Joule-Heating Annealing to Increase Organic Solar Cells Performance: A Comparative Study
Abstract
:1. Introduction
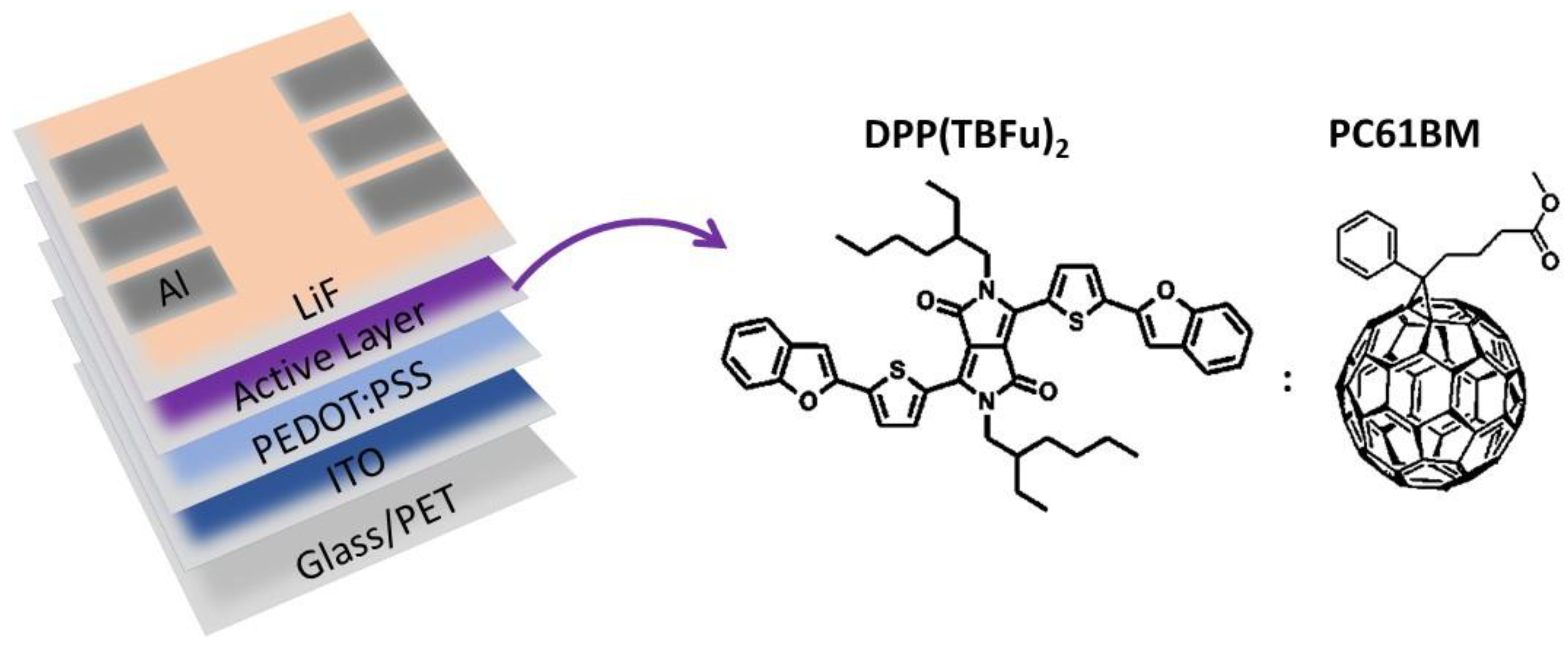
2. Materials and Methods
- Solvent vapor annealing (SVA) was achieved by exposing the films to a saturated vapor of solvent of THF or DCM in an airtight vessel [19]. The vessel (200 mL) was filled with 7 mL of an organic solvent and left sealed for 5 min prior to the SVA step to ensure that the headspace was saturated with the solvent. The substrates were exposed to the solvent vapors until the color of the films changed (between 1 and 2 min).
- Thermal annealing (TA) was done on a heating plate at 65−110 °C until the color changed.
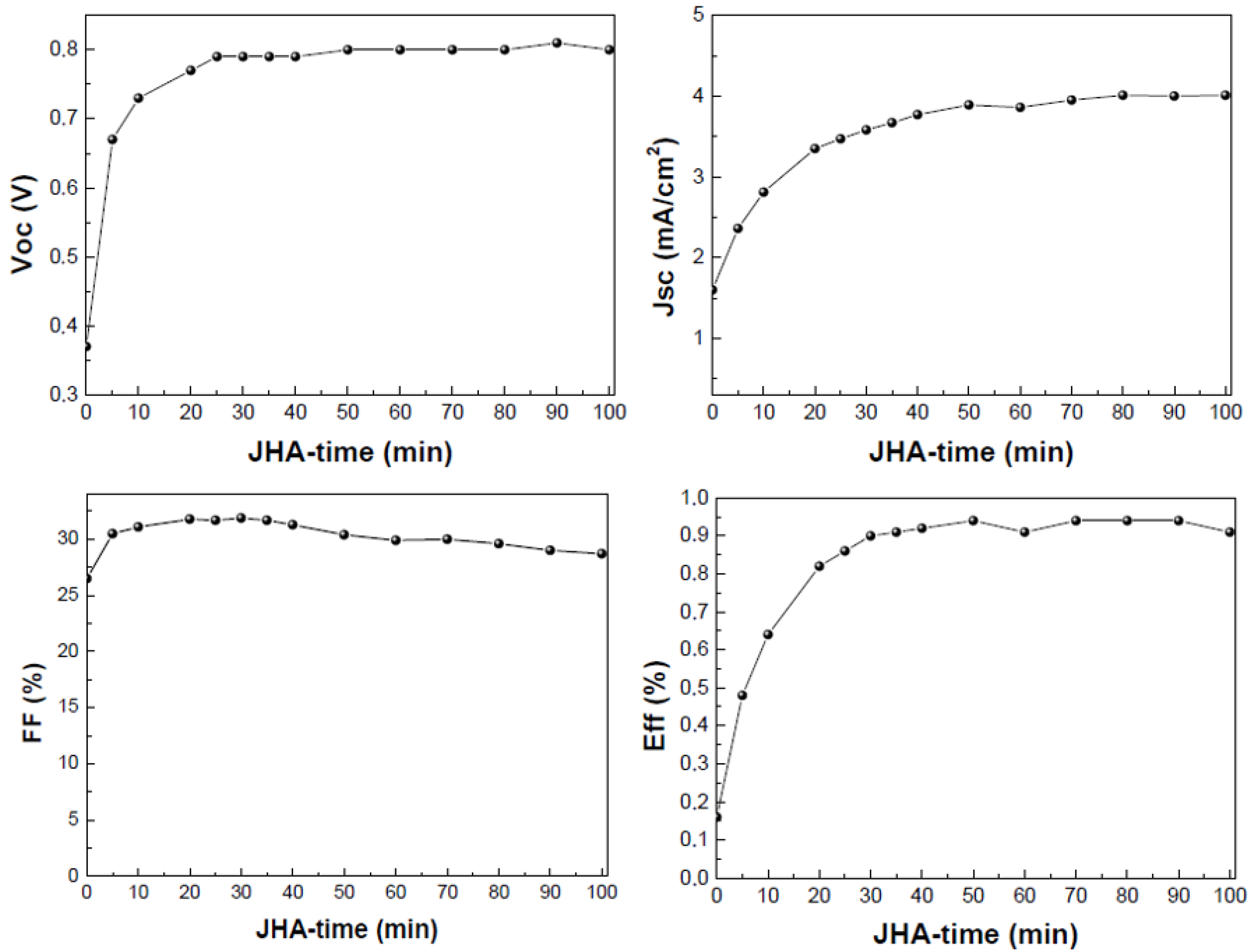
- Joule-Heating-Annealing (JHA) was also explored. This treatment is based on the heat released by the flow of an electric current generated after application of a constant voltage through the metallic contacts of the devices. The domains are formed by means of increasing the temperature due to the current flow through the active layer. The constant voltage applied was 5 V, and the current observed was 30–40 mA.
3. Results and Discussion
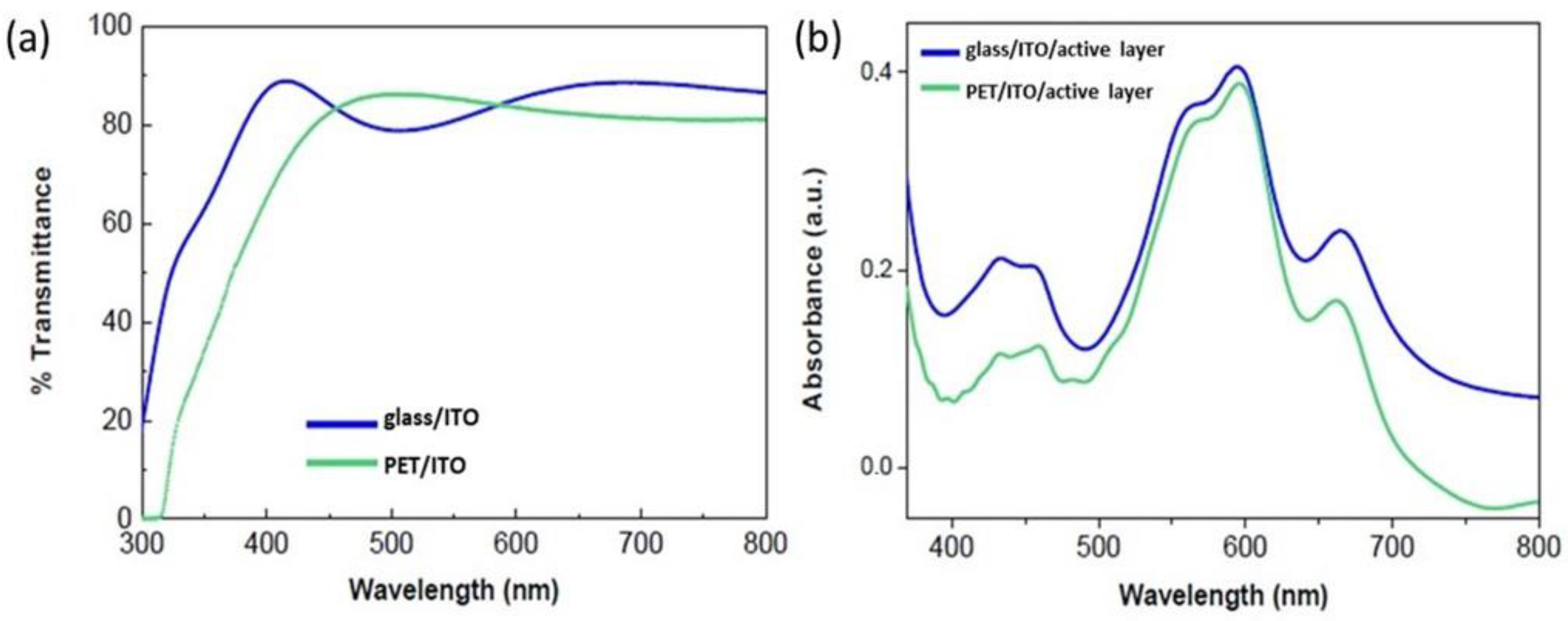
3.1. The Effects of Using PET
3.2. Post-Treatment of the Active Layer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A decade of perovskite photovoltaics. Nat. Energy 2019, 4, 1. [CrossRef] [Green Version]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, M.; Chai, Y.; Liu, P.; Zhang, B.; Liu, J.; Ye, L. Towards a bright future: The versatile applications of organic solar cells. Mater. Rep. Energy 2021, 1, 100062. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Liu, W.; Zhou, Z.; Yue, Q.; Zheng, W.; Sun, R.; Liu, W.; Xu, S.; Fan, H.; et al. Organic Solar Cells with 18% Efficiency Enabled by an Alloy Acceptor: A Two-in-One Strategy. Adv. Mater. 2021, 33, 2100830. [Google Scholar] [CrossRef] [PubMed]
- Burgués-Ceballos, I.; Stella, M.; Lacharmoise, P.; Martínez-Ferrero, E. Towards industrialization of polymer solar cells: Material processing for upscaling. J. Mater. Chem. A 2014, 2, 17711–17722. [Google Scholar] [CrossRef]
- Lucera, L.; Kubis, P.; Fecher, F.W.; Bronnbauer, C.; Turbiez, M.; Forberich, K.; Ameri, T.; Egelhaaf, H.-J.; Brabec, C.J. Guidelines for Closing the Efficiency Gap between Hero Solar Cells and Roll-To-Roll Printed Modules. Energy Technol. 2015, 3, 373–384. [Google Scholar] [CrossRef]
- Strohm, S.; Machui, F.; Langner, S.; Kubis, P.; Gasparini, N.; Salvador, M.; McCulloch, I.; Egelhaaf, H.-J.; Brabec, C.J. P3HT: Non-fullerene acceptor based large area, semi-transparent PV modules with power conversion efficiencies of 5%, processed by industrially scalable methods. Energy Environ. Sci. 2018, 11, 2225–2234. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, C.; Xie, C.; Li, W. Flexible organic solar cells: Materials, large-area fabrication techniques and potential applications. Nano Energy 2021, 89, 106399. [Google Scholar] [CrossRef]
- Cambarau, W.; Fritze, U.F.; Viterisi, A.; Palomares, E.; von Delius, M. Increased short circuit current in an azafullerene-based organic solar cell. Chem. Commun. 2015, 51, 1128–1130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Jiang, Z.; Yang, C.; Yu, J.; Feng, J.; Adil, M.A.; Deng, D.; Zou, W.; Zhang, J.; Lu, K.; et al. All-small-molecule organic solar cells with over 14% efficiency by optimizing hierarchical morphologies. Nat. Commun. 2019, 10, 5393. [Google Scholar] [CrossRef]
- Collins, S.D.; Ran, N.A.; Heiber, M.C.; Nguyen, T.-Q. Small is Powerful: Recent Progress in Solution-Processed Small Molecule Solar Cells. Adv. Energy Mater. 2017, 7, 1602242. [Google Scholar] [CrossRef]
- Kumar, C.V.; Cabau, L.; Koukaras, E.N.; Viterisi, A.; Sharma, G.D.; Palomares, E. Solution processed organic solar cells based on A–D–D′–D–A small molecule with benzo[1,2-b:4,5-b′]dithiophene donor (D′) unit, cyclopentadithiophene donor (D) and ethylrhodanine acceptor unit having 6% light to energy conversion efficiency. J. Mater. Chem. A 2015, 3, 4892–4902. [Google Scholar] [CrossRef]
- Walker, B.; Tamayo, A.B.; Dang, X.-D.; Zalar, P.; Seo, J.H.; Garcia, A.; Tantiwiwat, M.; Nguyen, T.-Q. Nanoscale Phase Separation and High Photovoltaic Efficiency in Solution-Processed, Small-Molecule Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 3063–3069. [Google Scholar] [CrossRef]
- De Luca, G.; Liscio, A.; Nolde, F.; Scolaro, L.M.; Palermo, V.; Müllen, K.; Samorì, P. Self-assembly of discotic molecules into mesoscopic crystals by solvent-vapour annealing. Soft Matter 2008, 4, 2064–2070. [Google Scholar] [CrossRef]
- El-Tantawy, F. Joule heating treatments of conductive butyl rubber/ceramic superconductor composites: A new way for improving the stability and reproducibility? Eur. Polym. J. 2001, 37, 565–574. [Google Scholar] [CrossRef]
- Tran, V.-T.; Wei, Y.; Du, H. On-Substrate Joule Effect Heating by Printed Micro-Heater for the Preparation of ZnO Semiconductor Thin Film. Micromachines 2020, 11, 490. [Google Scholar] [CrossRef]
- Znajdek, K.; Sibiński, M.; Strąkowska, A.; Lisik, Z. Polymer substrates for flexible photovoltaic cells application in personal electronic system. Opto-Electron. Rev. 2016, 24, 20–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, X.-D.; Kim, C.; Nguyen, T.-Q. Effect of Charge Recombination on the Fill Factor of Small Molecule Bulk Heterojunction Solar Cells. Adv. Energy Mater. 2011, 1, 610–617. [Google Scholar] [CrossRef]
- Viterisi, A.; Gispert-Guirado, F.; Ryan, J.W.; Palomares, E. Formation of highly crystalline and texturized donor domains in DPP(TBFu)2:PC71BM SM-BHJ devices via solvent vapour annealing: Implications for device function. J. Mater. Chem. 2012, 22, 15175–15182. [Google Scholar] [CrossRef]
- Kwong, C.Y.; Djurišić, A.B.; Chui, P.C.; Chan, W.K. CuPc/C60Solar Cells–Influence of the Indium Tin Oxide Substrate and Device Architecture on the Solar Cell Performance. Jpn. J. Appl. Phys. 2004, 43, 1305–1311. [Google Scholar] [CrossRef]
- Ajuria, J.; Chavhan, S.; Tena-Zaera, R.; Chen, J.; Rondinone, A.J.; Sonar, P.; Dodabalapur, A.; Pacios, R. Nanomorphology influence on the light conversion mechanisms in highly efficient diketopyrrolopyrrole based organic solar cells. Org. Electron. 2013, 14, 326–334. [Google Scholar] [CrossRef]
- Street, R.A.; Song, K.W.; Cowan, S. Influence of series resistance on the photocurrent analysis of organic solar cells. Org. Electron. 2011, 12, 244–248. [Google Scholar] [CrossRef]
- Guerrero, A.; Ripolles-Sanchis, T.; Boix, P.P.; Garcia-Belmonte, G. Series resistance in organic bulk-heterojunction solar devices: Modulating carrier transport with fullerene electron traps. Org. Electron. 2012, 13, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Li, L.; Yang, X. Creating a Uniform Distribution of Fullerene C60 Nanorods in a Polymer Matrix and its Photovoltaic Applications. Small 2008, 4, 601–606. [Google Scholar] [CrossRef]
- Swinnen, A.; Haeldermans, I.; vande Ven, M.; D’Haen, J.; Vanhoyland, G.; Aresu, S.; D’Olieslaeger, M.; Manca, J. Tuning the Dimensions of C60-Based Needlelike Crystals in Blended Thin Films. Adv. Funct. Mater. 2006, 16, 760–765. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, S.; Ahn, H.; Joe, S.; Kim, B.J.; Son, H.J. Low-Temperature Processable High-Performance D–A-Type Random Copolymers for Nonfullerene Polymer Solar Cells and Application to Flexible Devices. Adv. Energy Mater. 2018, 8, 1801601. [Google Scholar] [CrossRef]
- Hegde, R.; Henry, N.; Whittle, B.; Zang, H.; Hu, B.; Chen, J.; Xiao, K.; Dadmun, M. The impact of controlled solvent exposure on the morphology, structure and function of bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2012, 107, 112–124. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, B.; Qiao, X.; Luan, L.; Lu, K.; Hu, B. Revealing Underlying Processes Involved in Light Soaking Effects and Hysteresis Phenomena in Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500279. [Google Scholar] [CrossRef]
- Cabau, L.; Pellejà, L.; Clifford, J.N.; Kumar, C.V.; Palomares, E. Light soaking effects on charge recombination and device performance in dye sensitized solar cells based on indoline–cyclopentadithiophene chromophores. J. Mater. Chem. A 2013, 1, 8994–9000. [Google Scholar] [CrossRef]

| Substrates | Average Thickness of ITO Film (nm) | Sheet Resistance (Ω/sq) | RMS Average (nm) |
|---|---|---|---|
| ITO-glass | 205 | 10 | 1.07 |
| ITO-PET | 100 | 50 | 4.77 |
| Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | RS (Ω) | RP (Ω) | |
|---|---|---|---|---|---|---|
| ITO-glass | 0.77 ± 0.08 | 6.64 ± 1.18 | 42.85 ± 11.8 | 2.19 ± 0.8 | 129.23 ± 30 | 1653.90 ± 57 |
| ITO-PET | 0.64 ± 0.09 | 3.11 ± 1.46 | 36.82 ± 7.2 | 0.73 ± 0.65 | 469.63 ± 156 | 2133.71 ± 44 |
| SVA Exposure Time (min) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | RS (Ω) | RP (Ω) | |
|---|---|---|---|---|---|---|---|
| ITO-glass | 1.25 | 0.83 | 7.37 | 26.48 | 1.60 | 870.1 | 709.0 |
| 2 | 0.73 | 7.62 | 25.6 | 1.68 | 716.2 | 620.7 | |
| 3 | 0.73 | 7.23 | 23.46 | 1.23 | 943.5 | 672.2 | |
| 5 | 0.70 | 6.37 | 22.44 | 0.96 | 853.4 | 579.3 | |
| ITO-PET | 1.25 | 0.47 | 4.58 | 26.88 | 0.6 | 390.0 | 502.7 |
| 2 | 0.51 | 5.42 | 26.41 | 0.76 | 401.11 | 518.7 | |
| 3 | 0.61 | 4.01 | 26.05 | 0.56 | 1538.55 | 1784.6 | |
| 5 | 0.33 | 4.27 | 25.8 | 0.35 | 288.8 | 343.95 |
| Temperature | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | RS (Ω) | RP (Ω) |
|---|---|---|---|---|---|---|
| 110 °C | 0.64 | 3.11 | 36.82 | 0.73 | 469.63 | 2133.71 |
| 65–80 °C | 0.77 | 6.14 | 31.36 | 1.46 | 330.363 | 837.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez, M.; Fernández, D.; Viterisi, A.; Martínez-Ferrero, E.; Palomares, E. Joule-Heating Annealing to Increase Organic Solar Cells Performance: A Comparative Study. Appl. Sci. 2022, 12, 2552. https://doi.org/10.3390/app12052552
Méndez M, Fernández D, Viterisi A, Martínez-Ferrero E, Palomares E. Joule-Heating Annealing to Increase Organic Solar Cells Performance: A Comparative Study. Applied Sciences. 2022; 12(5):2552. https://doi.org/10.3390/app12052552
Chicago/Turabian StyleMéndez, Maria, Daniel Fernández, Aurelien Viterisi, Eugenia Martínez-Ferrero, and Emilio Palomares. 2022. "Joule-Heating Annealing to Increase Organic Solar Cells Performance: A Comparative Study" Applied Sciences 12, no. 5: 2552. https://doi.org/10.3390/app12052552
APA StyleMéndez, M., Fernández, D., Viterisi, A., Martínez-Ferrero, E., & Palomares, E. (2022). Joule-Heating Annealing to Increase Organic Solar Cells Performance: A Comparative Study. Applied Sciences, 12(5), 2552. https://doi.org/10.3390/app12052552








