RNA Interference-Based Pesticides and Antiviral Agents: Microbial Overproduction Systems for Double-Stranded RNA for Applications in Agriculture and Aquaculture
Abstract
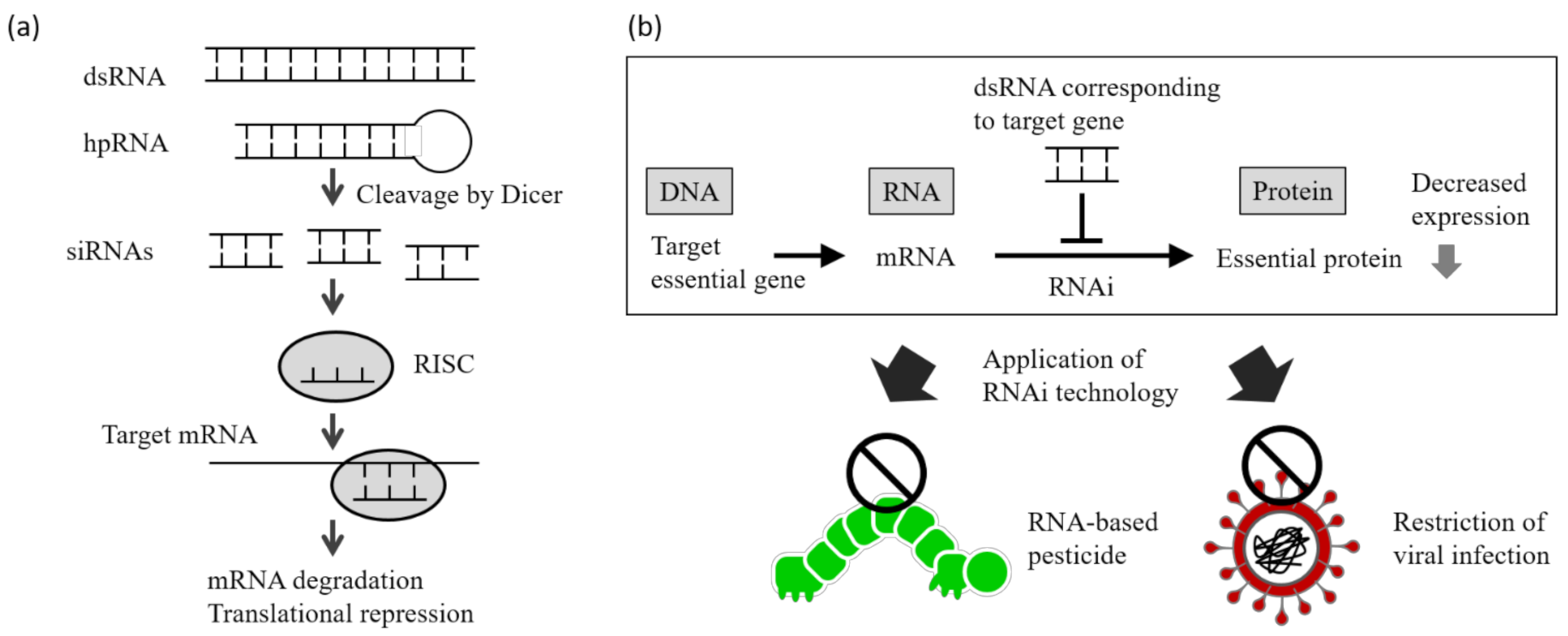
:1. Introduction
2. Production of Recombinant dsRNA in Microorganisms
2.1. Escherichia coli
2.2. Pseudomonas syringae
2.3. Corynebacterium glutamicum
2.4. Chlamydomonas reinhardtii
2.5. Other Microorganisms for dsRNA Production
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farm to Fork Strategy. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Available online: https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 29 September 2021).
- Michael, C.; Gil, E.; Gallart, M.; Stavrinides, M.C. Evaluation of the effects of spray technology and volume rate on the control of grape berry moth in mountain viticulture. Agriculture 2021, 11, 178. [Google Scholar] [CrossRef]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [Green Version]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Carbonell, A.; de Alba, Á.E.M.; Flores, R.; Gago, S. Double-stranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology 2008, 371, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Gebremichael, D.E.; Haile, Z.M.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA Interference strategies for future management of plant pathogenic fungi: Prospects and Challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef]
- Robinson, K.E.; Worrall, E.A.; Mitter, N. Double stranded RNA expression and its topical application for non-transgenic resistance to plant viruses. J. Plant Biochem. Biotechnol. 2014, 23, 231–237. [Google Scholar] [CrossRef]
- Itsathitphaisarn, O.; Thitamadee, S.; Weerachatyanukul, W.; Sritunyalucksana, K. Potential of RNAi applications to control viral diseases of farmed shrimp. J. Invertebr. Pathol. 2017, 147, 76–85. [Google Scholar] [CrossRef]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Jiang, C.; Uffman, J.; Levine, S.; Carson, D. Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef]
- Fischer, J.R.; Zapata, F.; Dubelman, S.; Mueller, G.M.; Uffman, J.P.; Jiang, C.; Jensen, P.D.; Levine, S.L. Aquatic fate of a double-stranded RNA in a sediment–water system following an over-water application. Environ. Toxicol. Chem. 2017, 36, 727–734. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Timmons, L.; Fire, A. Specific interference by ingested dsRNA. Nature 1998, 395, 854. [Google Scholar] [CrossRef]
- Timmons, L.; Court, D.L.; Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001, 263, 103–112. [Google Scholar] [CrossRef]
- Tenllado, F.; Martínez-García, B.; Vargas, M.; Díaz-Ruíz, J.R. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 2003, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Peng, H.; Yao, Q.; Chen, H.; Xie, Q.; Tang, B.; Zhang, W. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e6225. [Google Scholar] [CrossRef]
- Sun, Z.N.; Yin, G.H.; Song, Y.Z.; An, H.L.; Zhu, C.X.; Wen, F.J. Bacterially expressed double-stranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus Y genome have different ability to protect tobacco from viral infection. Appl. Biochem. Biotechnol. 2010, 162, 1901–1914. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Jiang, H.; Jiang, T.; Zhu, S.; Cheng, B. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef]
- Ganbaatar, O.; Cao, B.; Zhang, Y.; Bao, D.; Bao, W.; Wuriyanghan, H. Knockdown of Mythimna separata chitinase genes via bacterial expression and oral delivery of RNAi effectors. BMC Biotechnol. 2017, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Leelesh, R.S.; Rieske, L.K. Oral Ingestion of bacterially expressed dsRNA can silence genes and cause mortality in a highly invasive, tree-killing pest, the emerald ash borer. Insects 2020, 11, 440. [Google Scholar] [CrossRef]
- Holeva, M.C.; Sklavounos, A.; Rajeswaran, R.; Pooggin, M.M.; Voloudakis, A.E. Topical application of double-stranded RNA targeting 2b and CP genes of cucumber mosaic virus protects plants against local and systemic viral infection. Plants 2021, 10, 963. [Google Scholar] [CrossRef]
- Ongvarrasopone, C.; Roshorm, Y.; Panyim, S. A simple and cost effective method to generate dsRNA for RNAi studies in invertebrates. ScienceAsia 2007, 33, 35–39. [Google Scholar] [CrossRef]
- Kumar, D.R.; Kumar, P.S.; Gandhi, M.R.; Al-Dhabi, N.A.; Paulraj, M.G.; Ignacimuthu, S. Delivery of chitosan/dsRNA nanoparticles for silencing of wing development vestigial (vg) gene in Aedes aegypti mosquitoes. Int. J. Biol. Macromol. 2016, 86, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Guo, W.; Chen, S.; Guo, M.; Qiu, B.; Yang, C.; Zhang, Y.; Pan, H. Double-stranded RNAs targeting HvRPS18 and HvRPL13 reveal potential targets for pest management of the 28-spotted ladybeetle, Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2020, 76, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, M.; Yang, C.; Liu, Z.; Chen, S.; Lü, J.; Qiu, B.; Zhang, Y.; Zhou, X.; Pan, H. RNA interference-mediated silencing of vATPase subunits A and E affect survival and development of the 28-spotted ladybeetle, Henosepilachna vigintioctopunctata. Insect Sci. 2021, 28, 1664–1676. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Necira, K.; Makki, M.; Sanz-García, E.; Canto, T.; Djilani-Khouadja, F.; Tenllado, F. Topical Application of Escherichia coli-Encapsulated dsRNA Induces Resistance in Nicotiana benthamiana to Potato Viruses and Involves RDR6 and Combined Activities of DCL2 and DCL4. Plants 2021, 10, 644. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Govindaswamy, M.; Sidhu, G.S.; Singh, S.; Kaur, S.; Chhuneja, P. Ingestion of bacteria expressing dsRNA to maggots produces severe mortality and deformities in fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Egypt. J. Biol. Pest Control. 2021, 31, 1. [Google Scholar] [CrossRef]
- Thammasorn, T.; Sangsuriya, P.; Meemetta, W.; Senapin, S.; Jitrakorn, S.; Rattanarojpong, T.; Saksmerprome, V. Large-scale production and antiviral efficacy of multi-target double-stranded RNA for the prevention of white spot syndrome virus (WSSV) in shrimp. BMC Biotechnol. 2015, 15, 110. [Google Scholar] [CrossRef] [Green Version]
- Papić, L.; Rivas, J.; Toledo, S.; Romero, J. Double-stranded RNA production and the kinetics of recombinant Escherichia coli HT115 in fed-batch culture. Biotechnol. Rep. 2018, 20, e00292. [Google Scholar] [CrossRef]
- Yin, G.; Sun, Z.; Liu, N.; Zhang, L.; Song, Y.; Zhu, C.; Wen, F. Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. Appl. Microbiol. Biotechnol. 2009, 84, 323–333. [Google Scholar] [CrossRef]
- Sun, Z.N.; Song, Y.Z.; Yin, G.H.; Zhu, C.X.; Wen, F.J. HpRNAs derived from different regions of the NIb gene have different abilities to protect tobacco from infection with Potato virus Y. J. Phytopathol. 2010, 158, 566–568. [Google Scholar]
- Shen, W.; Yang, G.; Chen, Y.; Yan, P.; Tuo, D.; Li, X.; Zhou, P. Resistance of non-transgenic papaya plants to papaya ringspot virus (PRSV) mediated by intron-containing hairpin dsRNAs expressed in bacteria. Acta Virol. 2014, 58, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; He, J.; Luo, P.; Li, X.; Gao, Y. Production of functional double-stranded RNA using a prokaryotic expression system in Escherichia coli. MicrobiologyOpen 2019, 8, e00787. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Xu, Y.; Gao, J.; Song, Y.; Zhu, C. RNAi silencing of rice black-streaked dwarf virus P10 and two insect vector genes to reduce virus transmission protects rice plants against RBSDV. J. Plant Interact. 2021, 16, 83–92. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhou, H.; Wei, Y.L.; Yan, S.; Shen, J. A novel plasmid–Escherichia coli system produces large batch dsRNAs for insect gene silencing. Pest Manag. Sci. 2020, 76, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, J.; Deighan, P.; Kiner, E.; McReynolds, L.; Lieberman, J. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat. Biotechnol. 2013, 31, 350–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Lieberman, J. Production of highly potent recombinant siRNAs in Escherichia coli. Nat. Protoc. 2013, 8, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, L.; Dardel, F. Recombinant RNA technology: The tRNA scaffold. Nat. Methods 2007, 4, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponchon, L.; Dardel, F. Large scale expression and purification of recombinant RNA in Escherichia coli. Methods 2011, 54, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, F.H.; Leunissen, E.H.; van de Laar, L.; Tessari, M.; Heus, H.A.; Wijmenga, S.S. Fast production of homogeneous recombinant RNA—towards large-scale production of RNA. Nucleic Acids Res. 2012, 40, e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Potty, A.S.; Jackson, G.W.; Stepanov, V.; Tang, A.; Liu, Y.; Kourentzi, K.; Strych, U.; Fox, G.E.; Willson, R.C. Engineered 5S ribosomal RNAs displaying aptamers recognizing vascular endothelial growth factor and malachite green. J. Mol. Recognit. 2009, 22, 154–161. [Google Scholar] [CrossRef]
- Liu, Y.; Stepanov, V.G.; Strych, U.; Willson, R.C.; Jackson, G.W.; Fox, G.E. DNAzyme-mediated recovery of small recombinant RNAs from a 5S rRNA-derived chimera expressed in Escherichia coli. BMC Biotechnol. 2010, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.X.; Wang, W.P.; Zeng, S.; Urayama, S.; Yu, A.M. A general approach to high-yield biosynthesis of chimeric RNAs bearing various types of functional small RNAs for broad applications. Nucl. Acids Res. 2015, 43, 3857–3869. [Google Scholar] [CrossRef] [Green Version]
- Petrek, H.; Batra, N.; Ho, P.Y.; Tu, M.J.; Yu, A.M. Bioengineering of a single long noncoding RNA molecule that carries multiple small RNAs. Appl. Microbiol. Biotechnol. 2019, 103, 6107–6117. [Google Scholar] [CrossRef]
- Daròs, J.A.; Aragonés, V.; Cordero, T. A viroid-derived system to produce large amounts of recombinant RNA in Escherichia coli. Sci. Rep. 2018, 8, 1904. [Google Scholar] [CrossRef] [PubMed]
- Ortolá, B.; Cordero, T.; Hu, X.; Daròs, J.A. Intron-assisted, viroid-based production of insecticidal circular double-stranded RNA in Escherichia coli. RNA Biol. 2021, 18, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Aalto, A.P.; Sarin, L.P.; Van Dijk, A.A.; Saarma, M.; Poranen, M.M.; Arumäe, U.; Bamford, D.H. Large-scale production of dsRNA and siRNA pools for RNA interference utilizing bacteriophage ϕ6 RNA-dependent RNA polymerase. RNA 2007, 13, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.; Hirano, S.S.; Lindow, S.E. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 1999, 65, 1435–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poranen, M.M.; Bamford, D.H. Packaging and replication regulation revealed by chimeric genome segments of double-stranded RNA bacteriophage ϕ6. RNA 1999, 5, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiao, X.; Mindich, L. Construction of carrier state viruses with partial genomes of the segmented dsRNA bacteriophages. Virology 2004, 319, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, S.; Udaka, S.; Shimono, M. Studies on the amino acid fermentation. Part 1. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 1957, 3, 193–205. [Google Scholar] [CrossRef]
- Wolf, S.; Becker, J.; Tsuge, Y.; Kawaguchi, H.; Kondo, A.; Marienhagen, J.; Bott, M.; Wendisch, V.F.; Wittmann, C. Advances in metabolic engineering of Corynebacterium glutamicum to produce high-value active ingredients for food, feed, human health, and well-being. Essays Biochem. 2021, 65, 197–212. [Google Scholar]
- Lee, J.Y.; Na, Y.A.; Kim, E.; Lee, H.S.; Kim, P. The actinobacterium Corynebacterium glutamicum, an industrial workhorse. J. Microbiol. Biotechnol. 2016, 26, 807–822. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, P. Recombinant protein expression system in Corynebacterium glutamicum and its application. Front. Microbiol. 2018, 9, 2523. [Google Scholar] [CrossRef]
- Yasueda, H. Overproduction of L-glutamate in Corynebacterium glutamicum. In Microbial Production; Anazawa, H., Shimizu, S., Eds.; Springer: Tokyo, Japan, 2014; pp. 165–176. [Google Scholar]
- Ikeda, M.; Takeno, S. Amino acid production by Corynebacterium glutamicum. In Corynebacterium Glutamicum; Yukawa, H., Inui, M., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2013; pp. 107–147. [Google Scholar]
- Maeda, T.; Tanaka, Y.; Takemoto, N.; Hamamoto, N.; Inui, M. RNase III mediated cleavage of the coding region of mraZ mRNA is required for efficient cell division in Corynebacterium glutamicum. Mol. Microbiol. 2016, 99, 1149–1166. [Google Scholar] [CrossRef] [Green Version]
- Hashiro, S.; Mitsuhashi, M.; Yasueda, H. Overexpression system for recombinant RNA in Corynebacterium glutamicum using a strong promoter derived from corynephage BFK20. J. Biosci. Bioeng. 2019, 128, 255–263. [Google Scholar] [CrossRef]
- Koptides, M.; Barák, I.; ŠIšová, M.; Baloghová, E.; Ugorčaková, J.; Timko, J. Characterization of bacteriophage BFK20 from Brevibacterium flavum. Microbiology 1992, 138, 1387–1391. [Google Scholar] [CrossRef] [Green Version]
- Hashiro, S.; Mitsuhashi, M.; Yasueda, H. High copy number mutants derived from Corynebacterium glutamicum cryptic plasmid pAM330 and copy number control. J. Biosci. Bioeng. 2019, 127, 529–538. [Google Scholar] [CrossRef]
- Chikami, Y.; Kawaguchi, H.; Suzuki, T.; Yoshioka, H.; Sato, Y.; Yaginuma, T.; Niimi, T. Oral RNAi of diap1 results in rapid reduction of damage to potatoes in Henosepilachna vigintioctopunctata. J. Pest. Sci. 2021, 94, 505–515. [Google Scholar] [CrossRef]
- Hashiro, S.; Mitsuhashi, M.; Chikami, Y.; Kawaguchi, H.; Niimi, T.; Yasueda, H. Construction of Corynebacterium glutamicum cells as containers encapsulating dsRNA overexpressed for agricultural pest control. Appl. Microbiol. Biotechnol. 2019, 103, 8485–8496. [Google Scholar] [CrossRef] [Green Version]
- Hashiro, S.; Chikami, Y.; Kawaguchi, H.; Krylov, A.A.; Niimi, T.; Yasueda, H. Efficient production of long double-stranded RNAs applicable to agricultural pest control by Corynebacterium glutamicum equipped with coliphage T7-expression system. Appl. Microbiol. Biotechnol. 2021, 105, 4987–5000. [Google Scholar] [CrossRef]
- Hashiro, S.; Yasueda, H. Plasmid copy number mutation in repA gene encoding RepA replication initiator of cryptic plasmid pHM1519 in Corynebacterium glutamicum. Biosci. Biotechnol. Biochem. 2018, 82, 2212–2224. [Google Scholar] [CrossRef]
- Vatanparast, M.; Kim, Y. Optimization of recombinant bacteria expressing dsRNA to enhance insecticidal activity against a lepidopteran insect, Spodoptera exigua. PLoS ONE 2017, 12, e0183054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyard, S.; Erdelyan, C.N.; Partridge, A.L.; Singh, A.D.; Beebe, N.W.; Capina, R. Silencing the buzz: A new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit Vectors 2015, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Mendoza, S.; Paz-Maldonado, L.M.T.; Soria-Guerra, R.E. Chlamydomonas reinhardtii as a viable platform for the production of recombinant proteins: Current status and perspectives. Plant Cell Rep. 2012, 31, 479–494. [Google Scholar] [CrossRef]
- Scranton, M.A.; Ostrand, J.T.; Fields, F.J.; Mayfield, S.P. Chlamydomonas as a model for biofuels and bio-products production. Plant J. 2015, 82, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Scaife, M.A.; Nguyen, G.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.V. The penaeid shrimp viruses TSV, IHHNV, WSSV, and YHV: Current status in the Americas, available diagnostic methods, and management strategies. J. Appl. Aquac. 1999, 9, 27–52. [Google Scholar] [CrossRef]
- Almaraz-Delgado, A.L.; Flores-Uribe, J.; Pérez-España, V.H.; Salgado-Manjarrez, E.; Badillo-Corona, J.A. Production of therapeutic proteins in the chloroplast of Chlamydomonas reinhardtii. AMB Express 2014, 4, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somchai, P.; Jitrakorn, S.; Thitamadee, S.; Meetam, M.; Saksmerprome, V. Use of microalgae Chlamydomonas reinhardtii for production of double-stranded RNA against shrimp virus. Aquac. Rep. 2016, 3, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Charoonnart, P.; Worakajit, N.; Zedler, J.A.; Meetam, M.; Robinson, C.; Saksmerprome, V. Generation of microalga Chlamydomonas reinhardtii expressing shrimp antiviral dsRNA without supplementation of antibiotics. Sci. Rep. 2019, 9, 3164. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Wang, S.; Ou, R.; Samrakandi, M.; Beerntsen, B.T.; Sayre, R.T. Development of an RNAi based microalgal larvicide to control mosquitoes. Malar. World J. 2013, 4, 1–7. [Google Scholar]
- Thammasorn, T.; Jitrakorn, S.; Charoonnart, P.; Sirimanakul, S.; Rattanarojpong, T.; Chaturongakul, S.; Saksmerprome, V. Probiotic bacteria (Lactobacillus plantarum) expressing specific double-stranded RNA and its potential for controlling shrimp viral and bacterial diseases. Aquac. Int. 2017, 25, 1679–1692. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, A.R.; Romo-Quinones, C.; Rosas-Quijano, R.; Reyes, A.G.; Barraza, A.; Magallón-Barajas, F.; Angulo, C.H.; Mejía-Ruíz, C.H. Production of specific dsRNA against white spot syndrome virus in the yeast Yarrowia lipolytica. Aquac. Res. 2018, 49, 480–491. [Google Scholar] [CrossRef]
- Saksmerprome, V.; Charoonnart, P.; Gangnonngiw, W.; Withyachumnarnkul, B. A novel and inexpensive application of RNAi technology to protect shrimp from viral disease. J. Virol. Methods. 2009, 162, 213–217. [Google Scholar] [CrossRef]
- Whitten, M.M.; Facey, P.D.; Del Sol, R.; Fernández-Martínez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger O., G.; Dyson, P.J. Symbiont-mediated RNA interference in insects. Proc. R. Soc. B 2016, 283, 20160042. [Google Scholar] [CrossRef] [Green Version]
- Chanbusarakum, L.J.; Ullman, D.E. Distribution and ecology of Frankliniella occidentalis (Thysanoptera: Thripidae) bacterial symbionts. Environ. Entomol. 2009, 38, 1069–1077. [Google Scholar] [CrossRef]
- Facey, P.D.; Méric, G.; Hitchings, M.D.; Pachebat, J.A.; Hegarty, M.J.; Chen, X.; Morgan, L.V.; Hoeppner, J.E.; Whitten, M.M.; Kirk, W.D.; et al. Draft genomes, phylogenetic reconstruction, and comparative genomics of two novel cohabiting bacterial symbionts isolated from Frankliniella occidentalis. Genome Biol. Evol. 2015, 7, 2188–2202. [Google Scholar] [CrossRef] [Green Version]
- Mysore, K.; Li, P.; Wang, C.W.; Hapairai, L.K.; Scheel, N.D.; Realey, J.S.; Sun, L.; Roethele, J.B.; Severson, D.W.; Wei, N.; et al. Characterization of a yeast interfering RNA larvicide with a target site conserved in the synaptotagmin gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2019, 13, e0007422. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef] [Green Version]
- Van Ekert, E.; Powell, C.A.; Shatters Jr, R.G.; Borovsky, D. Control of larval and egg development in Aedes aegypti with RNA interference against juvenile hormone acid methyl transferase. J. Insect Physiol. 2014, 70, 143–150. [Google Scholar] [CrossRef]
- Borovsky, D.; Nauwelaers, S.; Van Mileghem, A.; Meyvis, Y.; Laeremans, A.; Theunis, C.; Bertier, L.; Boons, E. Control of mosquito larvae with TMOF and 60 kDa Cry4Aa expressed in Pichia pastoris. Pestycydy 2011, 1–4, 5–15. [Google Scholar]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Das, P.R.; Sherif, S.M. Application of exogenous dsRNAs-induced RNAi in agriculture: Challenges and triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef]
- Adeyinka, O.S.; Riaz, S.; Toufiq, N.; Yousaf, I.; Bhatti, M.U.; Batcho, A.; Olajide, A.A.; Nasir, I.A.; Tabassum, B. Advances in exogenous RNA delivery techniques for RNAi-mediated pest control. Mol. Biol. Rep. 2020, 47, 6309–6319. [Google Scholar] [CrossRef]
- Mehlhorn, S.G.; Geibel, S.; Bucher, G.; Nauen, R. Profiling of RNAi sensitivity after foliar dsRNA exposure in different European populations of Colorado potato beetle reveals a robust response with minor variability. Pestic. Biochem. Physiol. 2020, 166, 104569. [Google Scholar] [CrossRef]
- Taning, C.N.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg H., G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef]
- Kolliopoulou, A.; Taning, C.N.; Smagghe, G.; Swevers, L. Viral delivery of dsRNA for control of insect agricultural pests and vectors of human disease: Prospects and challenges. Front. Physiol. 2017, 8, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, W.J.; Cunningham, D.S.; MacEachran, D.; Gupta, M.; Abshire, J.R. Cell-free Production of Ribonucleic Acid. U.S. Patent 10954541 B2, 23 March 2021. [Google Scholar]
- Parsons, K.H.; Mondal, M.H.; McCormick, C.L.; Flynt, A.S. Guanidinium-functionalized interpolyelectrolyte complexes enabling RNAi in resistant insect pests. Biomacromolecules 2018, 19, 1111–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hu, Y.; Yan, S.; Zhou, H.; Song, D.; Yin, M.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef]
- Yong, J.; Zhang, R.; Bi, S.; Li, P.; Sun, L.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Sheet-like clay nanoparticles deliver RNA into developing pollen to efficiently silence a target gene. Plant Physiol. 2021, 187, 886–899. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C.; et al. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef] [Green Version]
- Caccia, S.; Astarita, F.; Barra, E.; Di Lelio, I.; Varricchio, P.; Pennacchio, F. Enhancement of Bacillus thuringiensis toxicity by feeding Spodoptera littoralis larvae with bacteria expressing immune suppressive dsRNA. J. Pest Sci. 2020, 93, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Palli, S.R.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef]
- Naito, Y.; Yamada, T.; Matsumiya, T.; Ui-Tei, K.; Saigo, K.; Morishita, S. dsCheck: Highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res. 2005, 33, W589–W591. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Adema, C.M.; Lane, T. A computational study of off-target effects of RNA interference. Nucl. Acids Res. 2005, 33, 1834–1847. [Google Scholar] [CrossRef] [Green Version]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef]
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied dsRNA-based Pesticides. Ser. Pestic. No. 104. ENV/JM/MONO 2020, 26. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)26&doclanguage=en (accessed on 4 March 2022).
- Lin, Y.H.; Huang, J.H.; Liu, Y.; Belles, X.; Lee, H.J. Oral delivery of dsRNA lipoplexes to German cockroach protects dsRNA from degradation and induces RNAi response. Pest Manag. Sci. 2017, 73, 960–966. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Hu, G.L.; Lu, L.Y.; Chen, X.L.; Gao, X.W. Silencing of CYP6AS160 in Solenopsis invicta Buren by RNA interference enhances worker susceptibility to fipronil. Bull. Entomol. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Choi, M.Y.; Vander Meer, R.K.; Coy, M.; Scharf, M.E. Phenotypic impacts of PBAN RNA interference in an ant, Solenopsis invicta, and a moth, Helicoverpa zea. J. Insect Physiol. 2012, 58, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wheeler, M.M.; Oi, F.M.; Scharf, M.E. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem. Mol. Biol. 2008, 38, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Gu, D.; Yan, S.; Li, Z. RNA interference of endoglucanases in the formosan subterranean termite Coptotermes formosanus shiraki (Blattodea: Rhinotermitidae) by dsRNA injection or ingestion. J. Insect Physiol. 2019, 112, 15–22. [Google Scholar] [CrossRef] [PubMed]




| Species of Host Microbe | Strain | RNA Type | Length of the dsRNA Region | Targeted Protein or Gene for RNAi | Targeted Organism or Virus | Expression System; Mode, Promoter, Vector | Titer (Culture Scale) | Reference |
|---|---|---|---|---|---|---|---|---|
| Escherichia coli | HT115(DE3) | hpRNA | 977 bp | Replicase | Pepper mild mottle virus | T7 promoter on pGEM | 4 mg/L (20 mL) | [22] |
| HT115(DE3) | hpRNA | 400 bp | Protease | Yellow head virus | T7 promoter on pET3a | 30 mg/L (50 mL) | [29] | |
| HT115(DE3) | dsRNA | 422 bp | Vestigial gene (vg) | Aedes aegypti | Convergent transcription, T7 promoters on pLitmus28i | 20 mg/L (25 mL) | [30] | |
| HT115(DE3) | dsRNA | 480 bp | Not specified | Not specified | Convergent transcription, T7 promoters on plasmid L4440 | 182 mg/L (initial volume of fed-batch culture, 2 L) | [37] | |
| M-JM109M-JM109lacY | hpRNA | 480 bp | Coat protein (CP) | Tobacco mosaic virus | T7 promoter on pET-22b or pGEM-T | Not specified | [38] | |
| T7 Express Iq | siRNA | ~21 bp | Enhanced green fluorescent protein (EGFP) Lamin A/C (LMNA)Polo-like kinase 1 (PLK1) Tumor protein p53 (TP53) Viral infectivity factor (vif) Group-specific antigen (gag) | Aequorea Victoria Homo sapiens Homo sapiens Homo sapiens Human immunodeficiency virus 1 (HIV1) Human immunodeficiency virus 1 (HIV1) | T7 promoter and GST-p19-His expression cassette on pGEX-4T-1 | ~4 µg/L (300 mL) | [44] | |
| HT115(DE3) | ELVd-hpRNA | 87 bp | Diabrotica virgifera smooth septate junction 1 (DvSSJ1) | Diabrotica virgifera | Murein lipoprotein (lpp) promoter on pLELVd-BZB and eggplant tRNA ligase expression cassette on p15LtRnlSm | Not specified | [54] | |
| Pseudomonas syringae | Cit7 | dsRNA | ~4.0 kbp | Enhanced green fluorescent protein (eGFP) | HeLa-eGFP cells | Conversion of ssRNA to dsRNA by RNA-dependent RNA polymerase of phi6 | 1.6 mg/g wet cells (10 L fermenter) | [55] |
| LM2691 | dsRNA | 2.6 kbp, 3.5 kbp | Replicase and movement protein (rep-MP) | Tobacco mosaic virus | Conversion of ssRNA to dsRNA by RNA-dependent RNA polymerase of phi6 | 7 mg/L (100 mL) | [59] | |
| Corynebacteirum glutamicum | 2256LΔrnc | dsRNA | 360 bp | Death-associated inhibitor of apoptosis protein 1 (Diap1) | Henosepilachna vigintioctopunctata | Convergent transcription, F1 promoter on pVC7H2 | 75 mg/L (jar fermenter) | [71] |
| 2256LΔrnc | dsRNA | 360 bp | Death-associated inhibitor of apoptosis protein 1 (Diap1) | Henosepilachna vigintioctopunctata | Convergent transcription, T7 promoter on pPK4H1 | 1.0 g/L (jar fermenter) | [72] | |
| Chlamydomonas reinhardtii | CC-503 (cw92 mt+) | hpRNA | 368 bp | RNA-dependent RNA polymerase (RdRp) | Yellow head virus | psaD promoter on nuclear expression plasmid pSL18-YHV | 450 ng/L (100 mL) | [81] |
| CC-5168 (cw15, ΔpsbH, SpecR) | dsRNA | 374 bp | RNA-dependent RNA polymerase (RdRp) | Yellow head virus | Convergent transcription, psaA promoters on pSR-PYP integrated into the chloroplast | Not specified | [82] | |
| CC-4147 (FUD7 mt+) | hpRNA | 328 bp | 3-Hydroxykynurenine transaminase (3-HKT) | Anopheles stephensi | atpA promoter on pCVAC108 integrated into the chloroplast | Not specified | [83] | |
| Yarrowia lipolytica | P01a | hpRNA | 416 bp | Putative regulatory protein (Orf89) | White spot syndrome virus | XPR2 promoter on pRRQ1 | 182 ng/L (1 L) | [85] |
| Host Microbe | Advantages | Disadvantages |
|---|---|---|
| Escherichia coli |
|
|
| Pseudomonas syringae |
|
|
| Corynebacteirum glutamicum |
|
|
| Chlamydomonas reinhardtii |
|
|
| Saccharomyces cerevisiae |
|
|
| Symbiotic bacteria (Rhodococcus rhodnii, BFo2) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashiro, S.; Yasueda, H. RNA Interference-Based Pesticides and Antiviral Agents: Microbial Overproduction Systems for Double-Stranded RNA for Applications in Agriculture and Aquaculture. Appl. Sci. 2022, 12, 2954. https://doi.org/10.3390/app12062954
Hashiro S, Yasueda H. RNA Interference-Based Pesticides and Antiviral Agents: Microbial Overproduction Systems for Double-Stranded RNA for Applications in Agriculture and Aquaculture. Applied Sciences. 2022; 12(6):2954. https://doi.org/10.3390/app12062954
Chicago/Turabian StyleHashiro, Shuhei, and Hisashi Yasueda. 2022. "RNA Interference-Based Pesticides and Antiviral Agents: Microbial Overproduction Systems for Double-Stranded RNA for Applications in Agriculture and Aquaculture" Applied Sciences 12, no. 6: 2954. https://doi.org/10.3390/app12062954
APA StyleHashiro, S., & Yasueda, H. (2022). RNA Interference-Based Pesticides and Antiviral Agents: Microbial Overproduction Systems for Double-Stranded RNA for Applications in Agriculture and Aquaculture. Applied Sciences, 12(6), 2954. https://doi.org/10.3390/app12062954






