Diamonds for Life: Developments in Sensors for Biomolecules
Abstract
:1. Introduction
2. Chemical Vapour Deposited Diamond Thin Films
Plasma Treatment of Diamond Thin Films
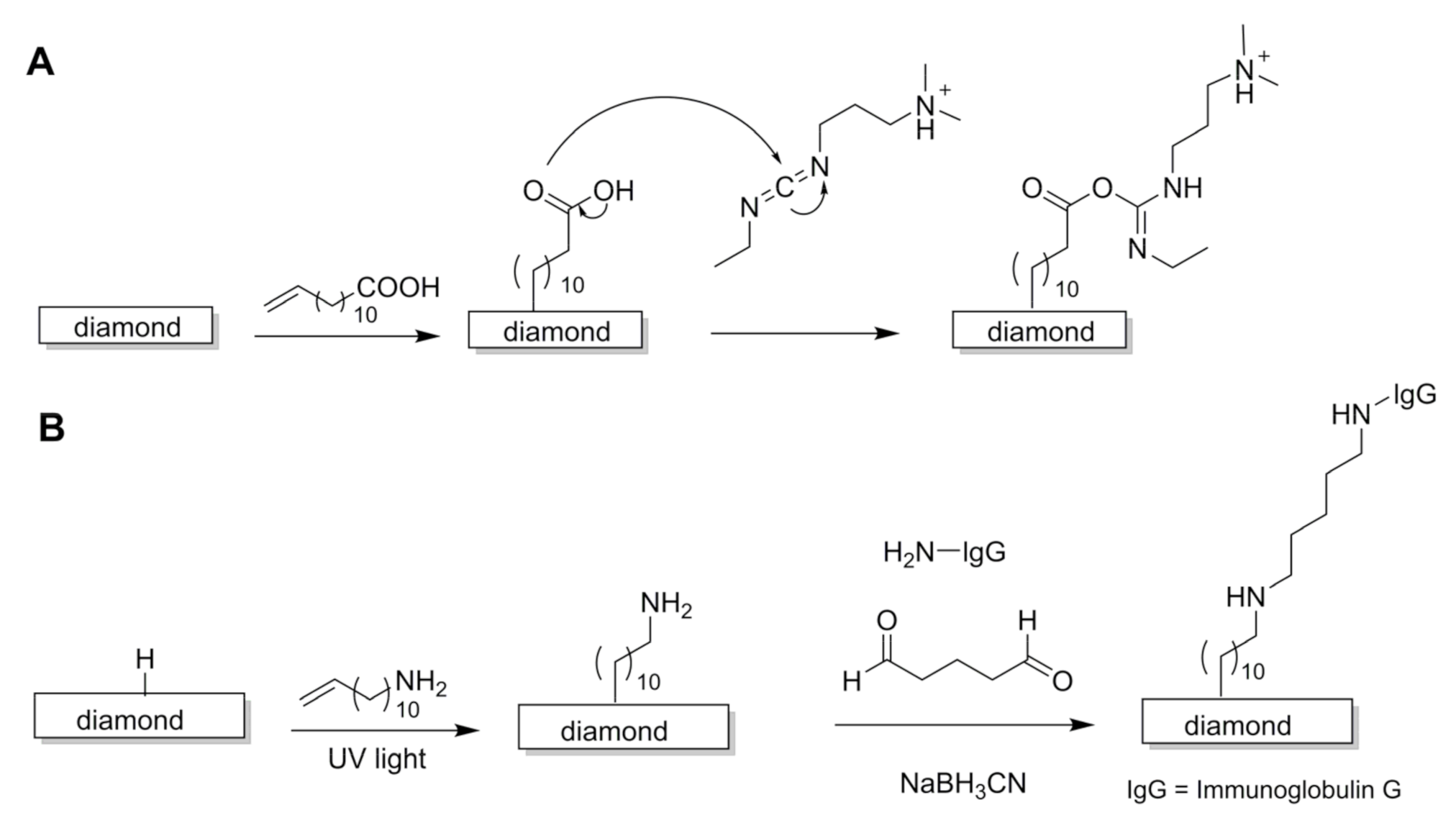
3. Surface Modification of Diamond Thin Films
3.1. Photochemical Reactions
3.2. Electrochemical Reactions
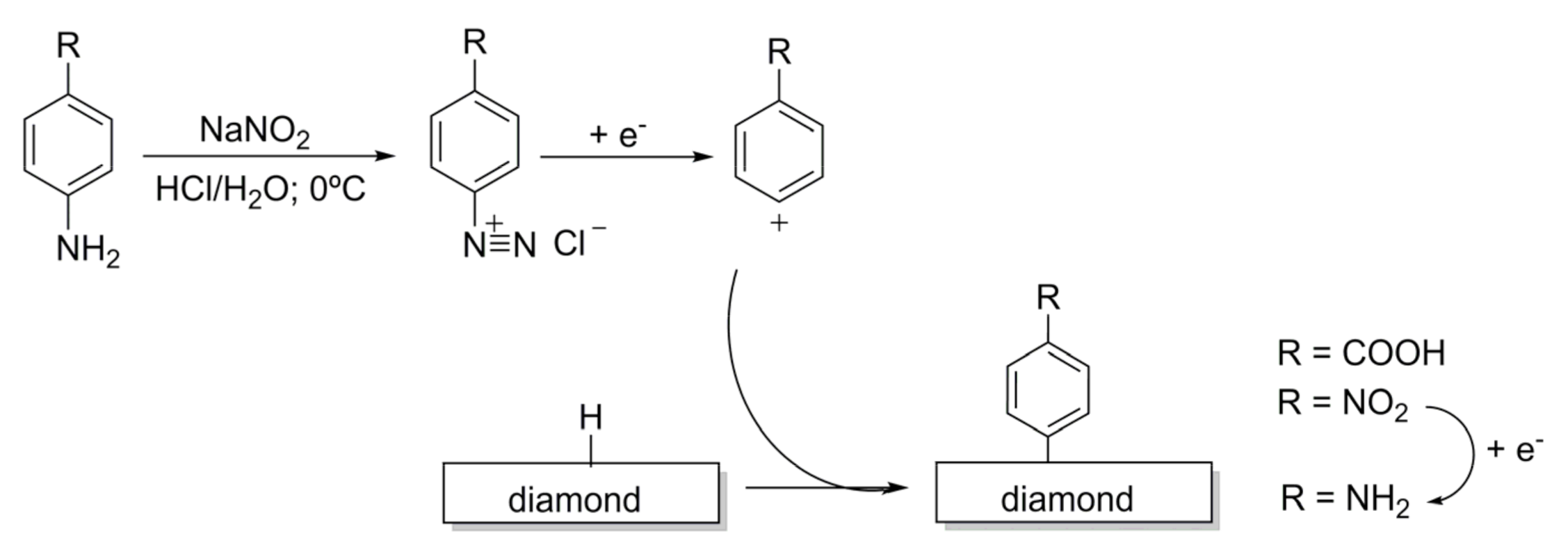
3.2.1. Reactions Involving Diazonium Salts
3.2.2. In Situ Electrochemical Polymerisation
3.3. Reactions Promoted by Ionic Liquids
4. Diamond-Based Electrodes
4.1. Histamine Detection
4.2. Serotonin Detection
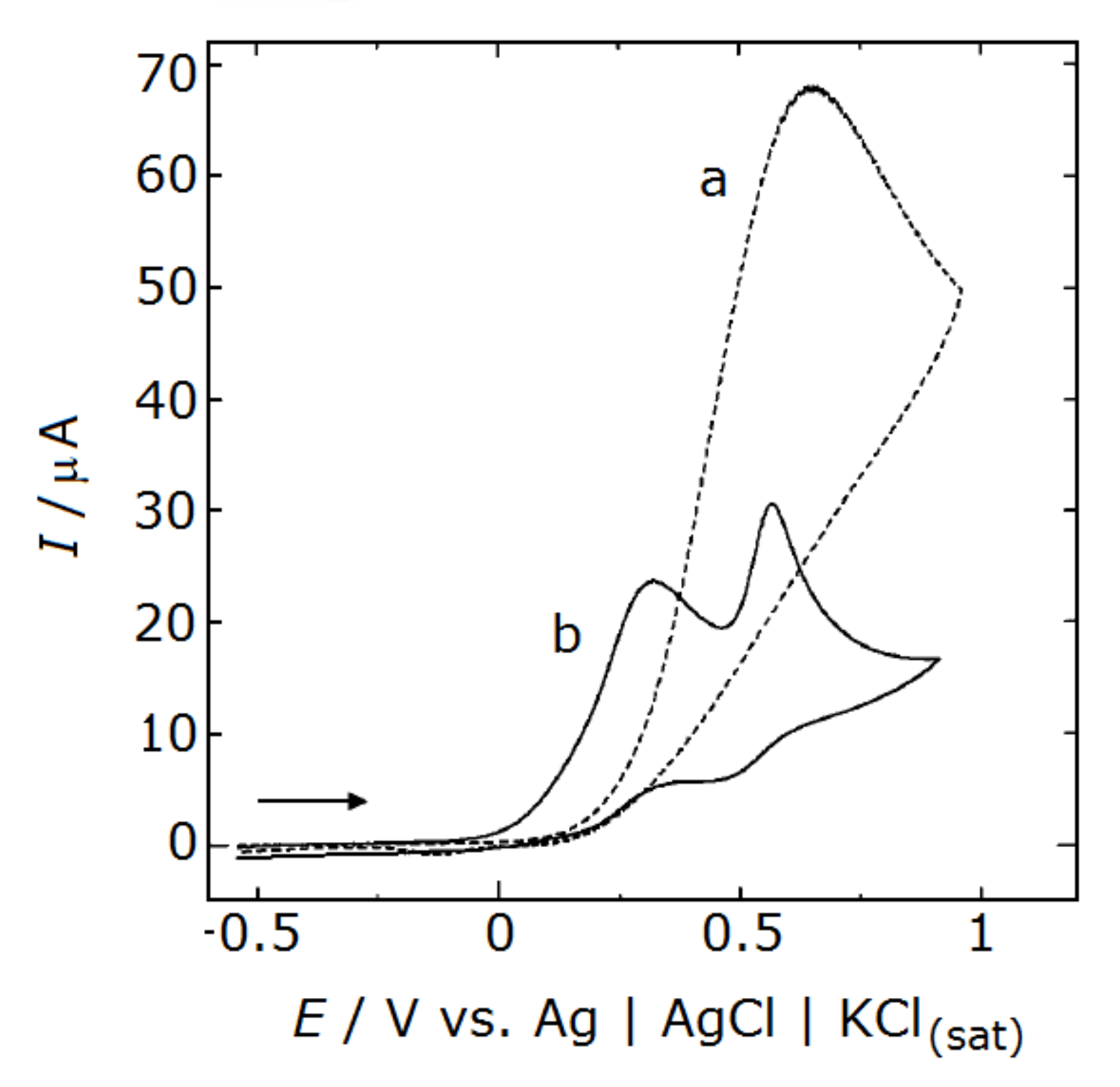
4.3. Dopamine Detection
4.4. Multi-Component Analysis: Dopamine, Adrenaline and Noradrenaline
4.5. Towards In Vivo Applications: Noradrenaline and Adenosine Detection in Excised Tissue
4.5.1. Noradrenaline
4.5.2. Adenosine
4.6. Detection of NADH, a Dinucleotide of Adenine and Nicotinamide
4.7. A Glutathione Electrode Operating In Vivo
5. Diamond-Based Electric Biosensors
5.1. Biosensors for DNA
5.2. Biosensors for Immunoglobulins
5.3. Biosensors for Influenza
5.3.1. Detecting M1 Protein as a Universal Influenza Biomarker
5.3.2. Detecting Whole Influenza Viral Particles via Hemagglutinin (HA) Recognition
6. Discussion and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Nebel, C.E.; Rezek, B.; Shin, D.; Uetsuka, H.; Yang, N. Diamond for bio-sensor applications. J. Phys. D Appl. Phys. 2007, 40, 6443–6466. [Google Scholar] [CrossRef]
- Tang, L.; Tsai, C.; Gerberich, C.C.; Kruckeberg, L.; Kania, D.R. Biocompatibility of chemical-vapour-deposited diamond. Biomaterials 1995, 16, 483–488. [Google Scholar] [CrossRef]
- Xue, Y.; Feng, X.; Roberts, S.C.; Chen, X. Diamond and carbon nanostructures for biomedical applications. Funct. Diam. 2021, 1, 221–242. [Google Scholar] [CrossRef]
- Szunerits, S.; Nebel, C.E.; Hamers, R.J. Surface functionalization and biological applications of CVD diamond. MRS Bull. 2014, 39, 517–524. [Google Scholar] [CrossRef]
- Field, J.E. The Properties of Natural and Synthetic Diamond, 1st ed.; Academic Press: London, UK, 1992. [Google Scholar]
- Luong, J.H.T.; Male, K.B.; Glennon, J.D. Boron-doped diamond electrode: Synthesis, characterization, functionalization and analytical applications. Analyst 2009, 134, 1965–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landstrass, M.I.; Ravi, K.V. Resistivity of chemical vapor-deposited diamond films. Appl. Phys. Lett. 1989, 55, 975–977. [Google Scholar] [CrossRef]
- Wenmackers, S.; Vermerren, V.; vandeVen, M.; Ameloot, M.; Bijnes, N.; Haenen, K.; Michiels, L.; Wagner, P. Diamond-based DNA sensors: Surface functionalization and read-out strategies. Phys. Status Solidi Appl. Mater. Sci. 2009, 206, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Raymakers, J.; Haenen, K.; Maes, W. Diamond surface functionalization: From gemstone to photoelectrochemical applications. J. Mater. Chem. C 2019, 7, 10134–10165. [Google Scholar] [CrossRef]
- Gracio, J.J.; Fan, Q.H.; Madaleno, J.C. Diamond growth by chemical vapour deposition. J. Phys. D Appl. Phys. 2010, 43, 43. [Google Scholar] [CrossRef]
- Sque, S.J.; Jones, R.; Briddon, P.R. Structure, electronics, and interaction of hydrogen and oxygen on diamond surfaces. Phys. Rev. B 2006, 73, 085313. [Google Scholar] [CrossRef]
- Eaton, S.C.; Anderson, A.B.; Angus, J.C.; Evstefeeva, Y.E.; Pleskov, Y.V. Co-doping of diamond with boron and sulfur. Electrochem. Solid State Lett. 2002, 5, G65–G68. [Google Scholar] [CrossRef]
- Siew, P.S.; Loh, K.P.; Poh, W.C.; Zhang, H. Biosensing properties of nanocrystalline diamond film grown on polycrystalline diamond electrodes. Diam. Relat. Mater. 2005, 14, 426–431. [Google Scholar] [CrossRef]
- Song, C.W.; Cho, D.S.; Lee, J.M.; Song, P.K. Effect of boron doping on diamond film and electrochemical properties of BDD according to thickness and morphology. Coatings 2020, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Klauser, F.; Ghodban, S.; Boukherroub, R.; Sznurits, S.; Steinmüller-Nethl, D.; Bertel, E.; Memmel, N. Comparison of different oxidation techniques on single-crystal and nanocrystalline diamond surfaces. Diam. Relat. Mater. 2010, 19, 474–478. [Google Scholar] [CrossRef]
- O’Donnell, K.M.; Edmonds, M.T.; Ristein, J.; Tadich, A.; Thomsen, L.; Wu, Q.; Pakes, C.I.; Ley, L. Diamond surfaces with air-stable negative electron affinity and giant electron yield enhancement. Adv. Funct. Mater. 2013, 23, 5608–5614. [Google Scholar] [CrossRef]
- Loh, K.P.; Xie, X.N.; Yang, S.W.; Zheng, J.C. Oxygen adsorption on (111)-oriented diamond: A study with ultraviolet photoelectron spectroscopy, temperature-programmed desorption, and periodic density functional theory. J. Phys. Chem. B 2002, 106, 5230–5240. [Google Scholar] [CrossRef]
- Nakamura, J.; Ito, T. Oxidization process of CVD diamond (100): H 2 × 1 surfaces. Appl. Surf. Sci. 2005, 244, 301–304. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, H.; Li, X.; Lu, S.; Hu, X. Thermal oxidation induced high electrochemical activity of boron-doped nanocrystalline diamond electrodes. Electrochim. Acta 2017, 258, 61–70. [Google Scholar] [CrossRef]
- Kuga, S.; Yang, J.H.; Takahashi, H.; Hirama, K.; Iwasaki, T.; Kawarada, H. Detection of mismatched DNA on partially negatively charged diamond surfaces by optical and potentiometric methods. J. Am. Chem. Soc. 2008, 130, 13251–13263. [Google Scholar] [CrossRef]
- Coffinie, Y.; Szuerits, S.; Jama, C.; Desmet, R.; Melnyk, O.; Marcus, B.; Gengembre, L.; Payen, E.; Delabouglise, D.; Boukherroub, R. Peptide immobilization on amine-terminated boron-doped diamond surfaces. Langmuir 2007, 3, 4494–4497. [Google Scholar] [CrossRef]
- Szunerits, S.; Jama, C.; Coffinier, Y.; Marcus, B.; Delabouglise, D.; Boukherroub, R. Direct amination of hydrogen-terminated boron doped diamond surfaces. Electrochem. Commun. 2006, 8, 1185–1190. [Google Scholar] [CrossRef]
- Chandran, M.; Shasha, M.; Michaelson, S.; Hoffman, A. Nitrogen termination of single crystal (100) diamond surface by radio frequency N2 plasma process: An in-situ x-ray photoemission spectroscopy and secondary electron emission studies. Appl. Phys. Lett. 2015, 107, 111602. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Chong, K.F.; May, P.W.; Chen, Z.K.; Loh, K.P. Optimizing biosensing properties on undecylenic acid-functionalized diamond. Langmuir 2007, 23, 5824–5830. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Brown, D.W. Properties of photochemically modified diamond films. Diam. Rel. Mater. 1995, 4, 435–440. [Google Scholar] [CrossRef]
- Yang, W.; Auciello, O.; Butler, J.E.; Cai, W.; Carlisle, J.A.; Gerbi, J.E.; Gruen, D.M.; Knickerbocker, T.; Lasseter, T.L.; Russel, J.N., Jr.; et al. DNA-modified nanocrystalline diamond thin-films as stable, biologically active substrates. Nature 2003, 1, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, P.; Vermeeren, V.; Wenmackers, S.; Daenen, M.; Haenen, K.; Nesládek, M.; VanDeVen, M.; Ameloot, M.; Michiels, L.; Wagner, P. EDC-mediated DNA attachment to nanocrystalline CVD diamond films. Biosens. Bioelectron. 2006, 22, 170–177. [Google Scholar] [CrossRef]
- Wenmackers, S.; Christiaens, P.; Daenen, M.; Haenen, K.; Nesládek, M.; van deVen, M.; Vermeeren, V.; Michiels, L.; Ameloot, M.; Wagner, P. DNA attachment to nanocrystalline diamond films. Phys. Status Solidi A 2005, 202, 2212–2216. [Google Scholar] [CrossRef]
- Yang, W.; Butler, J.E.; Russell, J.N.; Hamers, R.J. Direct electrical detection of antigen-antibody binding on diamond and silicon substrates using electrical impedance spectroscopy. Analyst 2007, 132, 296–306. [Google Scholar] [CrossRef]
- Kuo, T.C.; McCreery, R.L.; Swain, G.M. Electrochemical modification of boron-doped chemical vapor deposited diamond surfaces with covalently bonded monolayers. Electrochem. Solid-State Lett. 1999, 2, 288–290. [Google Scholar] [CrossRef]
- Nidzworski, D.; Siuzdak, K.; Niedziałkowski, P.; Bogdanowicz, R.; Sobaszek, M.; Ryl, J.; Weiher, P.; Sawczak, M.; Wnuk, E.; Goddard, W.A.; et al. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Yang, W.; Baker, S.E.; Butler, J.E.; Lee, C.S.; Russell, J.N.; Shang, L.; Sun, B.; Hamers, R.J. Electrically addressable biomolecular functionalization of conductive nanocrystalline diamond thin films. Chem. Mater. 2005, 17, 938–940. [Google Scholar] [CrossRef]
- Siuzdak, K.; Niedziałkowski, P.; Sobaszek, M.; Łęga, T.; Sawczak, M.; Czaczyk, E.; Dziąbowska, K.; Ossowski, T.; Nidzworski, D.; Bogdanowicz, R. Biomolecular influenza virus detection based on the electrochemical impedance spectroscopy using the nanocrystalline boron-doped diamond electrodes with covalently bound antibodies. Sens. Actuators B Chem. 2019, 280, 263–271. [Google Scholar] [CrossRef]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Olivia, H.; Sarada, B.V.; Shin, D.; Rao, T.N.; Fujishima, A. Selective amperometric detection of dopamine using OPPy-modified diamond microsensor system. Analyst 2002, 127, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.R.; Saha, M.S.; Okajima, T.; Park, S.-G.; Fujishima, A.; Ohsaka, T. Selective detection of dopamine and its metabolite, DOPAC, in the presence of ascorbic acid using diamond electrode modified by the polymer film. Electroanalysis 2004, 16, 1777–1784. [Google Scholar] [CrossRef]
- Shul, G.; Actis, P.; Marcus, B.; Opallo, M.; Boukherroub, R.; Szunerits, S. Solvent-free chemical functionalization of hydrogen-terminated boron-doped diamond electrodes with diazonium salts in ionic liquids. Diam. Relat. Mater. 2008, 17, 1394–1398. [Google Scholar] [CrossRef]
- Yano, T.; Tryk, D.A.; Hashimoto, K.; Fujishima, A. Electrochemical behavior of highly conductive boron-doped diamond electrodes for oxygen reduction in alkaline solution. J. Electrochem. Soc. 1998, 145, 1870–1876. [Google Scholar] [CrossRef]
- Strojek, J.W.; Granger, M.C.; Swain, G.M.; Dallas, T.; Holtz, M.W. Enhanced signal-to-background ratios in voltammetric measurements made at diamond thin-film electrochemical interfaces. Anal. Chem. 1996, 68, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Vinokur, N.; Miller, B.; Avyigal, Y.; Kalish, R. Electrochemical behavior of boron-doped diamond electrodes. J. Electrochem. Soc. 1996, 143, L238–L240. [Google Scholar] [CrossRef]
- Hébert, C.; Ruffinatto, S.; Bergonzo, P. Diamond Biosensors. In Carbon for Sensing Devices, 1st ed.; Demarchi, D., Tagliaferro, A., Eds.; Springer International Publishing: New York, NY, USA, 2015; pp. 227–264. [Google Scholar] [CrossRef]
- Xu, J.; Swain, G.M. Oxidation of azide anion at boron-doped diamond thin-film electrodes. Anal. Chem. 1998, 70, 1502–1510. [Google Scholar] [CrossRef]
- Rao, T.N.; Yagi, I.; Miwa, T.; Tryk, D.A.; Fujishima, A. Electrochemical oxidation of NADH at highly boron-doped diamond electrodes. Anal. Chem. 1999, 71, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, E.; de Sanoit, J.; Arnault, J.C.; Saada, C.; Mer, C.; Mailley, P.; Bergonzo, P.; Nesladek, M. Stability of H-terminated BDD electrodes: An insight into the influence of the surface preparation. Phys. Status Solidi 2007, 204, 2931–2939. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Simultaneous determination of dopamine and cysteamine by flow injection with multiple pulse amperometric detection using a boron-doped diamond electrode. Diam. Relat. Mater. 2018, 85, 68–73. [Google Scholar] [CrossRef]
- Shin, D.; Sarada, B.V.; Tryk, D.A.; Fujishima, A.; Wang, J. Application of diamond microelectrodes for end-column electrochemical detection in capillary electrophoresis. Anal. Chem. 2003, 75, 530–534. [Google Scholar] [CrossRef]
- Cvačka, J.; Quaiserová, V.; Park, J.W.; Show, Y.; Muck, A.; Swain, G.M. Boron-doped diamond microelectrodes for use in capillary electrophoresis with electrochemical detection. Anal. Chem. 2003, 75, 2678–2687. [Google Scholar] [CrossRef]
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical oxidation of histamine and serotonin at highly boron-doped diamond electrodes. Anal. Chem. 2000, 72, 1632–1638. [Google Scholar] [CrossRef]
- Park, J.; Quaiserová-Mocko, V.; Pecková, K.; Galligan, J.J.; Fink, G.D.; Swain, G.M. Fabrication, characterization, and application of a diamond microelectrode for electrochemical measurement of norepinephrine release from the sympathetic nervous system. Diam. Relat. Mater. 2006, 15, 761–772. [Google Scholar] [CrossRef]
- Park, J.; Show, Y.; Quaiserova, V.; Galligan, J.J.; Fink, G.D.; Swain, G.M. Diamond microelectrodes for use in biological environments. J. Electroanal. Chem. 2005, 583, 56–68. [Google Scholar] [CrossRef]
- Xie, S.; Shafer, G.; Wilson, C.G.; Martin, H.B. In vitro adenosine detection with a diamond-based sensor. Diam. Relat. Mater. 2006, 15, 225–228. [Google Scholar] [CrossRef]
- Fierro, S.; Yoshikawa, M.; Nagano, O.; Yoshimi, K.; Saya, H.; Einaga, Y. In vivo assessment of cancerous tumors using boron doped diamond microelectrode. Sci. Rep. 2012, 2, srep00901. [Google Scholar] [CrossRef]
- Yang, W.; Butler, J.E.; Russell, J.N.; Hamers, R.J. Interfacial electrical properties of DNA-modified diamond thin films: Intrinsic response and hybridization-induced field effects. Langmuir 2004, 20, 6778–6787. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, V.; Bijnens, N.; Wenmackers, S.; Daenen, M.; Haenen, K.; Williams, O.A.; Ameloot, M.; van de Ven, M.; Wagner, P.; Michiels, L. Towards a real-time, label-free, diamond-based DNA sensor. Langmuir 2007, 23, 13193–13202. [Google Scholar] [CrossRef]
- Tran, D.T.; Vermeeren, V.; Grieten, L.; Wenmackers, S.; Wagner, P.; Pollet, J.; Janssen, K.P.F.; Michiels, L.; Lammertyn, J. Nanocrystalline diamond impedimetric aptasensor for the label-free detection of human IgE. Biosens. Bioelectron. 2011, 26, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Hilsch, M.; Goldenbogen, B.; Sieben, C.; Höfer, C.T.; Rabe, J.P.; Klip, E.; Herrmann, A.; Chiantia, S. Influenza A matrix protein m1 multimerizes upon binding to lipid membranes. Biophys. J. 2014, 107, 912–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Ujie, M.; Yamamoto, T.; Akahori, M.; Einaga, Y.; Sato, T. Highly sensitive detection of influenza virus by boron-doped diamond electrode terminated with sialic acid-mimic peptide. Proc. Natl. Acad. Sci. USA 2016, 113, 8981–8984. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S.; Swoboda, K.J.; Miles, J.M.; Coppack, S.W.; Aneman, A.; Holmes, C.; Lamensdorf, I.; Eisenhofer, G. Sources and physiological significance of plasma dopamine sulphate. J. Clin. Endocrinol. Metab. 1999, 84, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Pillet, L.E.; Taccola, C.; Cotoni, J.; Thiriez, H.; André, K.; Verpillot, R. Correlation between cognition and plasma noradrenaline level in Alzheimer’s disease: A potential new blood marker of disease evolution. Transl. Psychiatry 2020, 10, 1–10. [Google Scholar] [CrossRef]
- DeBrosse, M.; Yuan, Y.; Brothers, M.; Karajic, A.; van Duren, J.; Kim, S.; Hussain, S.; Heikenfeld, J. A dual approach of an oil–membrane composite and boron-doped diamond electrode to mitigate biofluid interferences. Sensors 2021, 21, 8063. [Google Scholar] [CrossRef]
- Simard, T.; Jung, R.; Labinaz, A.; Faraz, M.A.; Ramirez, F.D.; Di Santo, P.; Perry-Nguyen, D.; Pitcher, I.; Motazedian, P.; Gaudet, C.; et al. Evaluation of plasma adenosine as a marker of cardiovascular risk: Analytical and biological considerations. J. Am. Heart Assoc. 2019, 8, e012228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhito, I.R.; Koo, K.-M.; Kim, T.-H. Recent advances in electrochemical sensors for the detection of biomolecules and whole cells. Biomedicines 2021, 9, 15. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.E.; Figueira, F.; Neto, M.; Paz, F.A.A.; Braga, S.S.; Mendes, J.C. Diamonds for Life: Developments in Sensors for Biomolecules. Appl. Sci. 2022, 12, 3000. https://doi.org/10.3390/app12063000
Santos NE, Figueira F, Neto M, Paz FAA, Braga SS, Mendes JC. Diamonds for Life: Developments in Sensors for Biomolecules. Applied Sciences. 2022; 12(6):3000. https://doi.org/10.3390/app12063000
Chicago/Turabian StyleSantos, Nádia E., Flávio Figueira, Miguel Neto, Filipe A. Almeida Paz, Susana Santos Braga, and Joana C. Mendes. 2022. "Diamonds for Life: Developments in Sensors for Biomolecules" Applied Sciences 12, no. 6: 3000. https://doi.org/10.3390/app12063000
APA StyleSantos, N. E., Figueira, F., Neto, M., Paz, F. A. A., Braga, S. S., & Mendes, J. C. (2022). Diamonds for Life: Developments in Sensors for Biomolecules. Applied Sciences, 12(6), 3000. https://doi.org/10.3390/app12063000









