Fingerprinting Organochlorine Groundwater Plumes Based on Non-Invasive ERT Technology at a Chemical Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Setting
2.2. Conceptual Model of ERT Method
2.3. ERT Layout and Detection
2.4. Groundwater Sampling and Analysis
2.5. Data Processing
3. Results and Discussion
3.1. Electrical Characteristics of Hydrogeological Background
3.2. Pollution Identification Based on ERT
3.3. Spatial Distribution of Organochlorine Pesticides
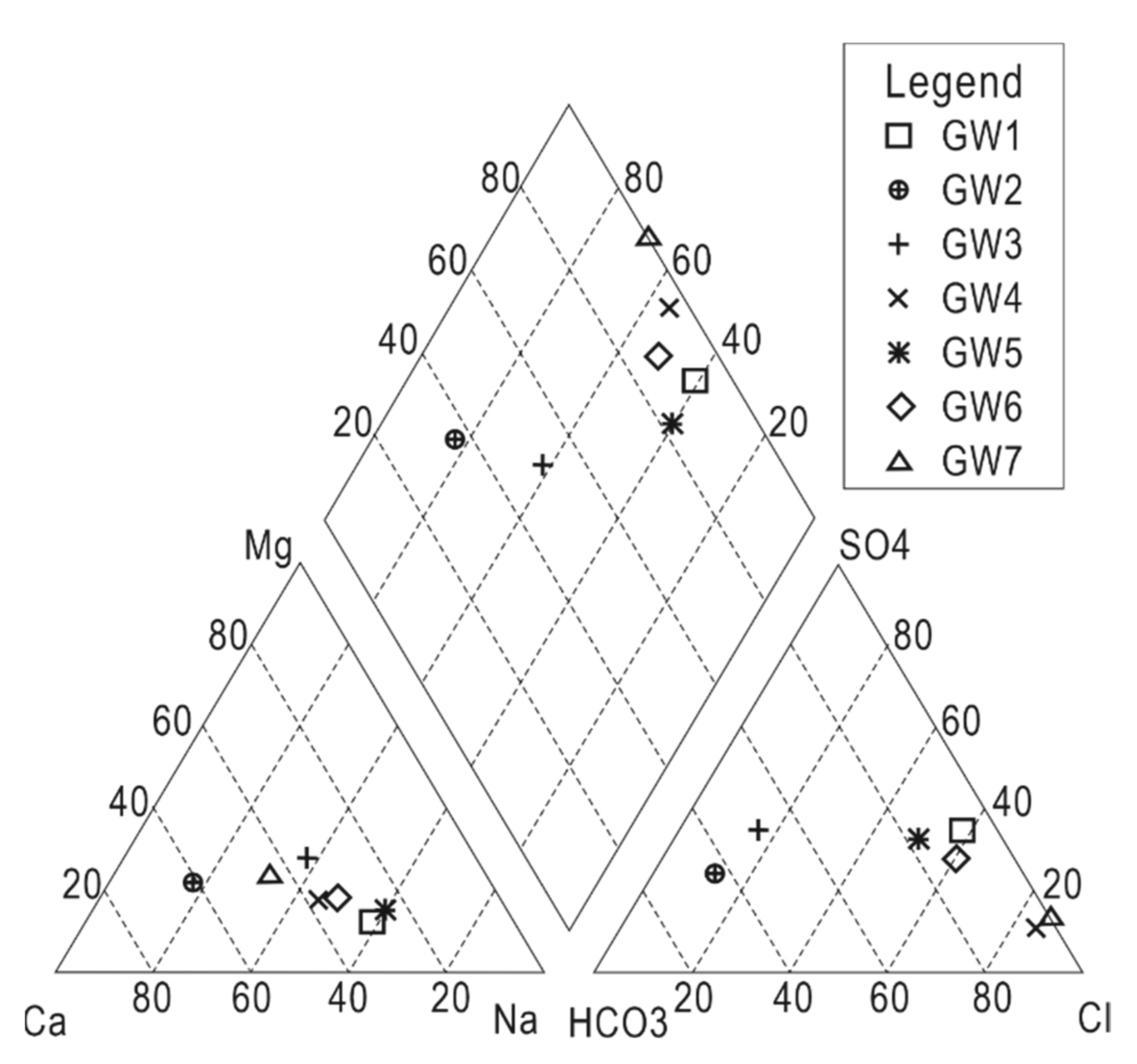
3.4. Hydrochemical Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghimire, N.; Woodward, R.T. Under- And over-Use of Pesticides: An International Analysis. Ecol. Econ. 2013, 89, 73–81. [Google Scholar] [CrossRef]
- Rafique, N.; Tariq, S.R. Distribution and Source Apportionment Studies of Heavy Metals in Soil of Cotton/Wheat Fields. Environ. Monit. Assess. 2016, 188, 309. [Google Scholar] [CrossRef] [PubMed]
- Shternshis, M.V.; Belyaev, A.A.; Matchenko, N.S.; Shpatova, T.V.; Lelyak, A.A. Possibility of Biological Control of Primocane Fruiting Raspberry Disease Caused by Fusarium Sambucinum. Environ. Sci. Pollut. Res. 2015, 22, 15656–15662. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Fang, J.; Wei, G. Compressed Sensing Reconstruction Algorithm Based on DCT Band Energy. J. S. China Univ. Technol. 2015, 43, 28–33. [Google Scholar]
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Impact of Spent Mushroom Substrates on the Fate of Pesticides in Soil, and Their Use for Preventing and/or Controlling Soil and Water Contamination: A Review. Toxics 2016, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Niu, L.; Xu, C.; Yao, Y.; Liu, K.; Yang, F.; Tang, M.; Liu, W. Status, Influences and Risk Assessment of Hexachlorocyclohexanes in Agricultural Soils Across China. Environ. Sci. Technol. 2013, 47, 12140–12147. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, X.; Zhao, L.; Zhou, B.; Weng, L.; Li, Y. Comparative Study on the Pollution Status of Organochlorine Pesticides (OCPs) and Bacterial Community Diversity and Structure between Plastic Shed and Open-Field Soils from Northern China. Sci. Total Environ. 2020, 741, 139620. [Google Scholar] [CrossRef]
- Wong, C.S.; Garrison, A.W.; Foreman, W.T. Enantiomeric Composition of Chiral Polychlorinated Biphenyl Atropisomers in Aquatic Bed Sediment. Environ. Sci. Technol. 2001, 35, 33–39. [Google Scholar] [CrossRef]
- Shang, X.; Dong, G.; Zhang, H.; Zhang, L.; Yu, X.; Li, J.; Wang, X.; Yue, B.; Zhao, Y.; Wu, Y. Polybrominated Diphenyl Ethers (PBDEs) and Indicator Polychlorinated Biphenyls (PCBs) in Various Marine Fish from Zhoushan Fishery, China. Food Control 2016, 67, 240–246. [Google Scholar] [CrossRef]
- Briz, V.; Molina-Molina, J.M.; Sánchez-Redondo, S.; Fernández, M.F.; Grimalt, J.O.; Olea, N.; Rodríguez-Farré, E.; Suñol, C. Differential Estrogenic Effects of the Persistent Organochlorine Pesticides Dieldrin, Endosulfan, and Lindane in Primary Neuronal Cultures. Toxicol. Sci. 2011, 120, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, H.; Pan, G.; Wang, J.; Chen, B. Factors Influencing the Persistence of Organochlorine Pesticides in Surface Soil from the Region around the Hongze Lake, China. Sci. Total Environ. 2013, 443, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; Vaishnavi, C. Bioaccumulative Potential and Toxicity of Endosulfan Insecticide to Non-Target Animals. Comp. Biochem. Physiol. C Comp. Pharmacol. 1993, 105, 347–361. [Google Scholar] [CrossRef]
- Kalra, S.; Dewan, P.; Batra, P.; Sharma, T.; Tyagi, V.; Banerjee, B.D. Organochlorine Pesticide Exposure in Mothers and Neural Tube Defects in Offsprings. Reprod. Toxicol. 2016, 66, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Megharaj, M. Local Applications but Global Implications: Can Pesticides Drive Microorganisms to Develop Antimicrobial Resistance. Sci. Total Environ. 2019, 654, 177–189. [Google Scholar] [CrossRef]
- Abdo, W.; Hirata, A.; Sakai, H.; El-Sawak, A.; Nikami, H.; Yanai, T. Combined Effects of Organochlorine Pesticides Heptachlor and Hexachlorobenzene on the Promotion Stage of Hepatocarcinogenesis in Rats. Food Chem. Toxicol. 2013, 55, 578–585. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Ayotte, P.; Diorio, C. Persistent Organic Pollutants and Breast Cancer: A Systematic Review and Critical Appraisal of the Literature. Cancers 2019, 11, 1063. [Google Scholar] [CrossRef] [Green Version]
- Mathur, V.; Bhatnagar, P.; Sharma, R.G.; Acharya, V.; Sexana, R. Breast Cancer Incidence and Exposure to Pesticides among Women Originating from Jaipur. Environ. Int. 2002, 28, 331–336. [Google Scholar] [CrossRef]
- Rathore, M.; Bhatnagar, P.; Mathur, D.; Saxena, G.N. Burden of Organochlorine Pesticides in Blood and Its Effect on Thyroid Hormones in Women. Sci. Total Environ. 2002, 295, 207–215. [Google Scholar] [CrossRef]
- Ennaceur, S.; Gandoura, N.; Driss, M.R. Distribution of Polychlorinated Biphenyls and Organochlorine Pesticides in Human Breast Milk from Various Locations in Tunisia: Levels of Contamination, Influencing Factors, and Infant Risk Assessment. Environ. Res. 2008, 108, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.N.; Humphries, M.S.; Buah-Kwofie, A.; Schleyer, M.H. Accumulation of Organochlorine Pesticides in Reef Organisms from Marginal Coral Reefs in South Africa and Links with Coastal Groundwater. Mar. Pollut. Bull. 2018, 137, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, Y.; Gui, T.; Huang, Y. Concentrations and Potential Health Hazards of Organochlorine Pesticides in Shallow Groundwater of Taihu Lake Region, China. Sci. Total Environ. 2014, 470–471, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liao, X.; Li, J.; Xu, L.; Liu, M.; Du, B.; Wang, Y. Influence of Long-Term Sewage Irrigation on the Distribution of Organochlorine Pesticides in Soil–Groundwater Systems. Chemosphere 2013, 92, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; Chidya, R.; Jadoon, W.; Sakugawa, H. Temporal Trends in Organophosphorus Pesticides Use and Concentrations in River Water in Japan, and Risk Assessment. J. Environ. Sci. 2019, 79, 135–152. [Google Scholar] [CrossRef]
- El-Nahhal, I. Pesticide Residues in Drinking Water, Their Potential Risk to Human Health and Removal Options. J. Environ. Manag. 2021, 299, 113611. [Google Scholar] [CrossRef]
- IPEP. International POPs Elimination Project. Fostering Active and Efficient Civil Society Participation in Preparation for Implementation of the Stockholm Convention, Country Situation on Persistent Organic Pollutants (POPs) in India; Toxics Link: New Delhi, India, 2006; pp. 1–57. [Google Scholar]
- Liu, Y.; Bashir, S.; Stollberg, R.; Trabitzsch, R.; Weiß, H.; Paschke, H.; Nijenhuis, I.; Richnow, H.-H. Compound Specific and Enantioselective Stable Isotope Analysis as Tools to Monitor Transformation of Hexachlorocyclohexane (HCH) in a Complex Aquifer System. Environ. Sci. Technol. 2017, 51, 8909–8916. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Atekwana, E.A. Geophysical Signatures of Microbial Activity at Hydrocarbon Contaminated Sites: A Review. Surv. Geophys. 2010, 31, 247–283. [Google Scholar] [CrossRef]
- Simyrdanis, K.; Papadopoulos, N.; Soupios, P.; Kirkou, S.; Tsourlos, P. Characterization and Monitoring of Subsurface Contamination from Olive Oil Mills’ Waste Waters Using Electrical Resistivity Tomography. Sci. Total Environ. 2018, 637–638, 991–1003. [Google Scholar] [CrossRef]
- Delaney, A.J.; Peapples, P.R.; Arcone, S.A. Electrical Resistivity of Frozen and Petroleum-Contaminated Fine-Grained Soil. Cold Reg. Sci. Technol. 2001, 32, 107–119. [Google Scholar] [CrossRef]
- Cardarelli, E.; Di Filippo, G. Electrical Resistivity and Induced Polarization Tomography in Identifying the Plume of Chlorinated Hydrocarbons in Sedimentary Formation: A Case Study in Rho (Milan—Italy). Waste Manag. Res. 2009, 27, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Renzi, B. Electrical Permittivity and Resistivity Time Lapses of Multiphase DNAPLs in a Lab Test. Water Resour. Res. 2015, 51, 377–389. [Google Scholar] [CrossRef]
- Cassidy, N.J. Evaluating LNAPL Contamination Using GPR Signal Attenuation Analysis and Dielectric Property Measurements: Practical Implications for Hydrological Studies. J. Contam. Hydrol. 2007, 94, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-P.; Chen, C.-C.; Tong, L.-T.; Chang, P.-Y.; Chen, Y.-C.; Dong, T.-H.; Liu, H.-C.; Lin, C.-P.; Yang, K.-H.; Ho, C.-J.; et al. Applying FDEM, ERT and GPR at a Site with Soil Contamination: A Case Study. J. Appl. Geophys. 2015, 121, 21–30. [Google Scholar] [CrossRef]
- Hudson, E.; Kulessa, B.; Edwards, P.; Williams, T.; Walsh, R. Integrated Hydrological and Geophysical Characterisation of Surface and Subsurface Water Contamination at Abandoned Metal Mines. Water Air Soil Pollut. 2018, 229, 256. [Google Scholar] [CrossRef] [Green Version]
- Vásconez-Maza, M.D.; Martínez-Segura, M.A.; Bueso, M.C.; Faz, Á.; García-Nieto, M.C.; Gabarrón, M.; Acosta, J.A. Predicting Spatial Distribution of Heavy Metals in an Abandoned Phosphogypsum Pond Combining Geochemistry, Electrical Resistivity Tomography and Statistical Methods. J. Hazard. Mater. 2019, 374, 392–400. [Google Scholar] [CrossRef]
- Pujari, P.R.; Pardhi, P.; Muduli, P.; Harkare, P.; Nanoti, M.V. Assessment of Pollution Near Landfill Site in Nagpur, India by Resistivity Imaging and GPR. Environ. Monit. Assess. 2007, 131, 489–500. [Google Scholar] [CrossRef]
- Bichet, V.; Grisey, E.; Aleya, L. Spatial Characterization of Leachate Plume Using Electrical Resistivity Tomography in a Landfill Composed of Old and New Cells (Belfort, France). Eng. Geol. 2016, 211, 61–73. [Google Scholar] [CrossRef]
- Azizi, F.; Vadiati, M.; Asghari Moghaddam, A.; Nazemi, A.; Adamowski, J. A Hydrogeological-Based Multi-Criteria Method for Assessing the Vulnerability of Coastal Aquifers to Saltwater Intrusion. Environ. Earth Sci. 2019, 78, 548. [Google Scholar] [CrossRef]
- Maury, S.; Balaji, S. Application of Resistivity and GPR Techniques for the Characterization of the Coastal Litho-Stratigraphy and Aquifer Vulnerability Due to Seawater Intrusion. Estuar. Coast. Shelf Sci. 2015, 165, 104–116. [Google Scholar] [CrossRef]
- Shao, S.; Gao, C.; Guo, X.; Wang, Y.; Zhang, Z.; Yu, L.; Tang, H. Mapping the Contaminant Plume of an Abandoned Hydrocarbon Disposal Site with Geophysical and Geochemical Methods, Jiangsu, China. Environ. Sci. Pollut. Res. 2019, 26, 24645–24657. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Xu, H.; Jiang, X.; Lan, J.; Chen, Y.; Zhang, Z. Use of Electrical Resistivity Tomography for Detecting the Distribution of Leachate and Gas in a Large-Scale MSW Landfill Cell. Environ. Sci. Pollut. Res. 2019, 26, 20325–20343. [Google Scholar] [CrossRef] [PubMed]
- Carretero, S.; Rapaglia, J.; Perdomo, S.; Albino Martínez, C.; Rodrigues Capítulo, L.; Gómez, L.; Kruse, E. A Multi-Parameter Study of Groundwater–Seawater Interactions along Partido de La Costa, Buenos Aires Province, Argentina. Environ. Earth Sci. 2019, 78, 513. [Google Scholar] [CrossRef]
- Alesse, B.; Orlando, L.; Palladini, L. Non-invasive Lab Test in the Monitoring of Vadose Zone Contaminated by Light Non-aqueous Phase Liquid. Geophys. Prospect. 2019, 67, 2161–2175. [Google Scholar] [CrossRef]
- Wang, Y. Study on Numerical Simulation and Inversion of Electrical Resistivity Method for Heavy Metal Contaminated Sites; China University of Mining & Technology: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Rosales, R.M.; Martínez-Pagan, P.; Faz, A.; Moreno-Cornejo, J. Environmental Monitoring Using Electrical Resistivity Tomography (ERT) in the Subsoil of Three Former Petrol Stations in SE of Spain. Water Air Soil Pollut. 2012, 223, 3757–3773. [Google Scholar] [CrossRef]
- Raji, W.O.; Obadare, I.G.; Odukoya, M.A.; Johnson, L.M. Electrical Resistivity Mapping of Oil Spills in a Coastal Environment of Lagos, Nigeria. Arab. J. Geosci. 2018, 11, 144. [Google Scholar] [CrossRef]
- Caterina, D.; Flores Orozco, A.; Nguyen, F. Long-Term ERT Monitoring of Biogeochemical Changes of an Aged Hydrocarbon Contamination. J. Contam. Hydrol. 2017, 201, 19–29. [Google Scholar] [CrossRef]
- Yang, C.; You, J. High Resistivities Associated with an LNAPL Plume Imaged by Geoelectrical Tomography. In Proceedings of the SEG Technical Program Expanded Abstracts 1999; Society of Exploration Geophysicists: Houston, TX, USA, 1999; pp. 469–472. [Google Scholar]
- Lago, A.L.; Elis, V.R.; Borges, W.R.; Penner, G.C. Geophysical Investigation Using Resistivity and GPR Methods: A Case Study of a Lubricant Oil Waste Disposal Area in the City of Ribeirão Preto, São Paulo, Brazil. Environ. Geol. 2009, 58, 407–417. [Google Scholar] [CrossRef]
- Wang, Y.; Anderson, N.; Torgashov, E. Condition Assessment of Building Foundation in Karst Terrain Using Both Electrical Resistivity Tomography and Multi-Channel Analysis Surface Wave Techniques. Geotech. Geol. Eng. 2019, 38, 1839–1855. [Google Scholar] [CrossRef]
- Heenan, J.; Slater, L.D.; Ntarlagiannis, D.; Atekwana, E.A.; Fathepure, B.Z.; Dalvi, S.; Ross, C.; Werkema, D.D.; Atekwana, E.A. Electrical Resistivity Imaging for Long-Term Autonomous Monitoring of Hydrocarbon Degradation: Lessons from the Deepwater Horizon Oil Spill. Geophysics 2015, 80, B1–B11. [Google Scholar] [CrossRef]
- Pan, Y.; Jia, Y.; Xu, Z.; Lei, X. The study on resistivity characteristics of oil contaminated soil in different influencing factors. Acta Sci. Circumst. 2015, 35, 880–889. (In Chinese) [Google Scholar]






| Experiment | Application | |||||
|---|---|---|---|---|---|---|
| Material | Resistivity (Ω·m) | Location | Geological Material | Resistivity (Ω·m) | References | |
| Lower Bound | Upper Bound | |||||
| Clay | 1 [46] | 100 1 | “El Trampolin” Petrol Station | Predominantly silts of reddish to brown shades. | 0–50 | [47] |
| Sand | 4 1 | 800 1 | Ijegun Community of Lagos Nigeria | Sand layer | 120–328 | [48] |
| Loam | 5 1 | 50 1 | Oil leakage in underground tanks in Liège (Belgium). | Quaternary clayey and loamy soils | <20 | [49] |
| Shale | 20 1 | 2000 1 | Ijegun Community of Lagos Nigeria | Shale/mudstone layer | 25–116 | [48] |
| Gravel/conglomerate | 2000 1 | 104 1 | LNAPL leak from an underground pipeline | Gravel layer | 170–250 | [50] |
| Coarse sandstone | 10 1 | 9.6 × 104 1 | “Lebor Campsa” Petrol Station in the southeast of the Iberian Peninsula | Quaternary alluviums (gravel, sand, and clay) | 70–350 | [47] |
| Siltstone | 1 1 | 1.5 × 104 1 | --- | --- | --- | --- |
| Sandstone | 50 1 | 8.0 × 103 1 | A lubricant oil waste disposal area in Brazil | Reddish sandstone layers, fine to medium, with well selected frosted grains, highly spherical | 185–3000 | [51] |
| Limestone | 80 1 | 1.0 × 103 1 | In foundation survey projects for hotel buildings in Kentucky | Limestone | <60 (identified where the moisture content was high) | [52] |
| Well | Groundwater Resistivity (Ω·m) | |
|---|---|---|
| June 2018 | April 2019 | |
| GW1 | 6.053 | 6.536 |
| GW2 | 32.787 | 15.221 |
| GW3 | 29.070 | 13.477 |
| GW4 | 3984.064 | --- |
| GW6 | 8.104 | --- |
| GW7 | 1131.222 | 2840.909 |
| Profile Number | Device | Layered Power Supply | Power Supply Time | Total Number of Electrodes | Electrode Distance | Repeated Collection Times | Total Data Points |
|---|---|---|---|---|---|---|---|
| ML-I | Wenner-Alpha | No | 1 s | 24 | 2 | 3 | 84 |
| ML-II | Wenner-Alpha | No | 1 s | 40 | 2 | 3 | 235 |
| ML-III | Wenner-Alpha | No | 1 s | 24 | 3 | 3 | 84 |
| ML-IV | Wenner-Alpha | No | 1 s | 32 | 4 | 3 | 155 |
| Groundwater Well | Trans-1,2-Dichloroethylene | 1,2,3-Trichlorobenzene | Hexachlorocyclohexane |
|---|---|---|---|
| GW1 | 703 | 23 | 1 |
| GW2 | 1.1 | <1.0 | 0 |
| GW3 | 1.7 | 41.2 | 13 |
| GW4 | 61.3 | 316 | 72 |
| GW7 | 9.4 | 103 | 783 |
| GW8 | 5.5 | 102 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Song, X.; Wu, Y.; Gao, C.; Wang, Y.; Yang, Y. Fingerprinting Organochlorine Groundwater Plumes Based on Non-Invasive ERT Technology at a Chemical Plant. Appl. Sci. 2022, 12, 2816. https://doi.org/10.3390/app12062816
Yan Z, Song X, Wu Y, Gao C, Wang Y, Yang Y. Fingerprinting Organochlorine Groundwater Plumes Based on Non-Invasive ERT Technology at a Chemical Plant. Applied Sciences. 2022; 12(6):2816. https://doi.org/10.3390/app12062816
Chicago/Turabian StyleYan, Zihan, Xiaoming Song, Yuhui Wu, Cuiping Gao, Yunlong Wang, and Yuesuo Yang. 2022. "Fingerprinting Organochlorine Groundwater Plumes Based on Non-Invasive ERT Technology at a Chemical Plant" Applied Sciences 12, no. 6: 2816. https://doi.org/10.3390/app12062816







