Construction and Degradation Performance Study of Polycyclic Aromatic Hydrocarbons (PAHs) Degrading Bacterium Consortium
Abstract
:1. Introduction
2. Experimental Methods and Materials
2.1. Materials
2.2. Experimental Procedures
2.2.1. Basic Bacterium Information Test
2.2.2. GC-MS Operating Conditions
2.2.3. Quantitative Analysis of Single and Mixture PAHs
2.2.4. Quantitative Analysis of Crude Oil
2.2.5. Sensitivity Analysis of P&A Bacterial Consortium to Heavy Metal Ions
2.2.6. Resistance Analysis of P&A Bacterial Consortium to Heavy Metal Ions
2.2.7. PAHs Degradation Experiment
3. Results and Discussion
3.1. Construction of P&A Bacterial Consortium
3.1.1. Optimization of PAHs Degradation by Individual Bacteria
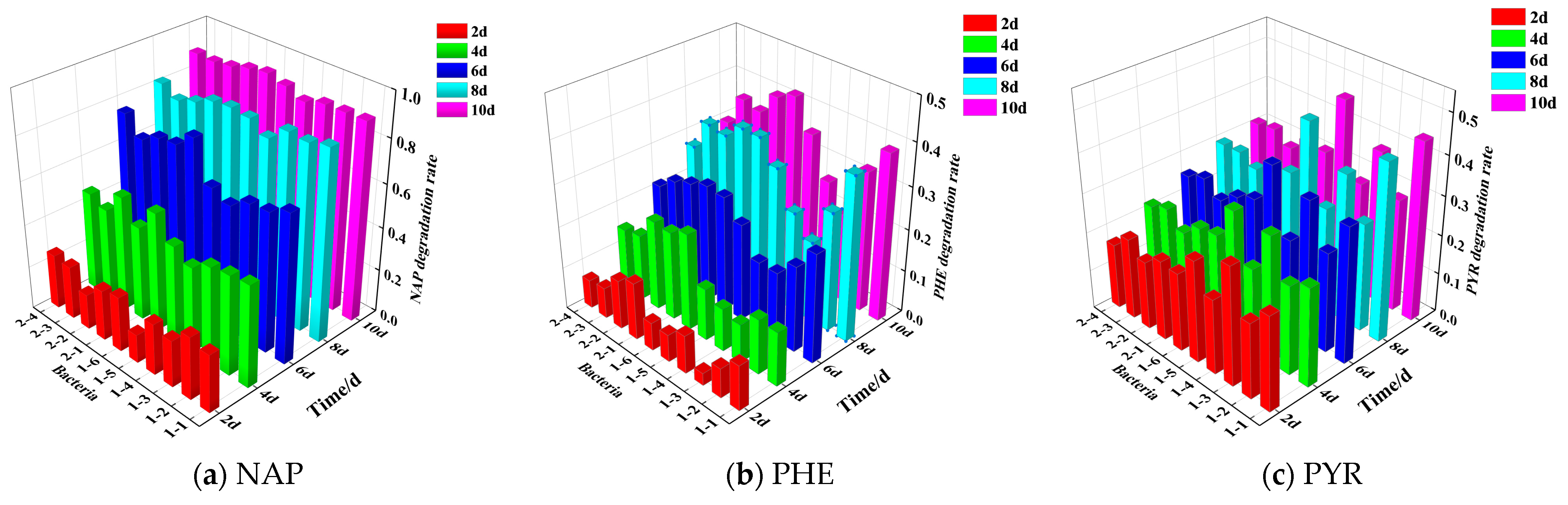
Optimization of Individual Bacteria by Single PAHs
Optimization of Individual Bacteria by Mixed PAHs
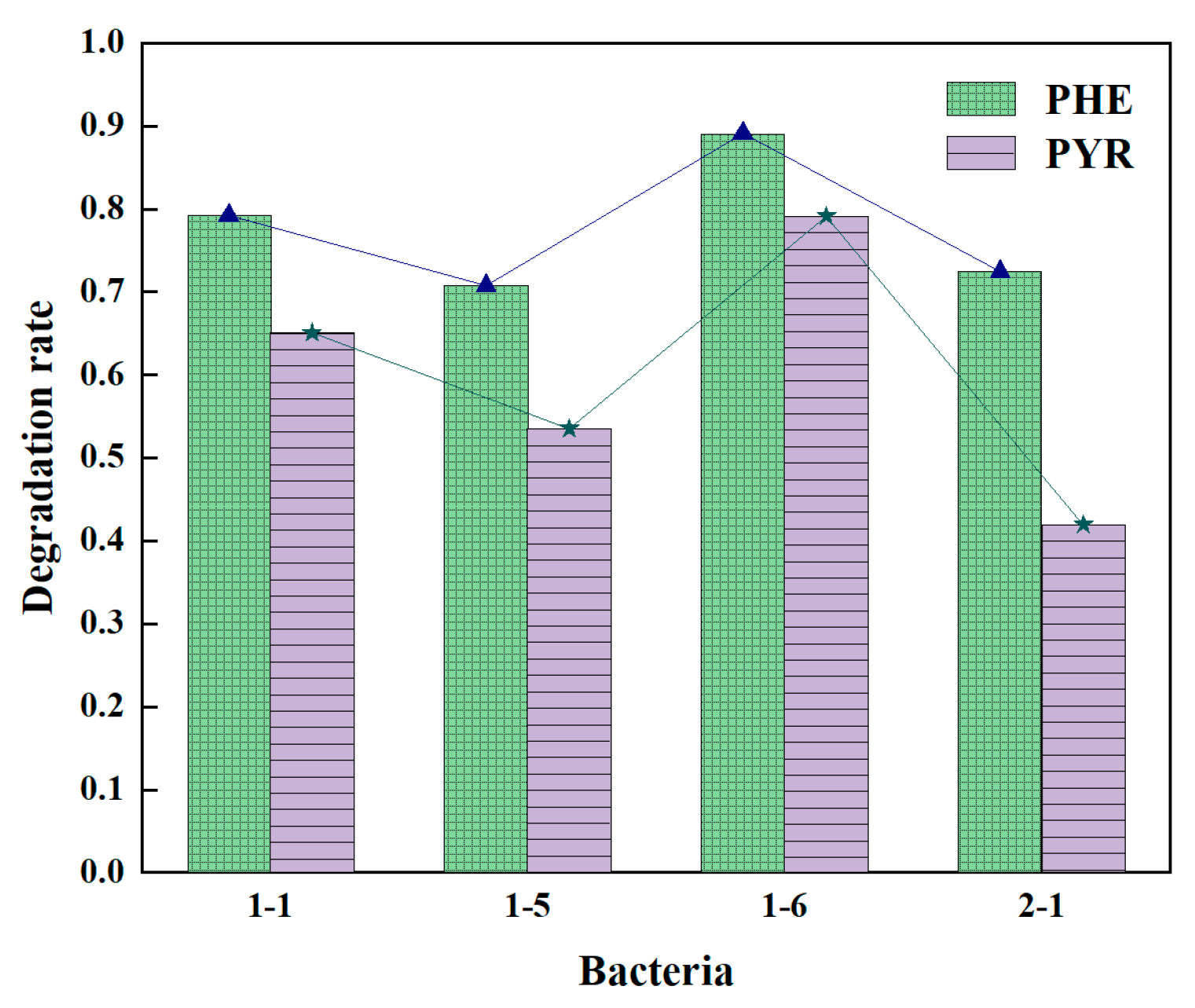
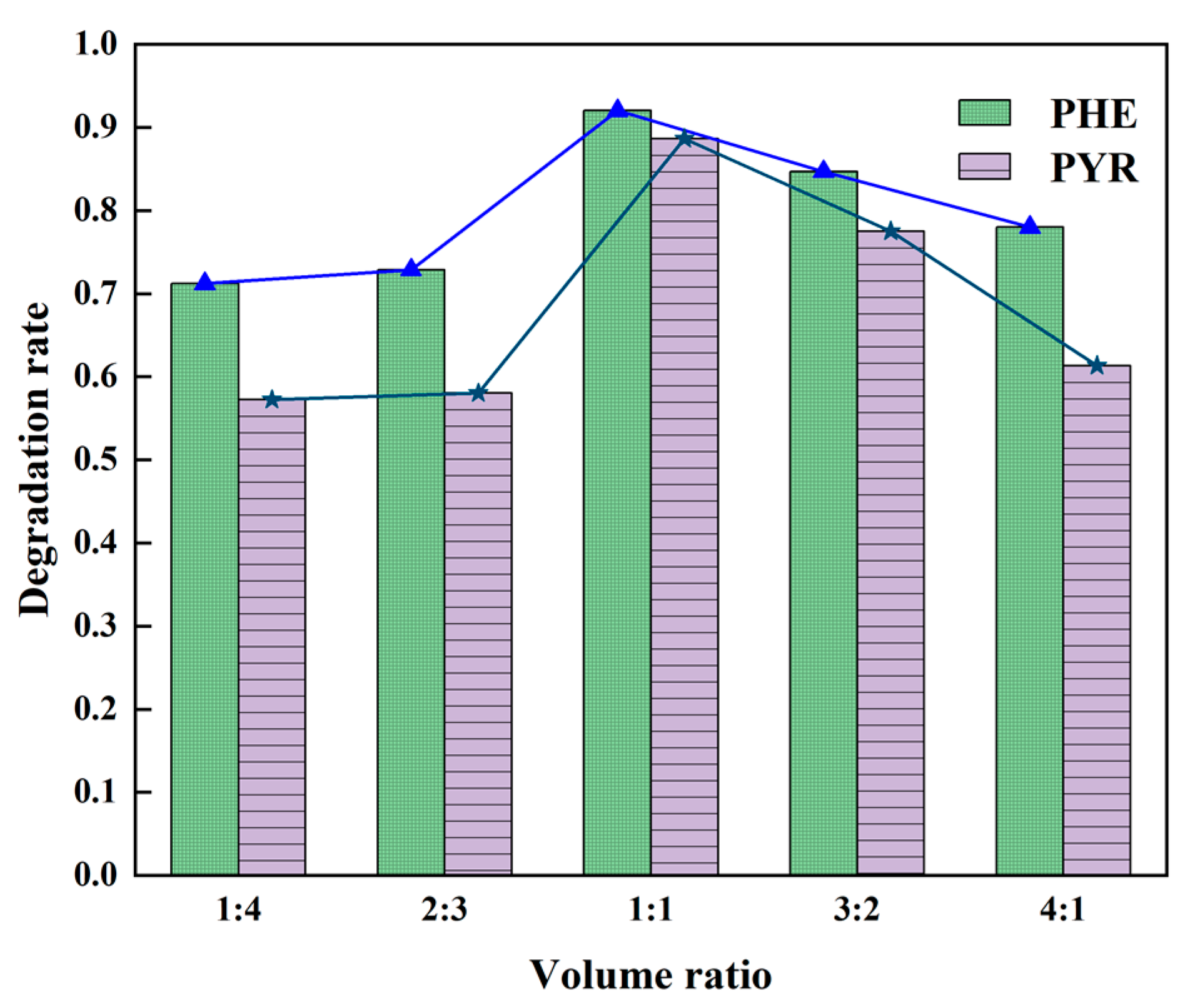
3.1.2. Optimization of Proportion of Two Individual Bacterium in Bacterial Consortium
3.2. Effect Analysis of Environmental Factors on Degradation
3.3. Effect Analysis of Exogenous Substances on PAHs Degradation
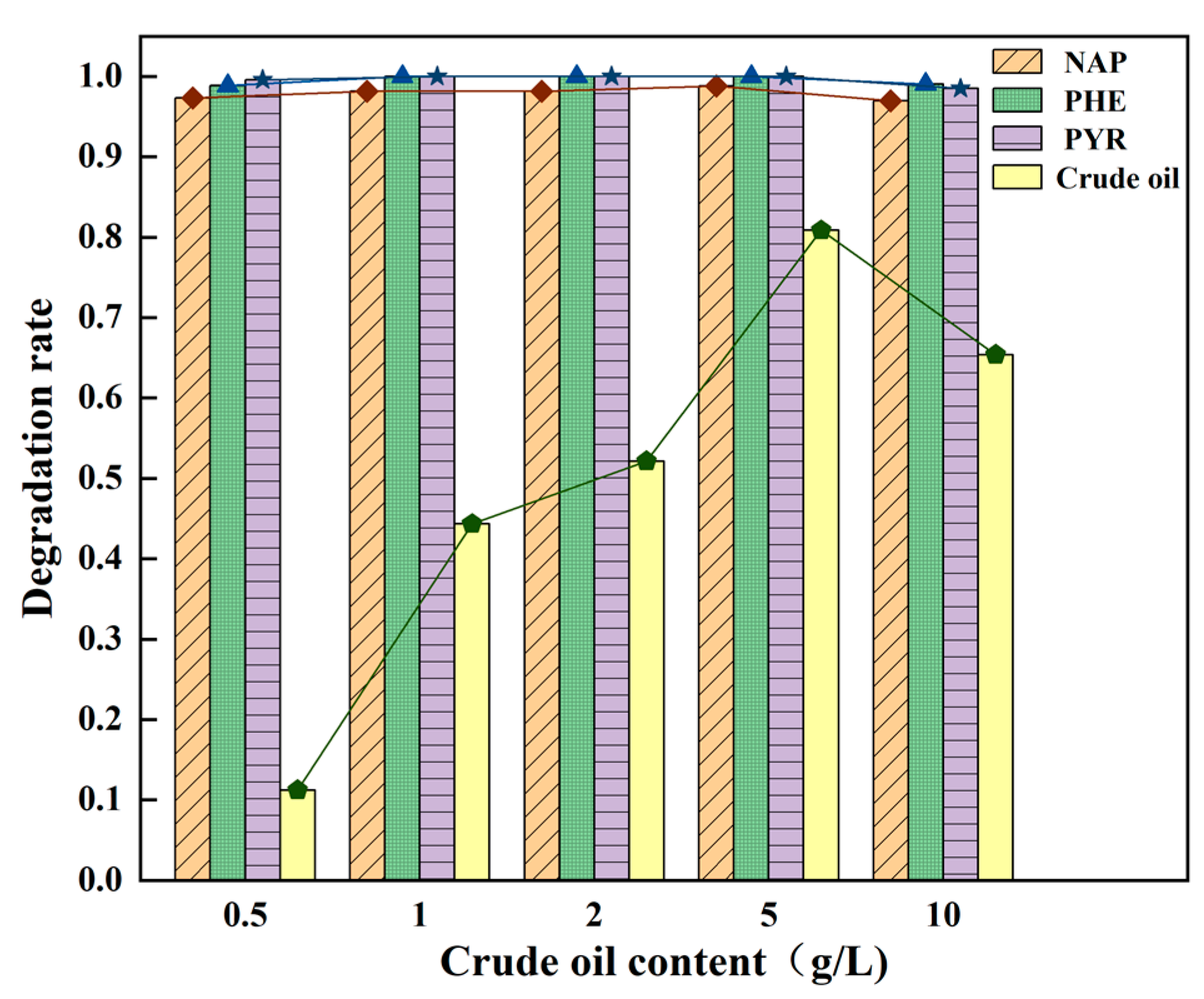
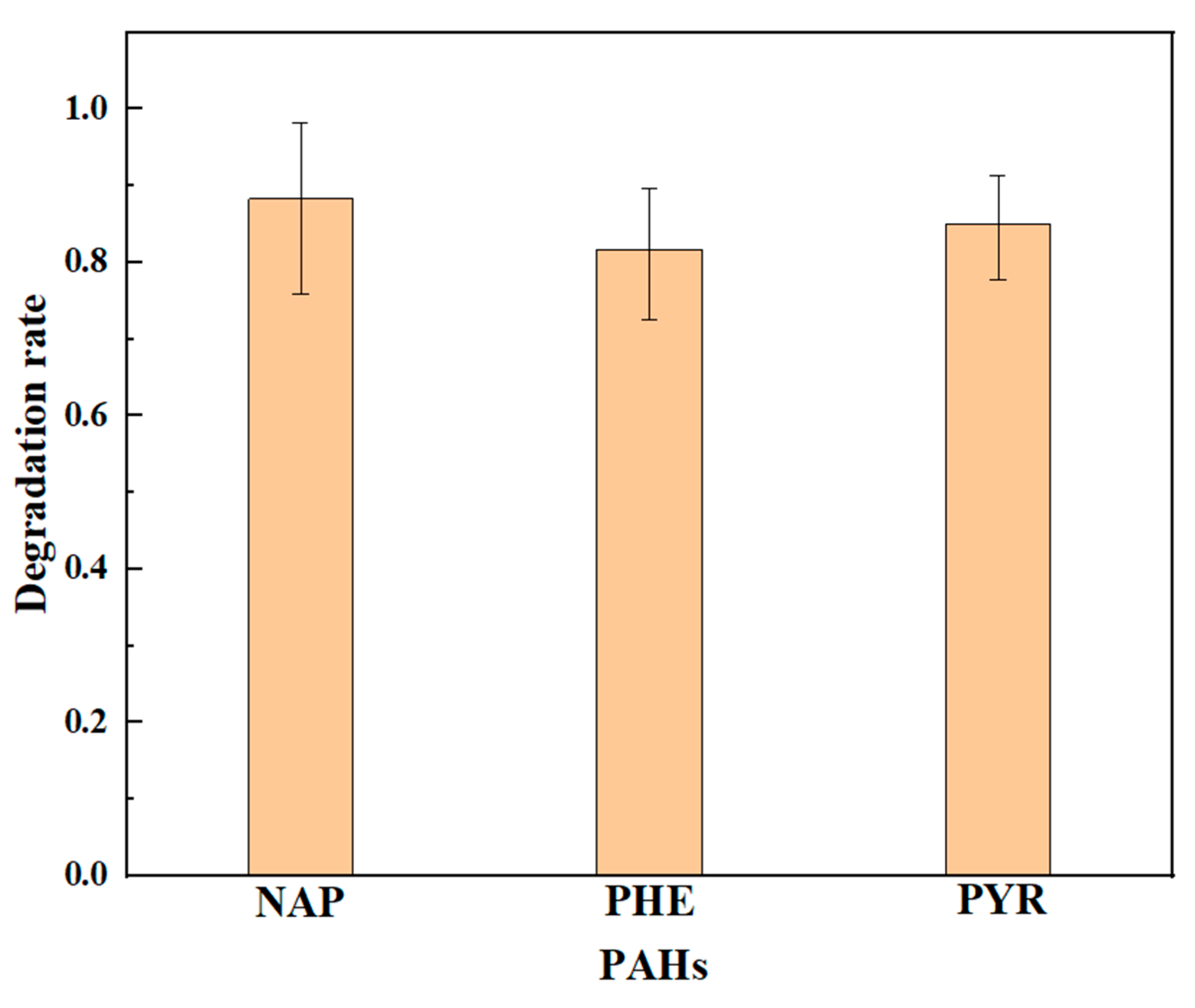
3.3.1. Crude Oil
3.3.2. Heavy Metal Ions
Sensitivity Analysis
Resistance Analysis
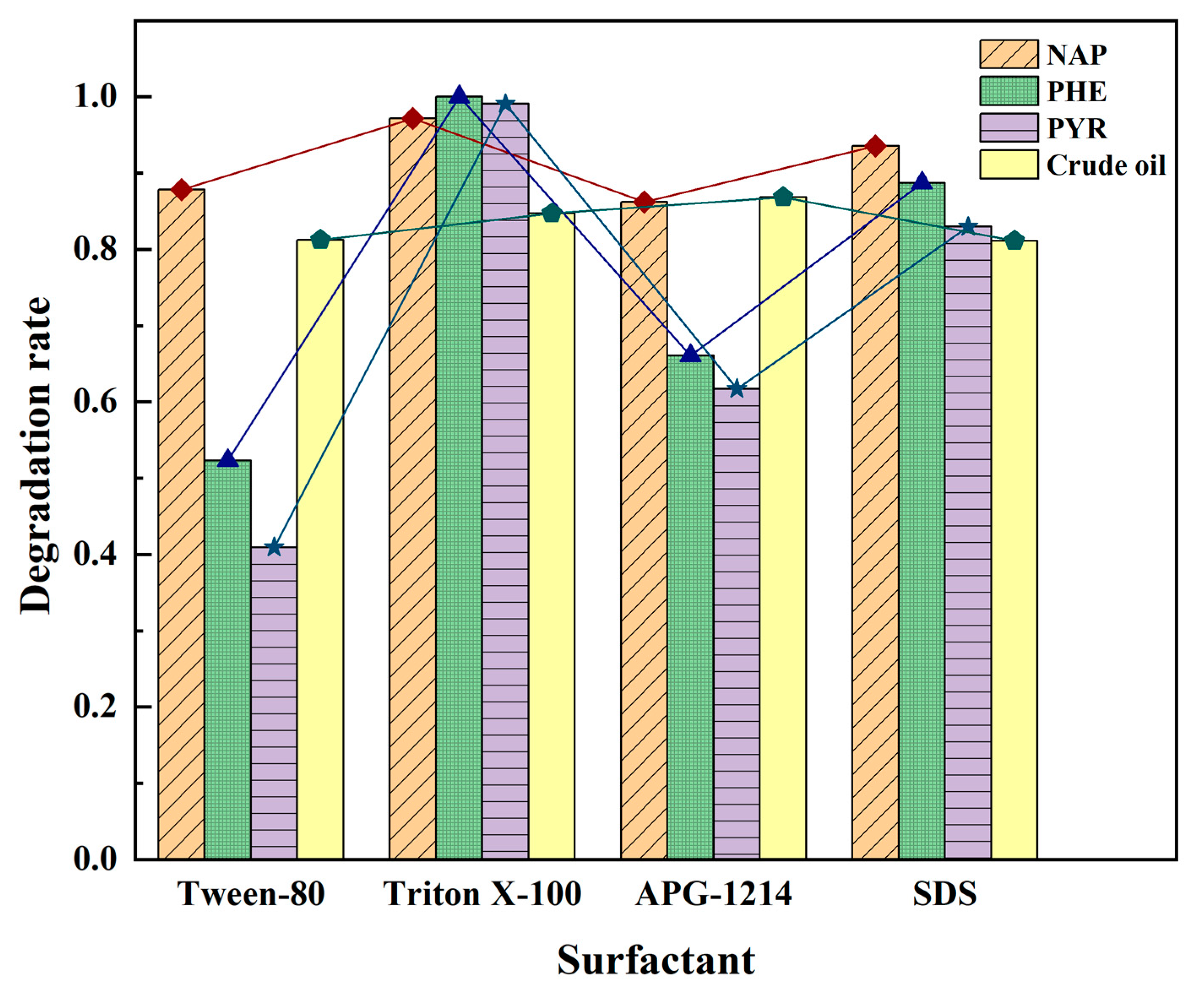
3.3.3. Surfactants
3.4. Degradation of PAHs in Oily Sludge by P&A Bacterial Consortium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, P.; Mukherji, S. Environmental contamination by heterocyclic Polynuclear aromatic hydrocarbons and their microbial degradation. Bioresour. Technol. 2021, 341, 125860. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Nieto, E.; Perales-Vargas-Machuca, J.A. Microbial degradation of PAHs: Organisms and environmental compartments. In Microbial Degradation of Xenobiotics; Environmental Science and Engineering; Springer: New York, NY, USA, 2012; pp. 263–290. [Google Scholar]
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic Microbial Degradation of Polycyclic Aromatic Hydrocarbons: A Comprehensive Review. Rev. Environ. Contam. Toxicol. 2020, 251, 25–108. [Google Scholar] [CrossRef] [PubMed]
- Achten, C.; Cheng, S.; Straub, K.L.; Hofmann, T. The lack of microbial degradation of polycyclic aromatic hydrocarbons from coal-rich soils. Environ. Pollut. 2011, 159, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Achten, C.; Hofmann, T. Native polycyclic aromatic hydrocarbons (PAH) in coals—A hardly recognized source of environmental contamination. Sci. Total Environ. 2009, 407, 2461–2473. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Qiao, P.; Cheng, Y.; Song, Y.; Sun, Z.; Zhang, T.; Wang, L.; Liu, Z. Enhanced degradation of polycyclic aromatic hydrocarbons in aged subsurface soil using integrated persulfate oxidation and anoxic biodegradation. Chem. Eng. J. 2020, 394, 125040. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Mawad, A.M.M.; Mostafa, Y.M.; Shoreit, A. Study of enhancement and inhibition phenomena and genes relating to degradation of petroleum polycyclic aromatic hydrocarbons in isolated bacteria. Microbiology 2014, 83, 599–607. [Google Scholar] [CrossRef]
- Jasmine, J.; Mukherji, S. Characterization of oily sludge from a refinery and biodegradability assessment using various hydrocarbon degrading strains and reconstituted consortia. J. Environ. Manag. 2015, 149, 118–125. [Google Scholar] [CrossRef]
- Thyssen, J.; Kimmerle, G.; Althoff, J.; Mohr, U. Inhalation Studies With Benzo[a]pyrene in Syrian Golden Hamsters. J. Natl. Cancer Inst. 1981, 66, 575–577. [Google Scholar] [CrossRef]
- Teixeira, E.C.; Pra, D.; Idalgo, D.; Henriques, J.A.; Wiegand, F. DNA-damage effect of polycyclic aromatic hydrocarbons from urban area, evaluated in lung fibroblast cultures. Environ. Pollut. 2012, 162, 430–438. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Oh, J.-E.; Chang, Y.-S. Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil. Sci. Total Environ. 2003, 311, 177–189. [Google Scholar] [CrossRef]
- Bumpus, J.A.; Aust, S.D. Biodegradation of environmental pollutants by the white rot fungus Phanerochaete chrysosporium: Involvement of the lignin degrading system. Bioessays 2010, 6, 166–170. [Google Scholar] [CrossRef]
- Miller, R.L.; Garfinkel, R.; Horton, M.; Camann, D.; Perera, F.P.; Whyatt, R.M.; Kinney, P.L. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 2004, 126, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency. An Exposure and Risk Assessment for Benzo(a)pyrene and Other Polycyclic Aromatic Hydrocarbons; United States Environmental Protection Agency: Washington, DC, USA, 1982. [Google Scholar]
- United States Environmental Protection Agency. Polycyclic Aromatic Hydrocarbons (PAHs); United States Environmental Protection Agency: Washington, DC, USA, 2008. [Google Scholar]
- European Comission. Opinion of the SCF on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food; SCF/CS/CNTM/PAH/29 Final; European Comission: Brussels, The Netherlands, 2002. [Google Scholar]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Kong, F.X.; Sun, G.D.; Liu, Z.P. Degradation of polycyclic aromatic hydrocarbons in soil mesocosms by microbial/plant bioaugmentation: Performance and mechanism. Chemosphere 2018, 198, 83–91. [Google Scholar] [CrossRef]
- Shao, B.; Tan, X.; Li, J.L.; He, M.; Tian, L.; Chen, W.J.; Lin, Y. Enhanced treatment of shale gas fracturing waste fluid through plant-microbial synergism. Environ. Sci. Pollut. Res. Int. 2021, 28, 29919–29930. [Google Scholar] [CrossRef]
- Robinson, S.L.; Novak, J.T.; Widdowson, M.A.; Crosswell, S.B.; Fetterolf, G.J. Field and Laboratory Evaluation of the Impact of Tall Fescue on Polyaromatic Hydrocarbon Degradation in an Aged Creosote-Contaminated Surface Soil. J. Environ. Eng. 2003, 129, 232–240. [Google Scholar] [CrossRef]
- Kamath, R.; Schnoor, A.J.L.; Alvarez, P.J.J. Effect of Root-Derived Substrates on the Expression ofnah-luxGenes in Pseudomonas fluorescens HK44: Implications for PAH Biodegradation in the Rhizosphere. Environ. Sci. Technol. 2004, 38, 1740–1745. [Google Scholar] [CrossRef]
- Muratova, A.; Hübner, T.; Tischer, S.; Turkovskaya, O.; Möder, M.; Kuschk, P. Plant—Rhizosphere-Microflora Association during Phytoremediation of PAH-Contaminated Soil. Int. J. Phytoremediat. 2003, 5, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.J.A.; Inoue, C.; Chien, M.F. Hydroponic approach to assess rhizodegradation by sudangrass (Sorghum × drummondii) reveals pH- and plant age-dependent variability in bacterial degradation of polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2020, 387, 121695. [Google Scholar] [CrossRef] [PubMed]
- Fortin Faubert, M.; Desjardins, D.; Hijri, M.; Labrecque, M. Willows Used for Phytoremediation Increased Organic Contaminant Concentrations in Soil Surface. Appl. Sci. 2021, 11, 2979. [Google Scholar] [CrossRef]
- Sakshi; Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Shahsavari, E.; Aburto-Medina, A.; Foda, M.F.; Clarke, B.; Roddick, F.; Ball, A.S. Bioremediation of biosolids with Phanerochaete chrysosporium culture filtrates enhances the degradation of polycyclic aromatic hydrocarbons (PAHs). Appl. Soil Ecol. 2018, 124, 163–170. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, X.; Huang, L.; Xu, J.; Wang, W.; Li, Z.; Xu, P.; Tang, H. Microbial degradation of multiple PAHs by a microbial consortium and its application on contaminated wastewater. J. Hazard. Mater. 2021, 419, 126524. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.; Stanley, G.; Britz, M. Microbial degradation and detoxification of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia strain VUN 10,003. Lett. Appl. Microbiol. 2010, 30, 396–401. [Google Scholar] [CrossRef]
- Trzesicka-Mlynarz, D.; Ward, O.P. Degradation of polycyclic aromatic hydrocarbons (PAHs) by a mixed culture and its component pure cultures, obtained from PAHmcontaminated soil. Can. J. Microbiol. 1995, 41, 470. [Google Scholar] [CrossRef]
- Tadros, M.G.; Hughes, J.B. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) by Indigenous Mixed and Pure Cultures Isolated from Coastal Sediments. Appl. Biochem. Biotechnol. 1997, 63, 865–870. [Google Scholar] [CrossRef]
- Muangchinda, C.; Rungsihiranrut, A.; Prombutara, P.; Soonglerdsongpha, S.; Pinyakong, O. 16S metagenomic analysis reveals adaptability of a mixed-PAH-degrading consortium isolated from crude oil-contaminated seawater to changing environmental conditions. J. Hazard. Mater. 2018, 357, 119–127. [Google Scholar] [CrossRef]
- Nana, X. Bioadsorption, Uptake and Biodegradation of Polycyclic Aromatic Hydrocarbons by PH Microbial Consortium; Ocean University of China: Qingdao, China, 2014. [Google Scholar]
- Zhao, X.; Wang, Y.; Ye, Z.; Borthwick, A.G.L.; Ni, J. Oil field wastewater treatment in Biological Aerated Filter by immobilized microorganisms. Process Biochem. 2006, 41, 1475–1483. [Google Scholar] [CrossRef]
- Agrawal, N.; Verma, P.; Shahi, S.K. Degradation of polycyclic aromatic hydrocarbons (phenanthrene and pyrene) by the ligninolytic fungi Ganoderma lucidum isolated from the hardwood stump. Bioresour. Bioprocess. 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Nanca, C.L.; Neri, K.D.; Ngo, A.C.R.; Bennett, R.M.; Dedeles, G.R. Degradation of polycyclic aromatic hydrocarbons by moderately halophilic bacteria from luzon salt beds degradation of pahs by moderately halophilic bacteria. J. Health Pollut. 2019, 8, 180915. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.B.; Mahala, K.; Jain, K.; Madamwar, D. Development of mixed bacterial cultures DAK11 capable for degrading mixture of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2018, 253, 288–296. [Google Scholar] [CrossRef]
- Barone, R.; Nastro, R.A.; Gambino, E.; Toscanesi, M.; Picciall, G.; Napoli, L.D.; Trifuoggi, M.; Piccialli, V.; Guida, M. Pseudomonas anguilliseptica Strain-A1 Degradation of Polycyclic Aromatic Hydrocarbons in Soil Microcosms: Focus on Detoxification Activity and Free Water-Soluble Protein Extracts Kinetics and Efficiency. J. Bioremediat. Biodegrad. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Smith, K.E.; Schwab, A.P.; Banks, M.K. Dissipation of PAHs in saturated, dredged sediments: A field trial. Chemosphere 2008, 72, 1614–1619. [Google Scholar] [CrossRef]
- Dezhong, S. Bioremediation of Polluted Environment; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Viamajala, S.; Peyton, B.M.; Richards, L.A.; Petersen, J.N. Solubilization, solution equilibria, and biodegradation of PAH’s under thermophilic conditions. Chemosphere 2007, 66, 1094–1106. [Google Scholar] [CrossRef]
- Hirahara, Y.; Ueno, H.; Nakamuro, K. Aqueous photodegradation of fenthion by ultraviolet B irradiation: Contribution of singlet oxygen in photodegradation and photochemical hydrolysis. Water Res. 2003, 37, 468–476. [Google Scholar] [CrossRef]
- Qunying, Z.; Shifen, W. Microbiology of Environmental Engineering; Springer: New York, NY, USA, 2015; pp. 155–156. [Google Scholar]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Al-Mutairi, N.; Bufarsan, A.; Al-Rukaibi, F. Ecorisk evaluation and treatability potential of soils contaminated with petroleum hydrocarbon-based fuels. Chemosphere 2008, 74, 142–148. [Google Scholar] [CrossRef]
- Gurav, R.; Lyu, H.; Ma, J.; Tang, J.; Liu, Q.; Zhang, H. Degradation of n-alkanes and PAHs from the heavy crude oil using salt-tolerant bacterial consortia and analysis of their catabolic genes. Environ. Sci. Pollut. Res. Int. 2017, 24, 11392–11403. [Google Scholar] [CrossRef] [PubMed]
- Hong, L. Construction of Salt-Tolerant Petroleum-Degrading Microbiol Consortium and Degradation Performance; Xi’an Shiyou University: Xi’an, China, 2020. [Google Scholar]
- Bordoloi, N.K.; Konwar, B.K. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf. B Biointerfaces 2008, 63, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sadler, W.R.; Trudinger, P.A. The inhibition of microorganisms by heavy metals. Miner. Depos. 1967, 2, 158–168. [Google Scholar] [CrossRef]
- Bauer, M.A.W.; Kirby, M.W.M.M.; Sherris, M.J.C.; Turck, M.M. Antibiotic susceptibility testing by a standardized single disk method. Tech. Bull. Regist. Med. Technol. 1966, 36, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Chengtun, Q.; Jinling, L.; Shidong, Z. Treatment Technology of Oily Sludge in Oil and Gas Field; Material Reports: Beijing, China, 2017; pp. 3–4. [Google Scholar]
- Fernando Bautista, L.; Sanz, R.; Carmen Molina, M.; González, N.; Sánchez, D. Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria. Int. Biodeterior. Biodegrad. 2009, 63, 913–922. [Google Scholar] [CrossRef]
- Ramirez, D.; Shaw, L.J.; Collins, C.D. Oil sludge washing with surfactants and co-solvents: Oil recovery from different types of oil sludges. Environ. Sci. Pollut. Res. Int. 2021, 28, 5867–5879. [Google Scholar] [CrossRef]
- Sponza, D.T.; Gok, O. Effect of rhamnolipid on the aerobic removal of polyaromatic hydrocarbons (PAHs) and COD components from petrochemical wastewater. Bioresour. Technol. 2010, 101, 914–924. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Prasanna, D.; Purushotham Reddy, B.; Sarma, P.N. Ex situ bioremediation of pyrene contaminated soil in bio-slurry phase reactor operated in periodic discontinuous batch mode: Influence of bioaugmentation. Int. Biodeterior. Biodegrad. 2008, 62, 162–169. [Google Scholar] [CrossRef]
- Arun, A.; Raja, P.P.; Arthi, R.; Ananthi, M.; Kumar, K.S.; Eyini, M. Polycyclic aromatic hydrocarbons (PAHs) biodegradation by basidiomycetes fungi, Pseudomonas isolate, and their cocultures: Comparative in vivo and in silico approach. Appl. Biochem. Biotechnol. 2008, 151, 132–142. [Google Scholar] [CrossRef]





| NO. | Name | Characteristics of Colony | ||||
|---|---|---|---|---|---|---|
| Colour | Transparency | Edge | Cell Morphology | GenBank Number | ||
| 1-1 | Pseudomonas aeruginosa A | White | Opaque | Neat | Bacilli | KX665604 |
| 1-2 | Microbacterium oxidans | Light yellow | Translucent | Neat | Bacilli | KX665599 |
| 1-3 | Ochrobactrum intermedium | Milky white | Opaque | Neat | Bacilli | KX665600 |
| 1-4 | Stenotrop Homonas | Milky white | Opaque | Neat | Bacilli | KX665603 |
| 1-5 | Brevibacillus laterosporus | Grayish white | Opaque | Untidy | Bacilli | KX665602 |
| 1-6 | Alcaligenes faecalis | White | Transparent | Untidy | Corynebacterium | KX665601 |
| 2-1 | Pseudomonas aeruginosa B | Grayish green | Translucent | Neat | Bacilli brevis | ----- |
| 2-2 | Achromobacter sp. | Yellow | Translucent | Neat | Bacilli brevis | ----- |
| 2-3 | Cellulomonas | Light yellow | Opaque | Untidy | Bacilli | ----- |
| 2-4 | Serratia marcescens | Dark red | Opaque | Untidy | Bacilli brevis | ----- |
| Serial NO. | NAP | PHE | PYR | |||
|---|---|---|---|---|---|---|
| NO. | Degradation Rates | NO. | Degradation Rates | NO. | Degradation Rates | |
| 1 | 1-1 | 87.40% | 1-1 | 38.58% | 1-5 | 45.83% |
| 2 | 1-3 | 86.05% | 2-1 | 37.15% | 1-1 | 44.88% |
| 3 | 1-2 | 85.36% | 1-6 | 37.10% | 1-3 | 37.42% |
| 4 | 1-6 | 85.25% | 2-3 | 34.35% | 1-6 | 31.02% |
| 5 | 1-5 | 84.36% | 2-2 | 33.69% | 2-1 | 30.29% |
| 6 | 2-1 | 84.28% | 1-5 | 31.99% | 2-3 | 29.73% |
| NO. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| NAP/mg/L | 1000 | 2000 | 3000 | 4000 | 5000 |
| PHE/mg/L | 10 | 20 | 30 | 40 | 50 |
| PYR/mg/L | 20 | 40 | 60 | 80 | 100 |
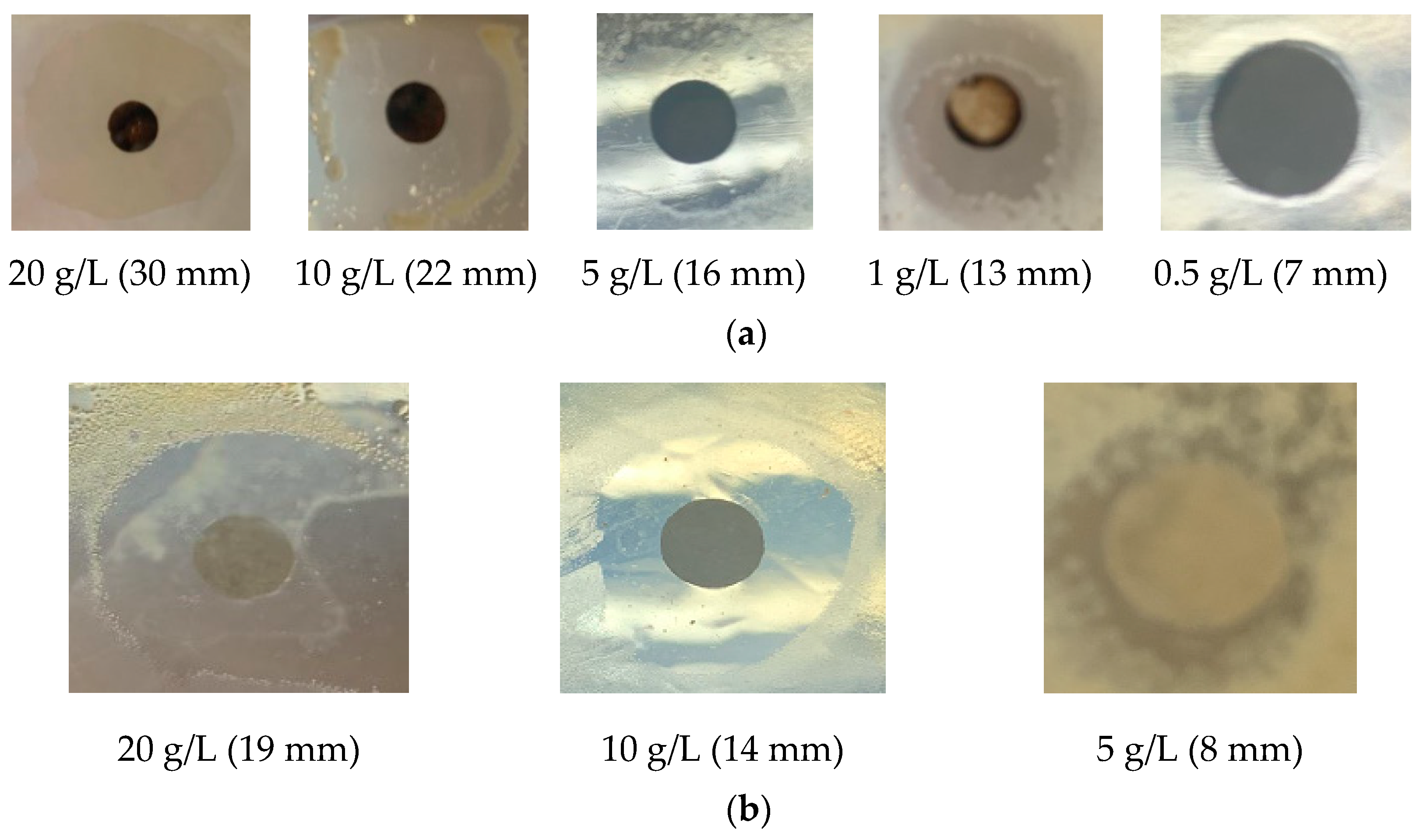
| Compound Concentration (g/L) | Average Diameter of Bacteriostatic Circles (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CuSO4·5H2O | ZnSO4·7H2O | Pb(NO3)2 | |||||||
| 1-1 | 1-6 | P&A | 1-1 | 1-6 | P&A | 1-1 | 1-6 | P&A | |
| 0.1 | —— | —— | —— | —— | —— | —— | —— | —— | —— |
| 0.5 | —— | 7 | 7 | —— | —— | —— | —— | —— | —— |
| 1 | —— | 8 | 13 | —— | —— | —— | —— | —— | —— |
| 5 | —— | 9 | 16 | —— | 10 | 5 | —— | —— | 7 |
| 10 | 15 | 16 | 22 | 8 | 12 | 10 | 8 | —— | 10 |
| 20 | 30 | 30 | 30 | 16 | 14 | 19 | 11 | 8 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yang, B.; Qu, C.; Chen, G.; Qi, F.; Yu, T.; Mustapha, A. Construction and Degradation Performance Study of Polycyclic Aromatic Hydrocarbons (PAHs) Degrading Bacterium Consortium. Appl. Sci. 2022, 12, 2354. https://doi.org/10.3390/app12052354
Zhang L, Yang B, Qu C, Chen G, Qi F, Yu T, Mustapha A. Construction and Degradation Performance Study of Polycyclic Aromatic Hydrocarbons (PAHs) Degrading Bacterium Consortium. Applied Sciences. 2022; 12(5):2354. https://doi.org/10.3390/app12052354
Chicago/Turabian StyleZhang, Long, Bo Yang, Chengtun Qu, Gang Chen, Feng Qi, Tao Yu, and Azlin Mustapha. 2022. "Construction and Degradation Performance Study of Polycyclic Aromatic Hydrocarbons (PAHs) Degrading Bacterium Consortium" Applied Sciences 12, no. 5: 2354. https://doi.org/10.3390/app12052354
APA StyleZhang, L., Yang, B., Qu, C., Chen, G., Qi, F., Yu, T., & Mustapha, A. (2022). Construction and Degradation Performance Study of Polycyclic Aromatic Hydrocarbons (PAHs) Degrading Bacterium Consortium. Applied Sciences, 12(5), 2354. https://doi.org/10.3390/app12052354







