETA program from the resting status with closed eyes with the condition during stimulation with closed eyes demonstrated a statistically significant difference at the level of statistical significance p ≤ 0.05 in the sense of increased current density: in the alpha-2 band in the cingulate cortex and in the premotor and supplementary motor area (BAs 24, 31, 23, 6); in the beta-1 band in the premotor, supplementary motor area, secondary sensory cortex, and the cingulate cortex (BAs 6, 7, 31); in the beta-2 band in the premotor, supplementary motor area, and secondary sensory cortex (BAs 6, 7) (Figure 1). We did not observe any statistically significant difference in the frequency bands gamma, delta, and theta.

Table 2.
Statistically significant Brodmann zones in status prior to stimulation (PRE CE) vs. stimulation (VOJTA CE).
Figure 1.
Statistically significant source activity in sLORETA imaging at the significance level p ≤ 0.05 for the alpha-2 band (top image), beta-1 band (bottom-left image), and beta-2 band (bottom-right image). Yellow and orange voxels indicate areas with increased electrical brain activity.
3.2. Statistical Comparison of the Resting Condition with Closed Eyes Prior to Stimulation with the Resting Condition with Closed Eyes until 5 min after the Stimulation
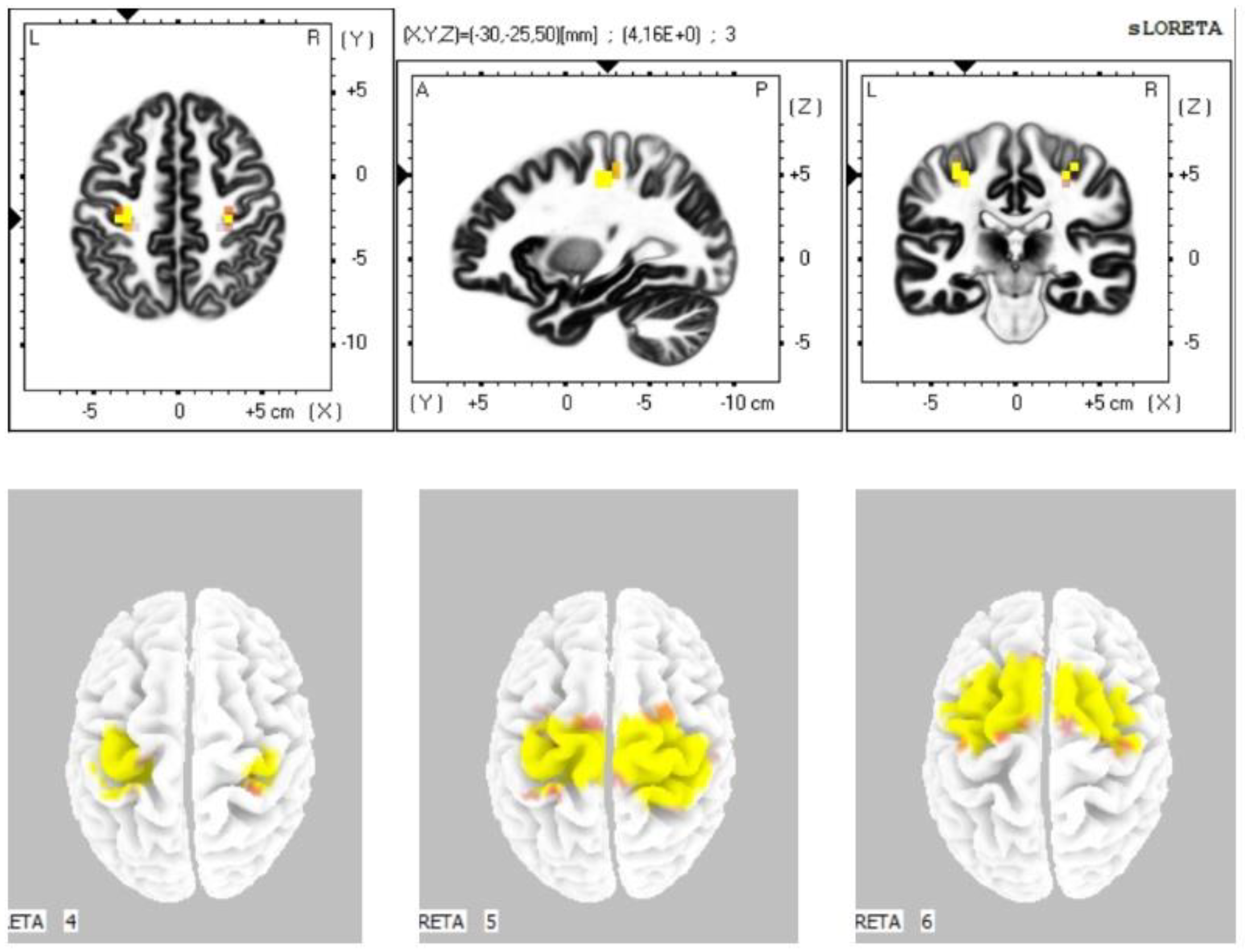
The statistical comparison of the data (Table 3) in the sLORETA program from the resting condition with closed eyes prior to stimulation with the resting condition with closed eyes until 5 min after the stimulation demonstrated a statistically significant difference at the significance level p ≤ 0.05 in the sense of increased current density: in the alpha-1 band in the primary motor cortex, primary sensory cortex, and the cingulate cortex (BA 4, 3, 24); in the alpha-2 band in the premotor and supplementary motor area, primary motor cortex, primary sensory cortex, and the cingulate cortex (BA 6, 4, 3, 24); in the beta-1 band in the premotor and supplementary motor area, primary motor cortex, and primary sensory cortex (BAs 6, 4, 3); in the beta-2 band in the premotor, supplementary motor area, and primary motor cortex (BAs 6, 4) (Figure 2). We did not observe any statistically significant difference in the frequency bands gamma, delta, and theta.

Table 3.
Statistically significant Brodmann zones in status prior to stimulation (PRE CE) vs. post-stimulation (POST CE 1).
Figure 2.
Statistically significant source activity in sLORETA imaging at the significance level p ≤ 0.05 for the alpha-1 band (top image), alpha-2 band (bottom-left image), beta-1 band (bottom-middle image), and beta-2 band (bottom-right image). Yellow and orange voxels indicate areas with increased electrical brain activity.
4. Discussion
The objective of our study was to evaluate changes of intracerebral activity using electromagnetic tomography (sLORETA) that arise during reflex locomotion stimulation of the breast zone. The analysis found statistically significant differences in the frequency bands alpha-2, beta-1, and beta-2 between actual stimulation and the resting condition prior to stimulation in BAs 6, 7, 23, 24, and 31 and between the resting condition after the stimulation was terminated and the resting condition prior to stimulation in the frequency bands alpha-1, alpha-2, beta-1, and beta-2 in BAs 3, 4, 6, and 24.
It follows from the data we obtained that the reflex locomotion stimulation (VOJTA CE X PRE CE) especially influenced the secondary/supplementary brain centers and the areas that are functionally associated with them. The activity of the premotor and supplementary motor cortex (BAs 6, 23, and 24) suggests the phase of movement planning and preparation. The functions of Brodmann Area 6 are especially preparation and planning of voluntary movements [24,25]. Brodmann Area 6 further contains the cortical supplementary motor area (SMA), which participates in movement programming and planning. The SMA is also associated with motor activity depending on the internal state of the organism [25,26,27,28,29,30]. BA 24 is part of the cingular premotor field and participates in movement planning and idea determination. The activity of BA 24 is also associated with attention [25,31,32].
Premotor and supplementary cortex areas have already demonstrated activity in movement planning or only in the mere idea of movement [24,25,27]. Electrical stimulation of the premotor cortex elicits coarse motor activity with a complex synergistic character. Electrical stimulation of the supplementary cortical area elicits complex movements, rhythmic movements of the limbs, and rotational movements [29,33,34]. The change in the activity of the secondary sensory cortex in BA 7 can be a reaction to the perception of pressure caused by reflex locomotion stimulation [35,36], though the area reacting to somatosensory stimuli is especially the primary sensory cortex (BAs 1, 2, and 3) [2,3,4,5,6,7,9,10,37,38]. This area further reacts to painful and non-painful stimuli, especially when the subject concentrates on the location or intensity of the stimuli [35,39,40]. The intense non-painful pressure stimulus, lasting several minutes, was generated during the reflex locomotion stimulation. It is exactly this pressure stimulation that can be the reason why we observed changes in BA 7 when the condition prior to stimulation was compared with that during stimulation. The activation changes in BA7 may also correlate with the finding of cortisol level changes in the saliva of infants after the reflex locomotion stimulation [41]. One of the reasons for the short-term increase in the cortisol levels may be a reaction to an unpleasant pressure stimulus. Modulation of the activity of BA7 can also be caused by the strong efferent connection with BA 6. BAs of the cingular premotor field (23 and 24) also correspond, as BA 6, to the planning and idea of movement. Areas 23 and 31 are further part of the anterior cingulum (ACC), which corresponds to attention. Area 24 is anatomically included in the posterior cingulum (PCC), which is associated with the regulation and directing of attention [29,31,32,42].
When we compared the changes in the cerebral cortex caused by the reflex locomotion stimulation with the changes that persisted after the stimulation, we found that we no longer observed the modulation of the activity in the cingulate cortex and the premotor field, but did observe a reduction of the current density in the primary motor cortex across the frequencies alpha-1, alpha-2, beta-1, as well as beta-2. We can therefore assume that early reflex locomotion stimulation influences especially the premotor cortex areas that prepare and plan movement. Subsequently, changes occur in the primary motor cortex, which elicits the motor manifestation of reflex locomotion stimulation, which can manifest as muscle fasciculation or directly as movement in the described movement patterns [1,43,44]. Changes in the locomotion apparatus can be observed also after the termination of reflex locomotion stimulation in the sense of increased muscle tone, which has been described by the author of the method, Václav Vojta, in his theory [1], and experimentally demonstrated [45]. The results we obtained in our study document the changes in the electrical activity of the brain caused by reflex locomotion stimulation and thus contribute to clarifying the principles of this concept, which has so far been supported by an unproven theoretical framework. Knowledge of the influence of Vojta’s therapy on individual cortical areas will enable the application of this method in practice in diseases affecting motor centers, whether acquired or congenital. Our experiment proposed the use of the reflex locomotion according to Vojta’s therapy in neurorehabilitation. Publications on this method in the field of neurorehabilitation focus mainly on the clinical effect [15,17,46]. By demonstrating the activation changes in cortical areas responsible for movement preparation, planning, and execution, reflex locomotion could be integrated into standard rehabilitation approaches in this field.
Furthermore, to standardize this method, it is important to determine the minimum time required for the stimulation to elicit a clinically relevant response. Even in this respect, the method relies only on empirical experience. The author did not specify the time interval of the stimulation needed to achieve a relevant effect. In this experiment, we decided 15 min of reflex locomotion stimulation time to ensure that a sufficient amount of data was collected for the statistical analysis. However, the duration of stimulation varies across different studies [11,12,13,14,15,16,17,18,47,48,49,50]. Another study [13] with a similar design showed changes in cingulate cortex, premotor, and supplementary cortical areas during an 8 min-long reflex locomotion stimulation. This study also showed that some of these activation changes remained during the post-stimulation resting state, which lasted one minute. Our study extends these findings. Our 15 min-long stimulation led to very similar response in cortical motor areas. We can further confirm that the activation changes remained observable at least 5 min after the end of the reflex locomotion stimulation according to our results. During our experiment, in some individuals, motor activity manifested as a response to the reflex locomotion stimulation, while in others, there were no observable motor reactions. Despite that, we observed statistically significant changes in cortical motor areas. Therefore, we have the opinion that the motor response to reflex locomotion stimulation is not a condition to induce changes in the activity of the motor cortex, and even more, motor reaction should not be the only aim of therapy.
A recent study [41] described changes in the cortisol levels in the saliva of infants after reflex locomotion stimulation. These findings could fundamentally affect the use of the reflex locomotion method. In case the cortisol levels are affected by the reflex locomotion stimulation, is it possible to observe this correlation in the cerebral cortex? The topic of the humoral response of the human body to reflex locomotion stimulation should be a research subject in this field.
We are also aware of the limitations of this study: First, in the present study, we used the method of standard low-resolution brain electromagnetic tomography (sLORETA) to evaluate changes in intracerebral electrical activity. Although sLORETA allowed us to monitor changes in the electrical brain activity with zero localization error, our observation was bound only to the cortical areas of the cerebral cortex. MRI should be used to observe subcortical structures as in the above-mentioned research. Second, the sample size needs to be increased in order to generalize our data.
5. Conclusions
Our findings showed that reflex locomotion stimulation according to Vojta’s therapy modulates electrical activity in the brain areas responsible for movement planning, regulation, and execution. Our data further proved that the statistically significant changes in electrical brain activity in secondary and supplementary cortical areas were also observable within 5 min after the end of the stimulation.
Author Contributions
Conceptualization, D.P. (David Pánek), M.M. and D.P. (Dagmar Pavlů); methodology, D.P. (David Pánek) and M.M.; investigation, M.M., D.P. (David Pánek) and T.N.; data curation, D.P. (David Pánek) and M.M.; writing—original draft preparation, M.M.; writing—review and editing, D.P. (David Pánek), M.M. and D.P. (Dagmar Pavlů); supervision, D.P. (David Pánek) and D.P. (Dagmar Pavlů); project administration, D.P. (David Pánek) and D.P. (Dagmar Pavlů). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Charles University FTVS (ID 90/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vojta, V.; Peters, A. Das Vojta-Prinzip: Muskelspiele in Reflexfortbewegung und motorischer Ontogenese. 3., vollst. Überarb. Aufl; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Eickhoff, S.B.; Grefkes, C.; Fink, G.R.; Zilles, K. Functional lateralization of face, hand, and trunk representation in anatomically defined human somatosensory areas. Cereb. Cortex 2008, 18, 2820–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastl, M.; Brünner, Y.F.; Wiesmann, M.; Freiherr, J. Depicting the Inner and outer nose: The representation of the nose and the nasal mucosa on the human primary somatosensory cortex (SI). Hum. Brain Mapp. 2014, 35, 4751–4766. [Google Scholar] [CrossRef] [PubMed]
- Fabri, M.; Polonara, G.; Salvolini, U.; Manzoni, T. Bilateral cortical representation of the trunk midline in human first somatic sensory area. Hum. Brain Mapp. 2005, 25, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.P.; Tamè, L. Locating primary somatosensory cortex in human brain stimulation studies: Systematic review and meta-analytic evidence. J. Neurophysiol. 2019, 121, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Custead, R.; Wang, Y.; Barlow, S. Neural encoding of saltatory pneumotactile velocity in human glabrous hand. PLoS ONE 2017, 12, e0183532. [Google Scholar] [CrossRef] [Green Version]
- Pfannmöller, J.P.; Greiner, M.; Balasubramanian, M.; Lotze, M. High-resolution fMRI investigation of the fingertip somatotopy and variability in BA3b and BA1 of the primary somatosensory cortex. Neuroscience 2016, 339, 667–677. [Google Scholar] [CrossRef]
- Sánchez-Panchuelo, R.M.; Besle, J.; Mougin, O.; Gowland, P.; Bowtell, R.; Francis, S. Regional structural differences across functionally parcellated Brodmann areas of human primary somatosensory cortex. NeuroImage 2014, 93, 221–230. [Google Scholar] [CrossRef]
- Akselrod, M.; Martuzzi, R.; Serino, A.; van der Zwaag, W.; Gassert, R.; Blanke, O. Anatomical and functional properties of the foot and leg representation inareas 3b, 1 and 2 of primary somatosensory cortex in humans: A 7T fMRI study. NeuroImage 2017, 159, 473–487. [Google Scholar] [CrossRef]
- Singh, H.; Bauer, M.; Chowanski, W.; Sui, Y.; Atkinson, D.; Baurley, S.; Fry, M.; Evans, J.; Bianchi-Berthouze, N. The brain’s response to pleasant touch: An EEG investigation of tactile caressing. Front. Hum. Neurosci. 2014, 8, 893. [Google Scholar] [CrossRef] [Green Version]
- Hok, P.; Opavský, J.; Kutín, M.; Tüdös, Z.; Kaňovský, P.; Hluštík, P. Modulation of the sesorimotor system by sustained manual pressure stimulation. Neuroscience 2017, 348, 11–22. [Google Scholar] [CrossRef]
- Sanz-Esteban, I.; Calvo-Lobo, C.; Ríos-Lago, M.; Álvarez-Linera, J.; Muñoz-García, D.; Rodríguez-Sanz, D. Mapping the human brain during a specific Vojta’stactile input: The ipsilateral putamen’s role. Medicine 2018, 97, 13–21. [Google Scholar] [CrossRef]
- Sanz-Esteban, I.; Cano-de-la-Cuerda, R.; San-Martín-Gómez, A.; Jiménez-Antona, C.; Monge-Periera, E.; Estrada-Barranco, C.; Serrano, J.I. Cortical activity during sensorial tactile stimulation in healthy adults through Vojta therapy. A randomized pilot controlled trial. J. Neuroeng. Rehabil. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Sung, Y.H. Changes of neural pathways after Vojta approach in a child with developmental delay. Children 2021, 8, 918. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Sung, Y.H. Vojta approach affects neck stability and static balance in sitting position of children with hypotonia. Int. Neurourol. J. 2021, 25, 90–95. [Google Scholar] [CrossRef] [PubMed]
- De-La-Barrera-Aranda, E.; Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Fernandez-Bueno, L.; Rodriguez-Blanco, C.; Bernal-Utrera, C. Vojta therapy in neuromotor development of pediatrics patients with periventricular leukomalacia: Case series. Medicina 2021, 57, 1149. [Google Scholar] [CrossRef] [PubMed]
- Epple, C.; Maurer-Burkhard, B.; Lichti, M.C.; Steiner, T. Vojta therapy improves postural control in very early stroke rehabilitation: A randomised controlled pilot trial. Neurol. Res. Pract. 2020, 2, 23. [Google Scholar] [CrossRef]
- Lopez, L.P.; Palmero, N.V.; Ruano, L.G.; San Leon Pascual, C.; Orile, P.W.; Down, A.V.; Gor Garcia-Fogeda, M.D.; Toré, S. The implementation of a reflex locomotion program according to Vojta produces short-term automatic postural control changes in patients with multiple sclerosis. J. Bodyw. Mov. Ther. 2021, 26, 401–405. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Review of Methods for Solving the EEG Inverse Problem. Int. J. Bioelectromagn. 1999, 1, 75–86. [Google Scholar]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): Review, new comparisons, and new validation. Jpn. J. Clin. Neurophysiol. 2002, 30, 81–94. [Google Scholar]
- Pánek, D. Elektroencefalografické Koreláty Pohybového Chování a Výkonnostní Zátěže; Nakladatelství Karolinum: Praha, Czech Republic, 2016; ISBN 978-80-246-3435-7. [Google Scholar]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunno, Y.; Suzuki, T. Motor imagery while viewing self-finger movements facilitates the excitability of spinal motor neurons. Exp. Brain Res. 2020, 238, 2077–2086. [Google Scholar] [CrossRef] [PubMed]
- Derek, W.Y.; Forshing, L. Physiology, Motor Cortical; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542188/ (accessed on 10 December 2021).
- Ardila, A. Supplementary motor area aphasia revisited. J. Neurolinguistics 2020, 54, 100888. [Google Scholar] [CrossRef]
- Busan, P. Developmental stuttering and the role of the supplementary motor cortex. J. Fluen. Disord. 2020, 64, 105763. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Wright, D.L. Transcranial direct current stimulation of supplementary motor region impacts the effectiveness of interleaved and repetitive practice schedules for retention of motor skills. Neuroscience 2020, 435, 58–72. [Google Scholar] [CrossRef]
- Švestková, O.; Angerová, Y.; Druga, R.; Pfeiffer, J.; Votava, J. Rehabilitace Motoriky člověka. Fyziologie a Léčebné Postupy; Grada Publishing: Praha, Czech Republic, 2017. [Google Scholar]
- Tsuru, D.; Watanabe, T.; Chen, X.; Kubo, N.; Sunagawa, T.; Mima, T.; Kirimoto, H. The effects of transcranial static magnetic fields stimulation over the supplementary motor area on anticipatory postural adjustments. Neurosci. Lett. 2020, 723, 134863. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Whitlock, J.R. Posterior parietal cortex. Curr. Biol. 2017, 27, 691–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruana, F.; Gerbella, M.; Avanzini, P.; Gozzo, F.; Pelliccia, V.; Mai, R.; Abdollahi, R.O.; Cardinale, F.; Sartori, I.; Lo Russo, G.; et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain 2018, 141, 3035–3051. [Google Scholar] [CrossRef]
- Fried, I.; Katz, A.; McCarthy, G.; Sass, K.J.; Williamson, P.; Spencer, S.S.; Spencer, D.D. Functional Organization of Human Supplementary Motor Cortex Studied by Electrical Stimulation. J. Neurosci. 1991, 11, 3656–3666. [Google Scholar] [CrossRef]
- Kropf, E.; Syan, S.K.; Minuzzi, L.; Frey, B.N. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry 2019, 41, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Hofbauer, R.K.; Rainville, P.; Duncan, G.H.; Bushnell, M.C. Cortical representation of the sensory dimension of pain. J. Neurophysiol. 2001, 86, 402–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravaja, N.; Harjunen, V.; Ahmed, I.; Jacucci, G.; Spape, M.M. Feeling touched: Emotional modulation of somatosensory potentials to interpersonal touch. Sci. Rep. 2017, 7, 40504. [Google Scholar] [CrossRef] [Green Version]
- Tamè, L.; Braun, C.; Holmes, N.P.; Farnè, A.; Pavani, F. Bilateral representations of touch in the primary somatosensory cortex. Cogn. Neuropsychol. 2016, 33, 48–66. [Google Scholar] [CrossRef]
- Ploner, M.; Schmitz, F.; Freund, H.J.; Schnitzler, A. Parallel activation of primary and secondary somatosensory cortices in human pain processing. J. Neurophysiol. 1999, 81, 3100–3104. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiebzak, W.; Zurawski, A.; Gluszek, S.; Kosztolowicz, M.; Bialek, W.A. Cortisol levels in infants with central coordination disorders during VOJTA therapy. Children 2021, 8, 1113. [Google Scholar] [CrossRef]
- Wang, Z.M.; Shan, Y.; Zhang, M.; Wei, P.H.; Li, Q.G.; Yin, Y.Y.; Lu, J. Projections of brodmann area 6 to the pyramidal tract in humans: Quantifications using high angular resolution data. Front. Nerual Circuits 2019, 13, 62. [Google Scholar] [CrossRef]
- Schellekens, W.; Petridou, N.; Ramsey, N.F. Detailed somatotopy in primary motor and somatosensory cortex revealed by Gaussian population receptive fields. NeuroImage 2018, 179, 337–347. [Google Scholar] [CrossRef]
- Huber, L.; Finn, E.S.; Handwerker, D.A.; Bönstrup, M.; Glen, D.R.; Kashyap, S.; Ivanov, D.; Petridou, N.; Marrett, S.; Goense, J.; et al. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. NeuroImage 2020, 208, 116463. [Google Scholar] [CrossRef]
- Gajewska, E.; Huber, J.; Kulczyk, A.; Lipiec, J. An attempt to explain the Vojta therapy mechanism of action using the surface polyelectromyography in healthy subjects: A pilot study. J. Bodyw. Mov. Ther. 2018, 22, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Franki, I.; Desloovere, K.; De Cat, J.; Feys, H.; Molenaers, G.; Calders, P.; Vanderstraeten, G.; Himpens, E.; Van Den Broeck, C. The evidence-base for conceptual approaches and additional therapies targeting lower limb function in children with cerebral palsy: A systematic review using the international classification of functioning, disability and health as a framework. J. Rehabil. Med. 2012, 44, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Albuixech, M.L.; Redondo-Gonzáles, O.; Tello, I.; Collado-Vázquez, S.; Jiménez-Antona, C. Vojta Therapy versus transcutaneous electrical nerve stimulation for lumbosciatica syndrome: A quasi-experimental pilot study. J. Bodyw. Mov. Ther. 2020, 24, 39–46. [Google Scholar] [CrossRef]
- Lim, H.; Kim, T. Effects of Vojta Therapy on gait of children with spastic diplegia. J. Phys. Ther. Sci. 2013, 25, 1605–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmyślna, A.; Kiebzak, W.; Zurawski, A.; Pogorzelska, J.; Kotela, I.; Kowalski, T.J.; Śliwiński, Z.; Śliwiński, G. Effect of physiotherapy on spinal alignment in children with postural defects. Int. J. Occup. Med. Environ. Health 2019, 32, 25–32. [Google Scholar] [CrossRef]
- Zurawski, A.; Kiebzak, W.; Zmyślna, A.; Pogorzelska, J.; Kotela, I.; Kowalski, T.J.; Śliwiński, Z.; Śliwiński, G. Efficacy of the use of the McKenzie and Vojta methods to treat discopathy-associated syndromes in the pediatric population. Int. J. Occup. Med. Environ. Health 2019, 32, 33–41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).