Modified Compositions of Micelle–Clay and Liposome–Clay Composites for Optimal Removal from Water of Bacteria and Hydrophobic Neutral Chemicals
Abstract
:1. Introduction
Role of Released Cations during Filtration
- An increase in concentration of released cations, which is expected for composites formed with a larger added amount of cation per clay than in the ordinary one [12,14], will result in a larger biocidal/biostatic effect and enable the micelle–clay complex to remove more TBC from water by filtration. The tests will involve a synthesis of a granulated complex obtained by adding the salt of ODTMA at a larger ratio to bentonite than in the ordinary complex during preparation of the complex.
- In the case of optimization of removal of neutral hydrophobic pollutants. the tested modification will deal with both the micelle–clay and liposome–clay [12,14,16] composites. Liposome–clay complexes are similar to the micelle–clay ones but are prepared by adsorption on the clay of vesicles whose alkyl ammonium surfactants have two long alkyl chains. In this case, the concentrations of monomers in solution are significantly smaller (Undabeytia et al.) [17] than those of micelle-forming surfactants with one long alkyl chain of the same length. As an example, the surfactant didodecyldimethylammonium bromide (DDAB) remains in solution as monomers up to 35 µM [18], whereas the CMC of dodecyltrimehylammonium bromide is 3.49 mM [19].
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Granulated Complexes
2.2.2. Filtration Procedure
2.2.3. Analysis of TBC
2.2.4. Regeneration
2.2.5. Analysis of Non-Ionic Herbicides
3. Results
3.1. Removal of TBC from Drinking Water by Filtration
3.1.1. Filtration at a Flow Rate of 1 L/min (Flow Velocity 18 m/h; Retention Time 0.67 min)
3.1.2. Removal of TBC from Drinking Water at a Flow Rate of 6 mL/min
3.1.3. Results of First Regeneration
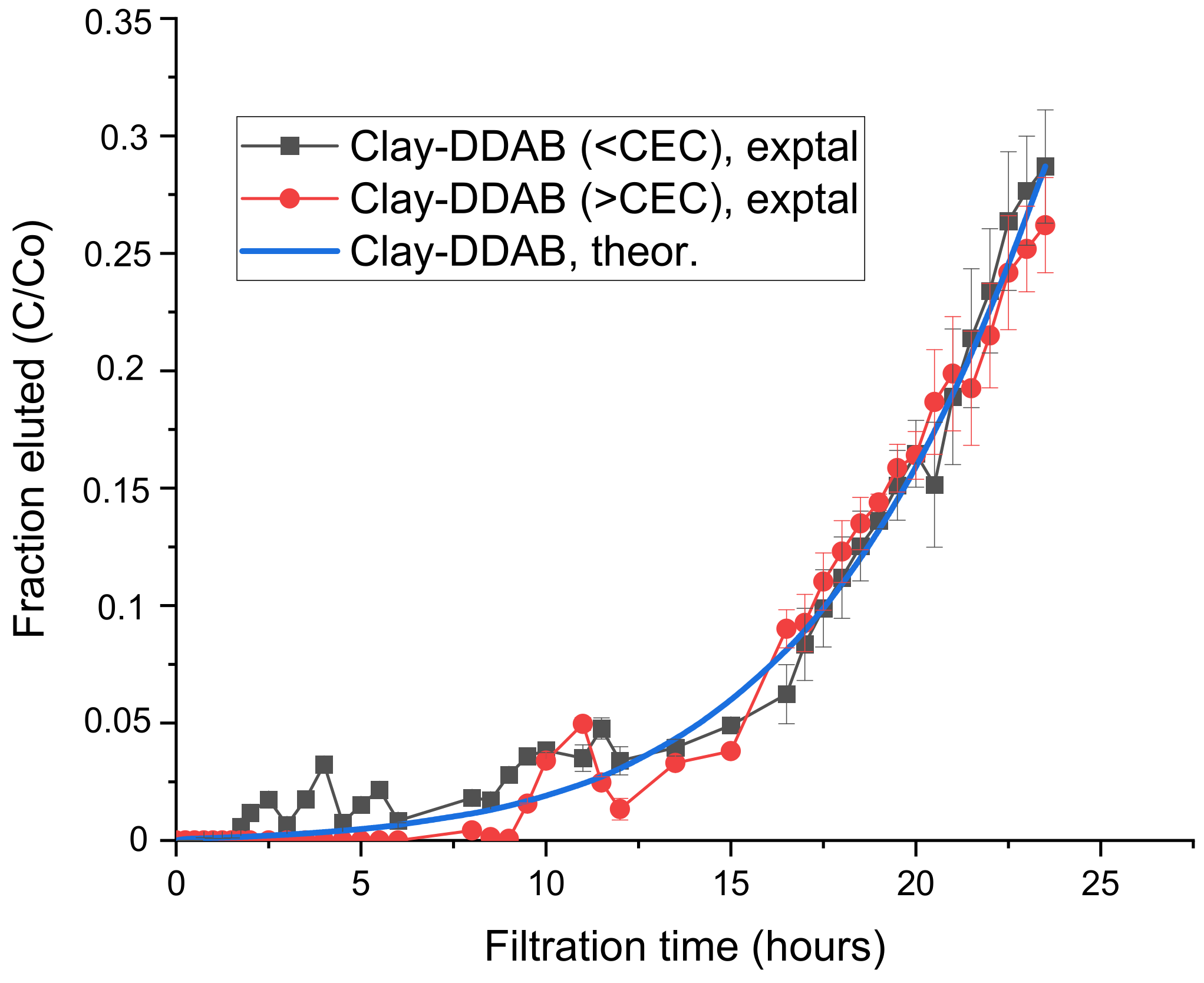
3.2. Tests of the Efficiency of the Ordinary vs. Neutral Complex in Removal from Water of the Non-Ionic Herbicide Metolachlor
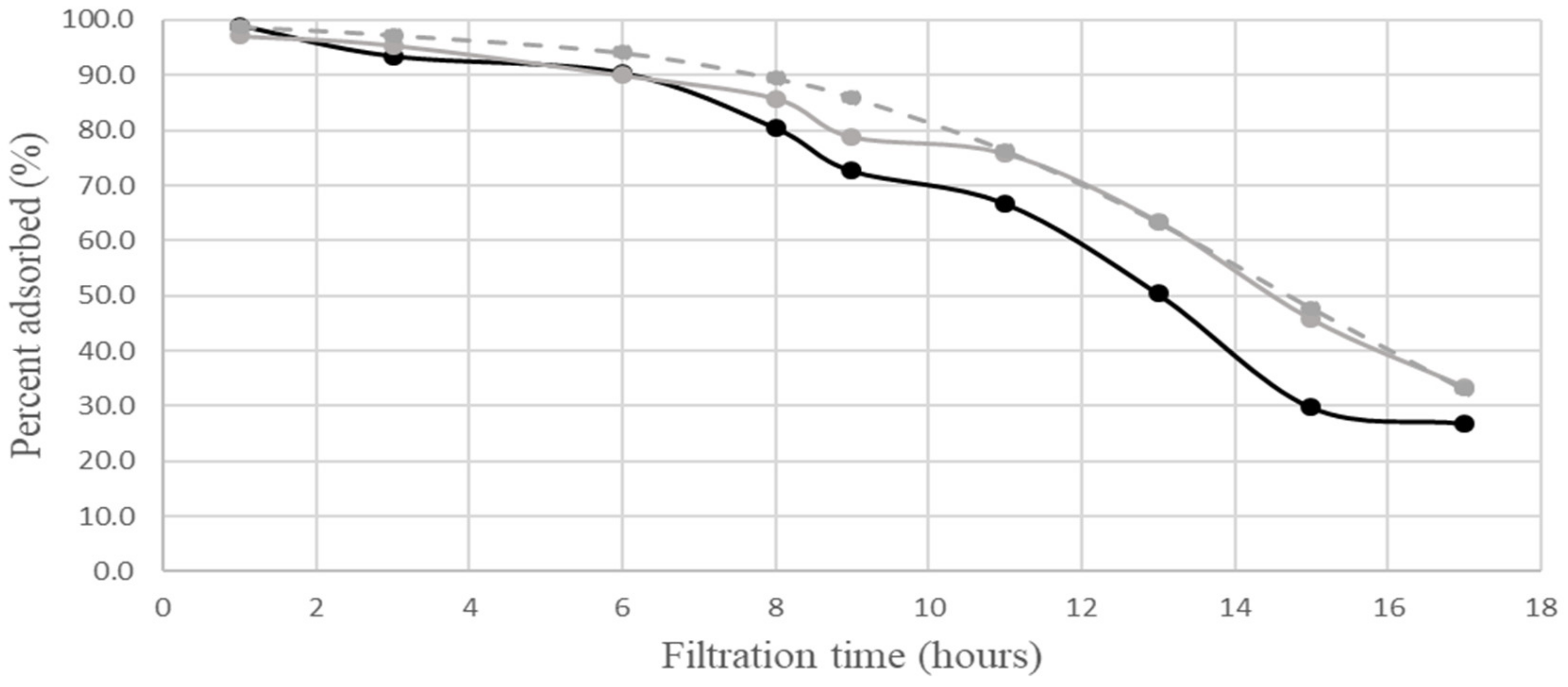
 ordinary complex; (exp.)
ordinary complex; (exp.)  neutral complex;
neutral complex;  calculated for neutral complex. The calculations according to [3] employed the following values of the parameters: R0 = 0.042 M; C1 = 30 M−1 min−1; D1 = 0.0004 min−1. The relative experimental errors in percent adsorbed were less than 10% during the first 10 h of filtration, about 20% at 11–14 h, and about 30% at a later time.
calculated for neutral complex. The calculations according to [3] employed the following values of the parameters: R0 = 0.042 M; C1 = 30 M−1 min−1; D1 = 0.0004 min−1. The relative experimental errors in percent adsorbed were less than 10% during the first 10 h of filtration, about 20% at 11–14 h, and about 30% at a later time. ordinary complex; (exp.)
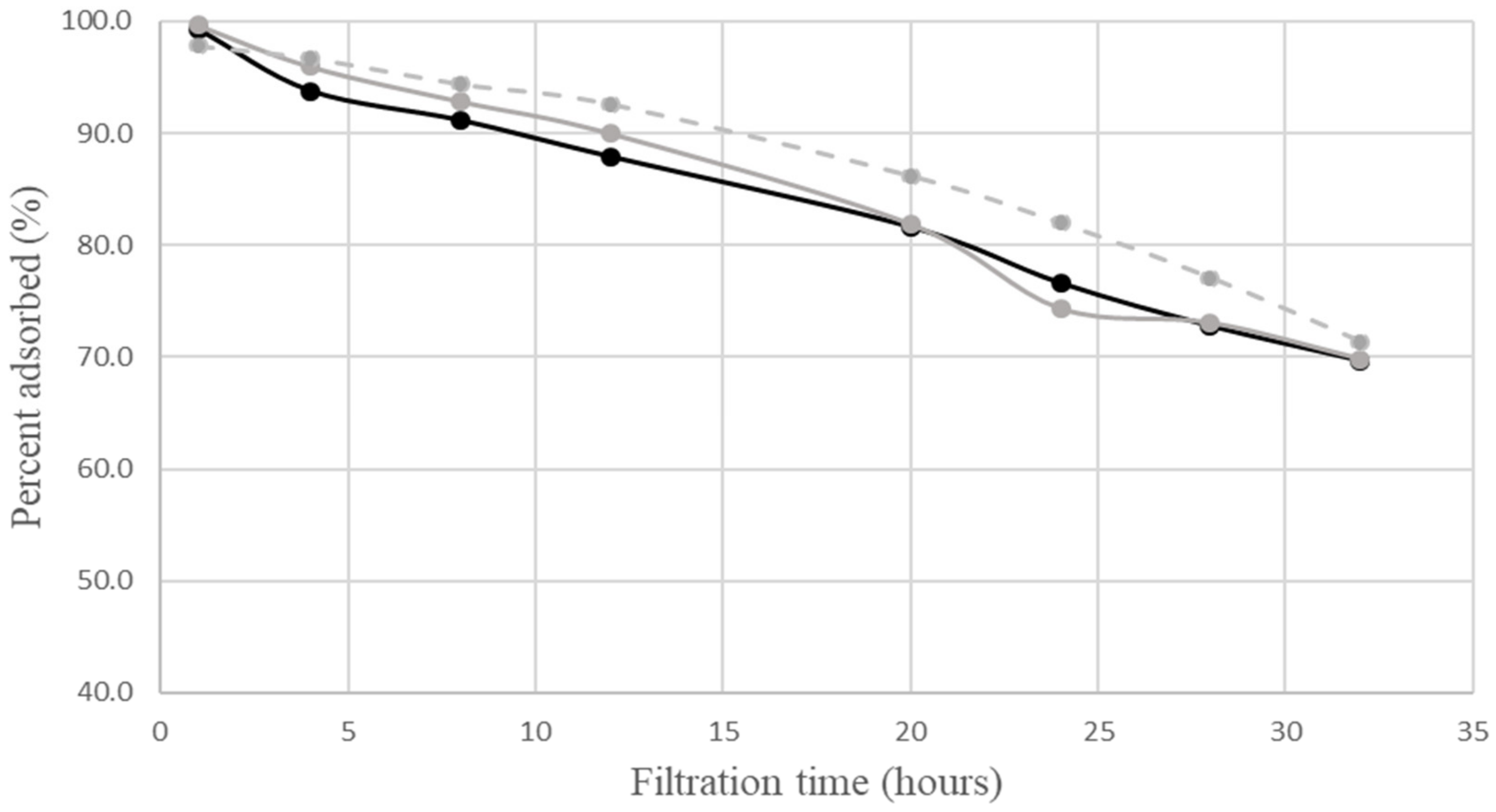
ordinary complex; (exp.)  neutral complex;
neutral complex;  calculated percent adsorbed by neutral complex. The calculations employed the same values of parameters as in Figure 1. The relative experimental errors in percent adsorbed were less than 5% during the first 24 h of filtration, and about 5 to 20% later.
calculated percent adsorbed by neutral complex. The calculations employed the same values of parameters as in Figure 1. The relative experimental errors in percent adsorbed were less than 5% during the first 24 h of filtration, and about 5 to 20% later.4. Discussion
4.1. Filtration of Total Count Bacteria
4.2. On the Choice of Optimal Composites for Removal from Water of Neutral Hydrophobic Molecules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishael, Y.G.; Undabeytia, T.; Rabinovitz, O.; Rubin, B.; Nir, S. Slow-Release Formulations of Sulfometuron Incorporated in Micelles Adsorbed on Montmorillonite. J. Agric. Food Chem. 2002, 50, 2864–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polubesova, T.; Nir, S.; Zadaka, D.; Rabinovitz, O.; Serban, C.; Groisman, L.; Rubin, B. Water Purification from Organic Pollutants by Optimized Micelle−Clay Systems. Environ. Sci. Technol. 2005, 39, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Nir, S.; Zadaka-Amir, D.; Kartaginer, A.; Gonen, Y. Simulation of adsorption and flow of pollutants in a column filter: Application to micelle–montmorillonite mixtures with sand. Appl. Clay Sci. 2012, 67–68, 134–140. [Google Scholar] [CrossRef]
- Lelario, F.; Gardi, I.; Mishael, Y.; Dolev, N.; Undabeytia, T.; Nir, S.; Scrano, L.; Bufo, S.A. Pairing micro pollutants and clay-composite sorbents for efficient water treatment: Filtration and modeling at a pilot scale. Appl. Clay Sci. 2017, 137, 225–232. [Google Scholar] [CrossRef]
- Polubesova, T.; Zadaka, D.; Groisman, L.; Nir, S. Water remediation by micelle–clay system: Case study for tetracycline and sulfonamide antibiotics. Water Res. 2006, 40, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R.; Khamis, M.; Quried, M.; Halabieh, R.; Makharzeh, I.; Manassra, A.; Abbadi, J.; Qtait, A.; Bufo, S.A.; Nasser, A.; et al. Removal of diclofenac potassium from wastewater using clay-micelle complex. Environ. Technol. 2012, 33, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, S.; Al-Rimawi, F.; Khamis, M.; Nir, S.; Bufo, S.A.; Scrano, L.; Mecca, G.; Karaman, R. Efficiency of membrane technology, activated charcoal, and a micelle-clay complex for removal of the acidic pharmaceutical mefenamic acid. J. Environ. Sci. Health Part A 2013, 48, 1655–1662. [Google Scholar] [CrossRef]
- Qurie, M.; Khamis, M.; Manassra, A.; Ayyad, I.; Nir, S.; Scrano, L.; Bufo, S.A.; Karaman, R. Removal of Cr (VI) from Aqueous Environments Using Micelle-Clay Adsorption. Sci. World J. 2013, 2013, 942703. [Google Scholar] [CrossRef]
- Sulaiman, S.; Khamis, M.; Nir, S.; Lelario, F.; Scrano, L.; Bufo, S.A.; Karaman, R. Stability and removal of dexamethasone sodium phosphate from wastewater using modified clays. Environ. Technol. 2014, 35, 1945–1955. [Google Scholar] [CrossRef]
- Awwad, M.; Al-Rimawi, F.; Dajani, K.J.K.; Khamis, M.; Nir, S.; Karaman, R. Removal of amoxicillin and cefuroxime axetil by advanced membranes technology, activated carbon and micelle–clay complex. Environ. Technol. 2015, 36, 2069–2078. [Google Scholar] [CrossRef]
- Karaman, R.; Khamis, M.; Abbadi, J.; Amro, A.; Qurie, M.; Ayyad, I.; Ayyash, F.; Hamarsheh, O.; Yaqmour, R.; Nir, S.; et al. Paracetamol biodegradation by activated sludge and photocatalysis and its removal by a micelle–clay complex, activated charcoal, and reverse osmosis membranes. Environ. Technol. 2016, 37, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Nir, S.; Brook, I.; Anavi, Y.; Ryskin, M.; Ari, J.B.; Huterer, R.S.; Etkin, H.; Zadaka-Amir, D.; Shuali, U. Water purification from perchlorate by a micelle–clay complex: Laboratory and pilot experiments. Appl. Clay Sci. 2015, 114, 151–156. [Google Scholar] [CrossRef]
- Kalfa, A.; Rakovitsky, N.; Tavassi, M.; Ryskin, M.; Ben-Ari, J.; Etkin, H.; Shuali, U.; Nir, S. Removal of Escherichia coli and total bacteria from water by granulated micelle-clay complexes: Filter regeneration and modeling of filtration kinetics. Appl. Clay Sci. 2017, 147, 63–68. [Google Scholar] [CrossRef]
- Zadaka, D.; Polubesova, T.; Mishael, Y.; Spitzy, A.; Koehler, H.; Wakshal, E.; Rabinovitz, O.; Nir, S. Determination of release of organic cations from micelle- clay complexes and their re-adsorption in sand/clay columns. Appl. Clay Sci. 2005, 29, 282–286. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Tavassi, M.; Nir, S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation octadecyltrimethyl ammonium (ODTMA). Water Res. 2017, 120, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Undabeytia, T.; Mishael, G.Y.; Nir, S.; Papahadjopoulos-Sternberg, B.; Rubin, B.; Morillo, E.; Maqueda, C. A novel system for reducing leaching from formulations of anionic herbicides: Clay-liposomes. Environ. Sci. Technol. 2003, 37, 4475–4480. [Google Scholar] [CrossRef] [Green Version]
- Undabeytia, T.; Nir, S.; Sánchez-Verdejo, T.; Villaverde, J.; Maqueda, C.; Morillo, E. A clay-vesicle system for water purification from organic pollutants. Water Res. 2008, 42, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Svitova, T.F.; Smirnova, Y.P.; Pisarev, S.A.; Berezina, N.A. Self-assembly in double-tailed surfactants in dilute aqueous solutions. Colloids Surf. A Physicochem. Eng. Aspects 1995, 98, 107–115. [Google Scholar] [CrossRef]
- Inacio, A.S.; Domingues, S.N.; Nunes, A.; Martins, P.T.; Moreno, M.J.; Estronca, L.M.; Fernandes, R.; Moreno, A.J.M.; Borrego, M.J.; Gomes, J.P.; et al. Quaternary ammonium surfactant structure determines selective toxicity towards bacteria: Mechanisms of action and clinical implications in antibacterial prophylaxis. J. Antimicrob. Chemother. 2016, 71, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nir, S.; Ryskin, M. Method of Production of Granulated Micelle-Clay Complexes: Application for Removal of Organic, Inorganic Anionic Pollutants and Microorganisms from Contaminated Water. U.S. Patent 10384959, 20 August 2019. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Kaya, A.U.; Güner, S.; Ryskin, M.; Lameck, A.S.; Benitez, A.R.; Shuali, U.; Nir, S. Effect of Microwave Radiation on Regeneration of a Granulated Micelle–Clay Complex after Adsorption of Bacteria. Appl. Sci. 2020, 10, 2530. [Google Scholar] [CrossRef] [Green Version]
- Goldreich, O.; Goldwasser, Y.; Mishael, Y.G. Effect of Soil Wetting and Drying Cycles on Metolachlor Fate in Soil Applied as a Commercial or Controlled-Release Formulation. J. Agric. Food Chem. 2011, 59, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Rakovitsky, N.; Brook, I.; Rijn, J.V.; Ryskin, M.; Mkhweli, Z.; Etkin, H.; Nir, S. Purification of greywater by a moving bed reactor followed by a filter including a granulated micelle-clay composite. Appl. Clay Sci. 2016, 132–133, 267–272. [Google Scholar] [CrossRef]
- Khamis, M.; Karaman, R.; Qurie, M.; Abbadi, J.; Nusseibeh, S.; Manassra, A.; Nir, S. Performance of micelle-clay filters for removing pollutants and bacteria from tertiary treated wastewater. J. Environ. Sci. Eng. A 2012, 1, 160–168. [Google Scholar]
- Shtarker-Sasi, A.; Castro-Sowinski, S.; Matan, O.; Kagan, T.; Nir, S.; Okon, Y.; Nasser, A.M. Removal of bacteria and Cryptosporidium from water by micelle-montmorillonite complexes. Desalin. Water Treat. 2013, 51, 7672–7680. [Google Scholar] [CrossRef]
- Brook, I.; Malchi, T.; Nir, S. Removal of anionic detergents from water and treatment of greywater by micelle-clay composites. Desalin. Water Treat. 2015, 53, 2184–2192. [Google Scholar] [CrossRef]
- Brienza, M.; Nir, S.; Plantard, G.; Goetz, V.; Chiron, S. Combining micelle-clay sorption to solar photo- fenton processes for domestic wastewater treatment. Environ. Sci. Pollut. Res. 2018, 26, 18971–18978. [Google Scholar] [CrossRef]
- Undabeytia, T.; Posada, R.; Nir, S.; Golindo, I.; Laiz, L.; Saiz-Jimenez, C.; Morillo, E. Removal of waterborne microorganisms by filtration using clay polymer complexes. J. Hazard. Mater. 2014, 279, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Masson, M.; Kristinsson, K.G.; Hjalmarsdottir, M.A.; Hilmarsson, H.; Loftsson, T. Soft antibacterial agents: Synthesis and activity of labile environmentally friendly long chain quaternary ammonium compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef]
- Shuali, U.; Nir, S. Role of Micelle-Clay Complexes and Quaternary Amine Cations in Removal of Bacteria from Water: Adsorption, Biostatic and Biocidal Effects. Clays Clay Miner. 2018, 66, 485–492. [Google Scholar] [CrossRef]
- Nir, S.; Shuali, U. Water Purification by Micelle-Clay Nano-Particles; NOVA SCIENCE Publishers: New York, NY, USA, 2019. [Google Scholar]
- Wu, X.; Viner-Mozzini, Y.; Jia, Y.; Song, L.; Sukenik, A. Alkyltrimethylammonium (ATMA) surfactants as cyanocides—Effects on photosynthesis and growth of cyanobacteria. Chemosphere 2021, 274, 129778. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Liu, D. Effect of flow rate on biofilm accumulation in open channels. Water Res. 1993, 27, 355–360. [Google Scholar] [CrossRef]
- Soini, S.M.; Koskinen, K.T.; Vilenius, M.J.; Puhakka, J.A. Effects of fluid-flow velocity and water quality on planktonic and sessile microbial growth in water hydraulic system. Water Res. 2002, 36, 3812–3820. [Google Scholar] [CrossRef]
| Total Count of Bacteria Emerging from Filters per mL | ||||||
|---|---|---|---|---|---|---|
| Enriched Granules | Ordinary Granules | Bacteria per mL at Entry to Filters | Volume Filtered | Day | ||
| 1 | 2 | 1 | 2 | (CFU/mL) | (L) | |
| <1 | <1 | 5 | <1 | <1 | 180 | Mon. |
| 2 | 8 | 1 | <1 | 28 | Tue. M | |
| <1 | <1 | <1 | <1 | 1 | 360 | Tue. |
| <1 | <1 | <1 | <1 | <1 | Wed. M | |
| <1 | <1 | <1 | <1 | <1 | 540 | Wed. |
| <1 | 3 | <1 | 3 | 140 | Thu. M | |
| <1 | <1 | <1 | <1 | <1 | 720 | Thu. |
| <1 | 8 | <1 | 1600 b | 370 | Sun. M | |
| <1 | <1 | <1 | 3 | 220 | 900 | Sun. |
| <1 | 8 | 2 | 42,000 a | 630 | Mon. M | |
| <1 | <1 | <1 | 16 | 10 | 1080 | Mon. |
| <1 | 2 | <1 | 240 c | 210 | Tue. M | |
| <1 | <1 | 68 c | 2 | 16 | 1260 | Tue. |
| 55 c | <1 | <1 | 88,000 a | 6 | Wed. M | |
| <1 | <1 | <1 | 85 c | 95 | 1440 | Wed. |
| 37 c | 32 c | 1 | 150,000 a | 120 | Thu. M | |
| <1 | <1 | <1 | 190 c | 110 | 1630 | Thu. |
| TBC Emerging from Filters per mL | ||||||
|---|---|---|---|---|---|---|
| Enriched Granules | Ordinary Granules | Bacteria per mL at Entry to Filters | Volume Filtered | Day | ||
| 1 | 2 | 1 | 2 | (CFU/mL) | (L) | |
| 1 | <1 | <1 | <1 | 1 | 180 | Sun. |
| 96c | 32 c | 1200 b | 11 | <1 | 180 | Mon. M |
| 78c | 87 c | 5200 b | 73 c | 350 | 360 | Tue. M |
| 87c | 670 b | 29,000 a | 4800 b | 23,000 a | 540 | Wed. M |
| 150b | 1400 b | 15,000 a | 600 b | 12,000 | 720 | Thu. M |
| 1 | 4 | 15,000 a | 120 b | 11,000 | 900 | Thu. |
| 43,000 a | 7100 a | >57,000 a | 6700 a | 15,000 | Sun. M | |
| 55 c | 110 c | 95 c | 7 | 62,000 | 1080 | Sun. |
| 620 b | 610 b | >57,000 a | 1100 b | 6900 | Mon. M | |
| 220 b | 3000 b | 2900 b | 710 b | 15,000 | 1270 | Mon. |
| Source of Sample | Volumes Filtered (L) | |||
|---|---|---|---|---|
| 43.2 | 77.8 | 112.3 | 138.2 | |
| Tap | 131 | 1 | 1 | <1 |
| Container | 475 | 2 | 8 | <1 |
| R1 | 1600 b | 253 b | 2300 b | 3400 b |
| R2 | 2 | 67 c | 1000 b | 18,000 a |
| R3 | 7100 a | 2500 b | 3500 b | 20,000 a |
| E1 | 475 b | 40 c | 1400 b | 11,000 a |
| E2 | 17 | 58 c | 3200 b | 29,000 a |
| E3 | 1300 b | 169 b | 5300 b | 54,000 a |
| Source of Sample | Volumes Filtered (L) | ||
|---|---|---|---|
| 25.9 | 51.8 | 86.4 | |
| Tap | 89 | 11 | 1 |
| Container | 82 | 5 | 1 |
| R1 | 1 | 4600 b | 19,000 a |
| R2 | 90 c | 755 b | 15,000 a |
| R3 | 18 | 435 b | 905 b |
| E1 | <1 | 11 | 610 b |
| E2 | 4 | 222 b | 3400 b |
| E3 | 52 c | 6400 a | 180,000 a |
| Metolachlor, Micelle–Clay | ||||||
|---|---|---|---|---|---|---|
| Ro (M) | C1 (M−1 min−1) | D1 (min−1) | K (M−1) | Ro·K | R2 | RMSE |
| 0.042 | 30 | 4·10−4 | 7.5·104 | 3150 | 0.97 | 1.8 |
| Alachlor, liposome–clay | ||||||
| Ro (M) | C1 (M−1 min−1) | D1 (min−1) | K (M−1) | Ro·K | R2 | RMSE |
| 0.15 | 20 | 1.1·10−3 | 1.8·104 | 2700 | 0.98 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez, A.R.; Margalit, D.; Ryskin, M.; Dor, M.; Shuali, U.; Nir, S.; Polubesova, T.; Ben-Ari, J.; Kertsnus-Banchik, J.; Undabeytia, T. Modified Compositions of Micelle–Clay and Liposome–Clay Composites for Optimal Removal from Water of Bacteria and Hydrophobic Neutral Chemicals. Appl. Sci. 2022, 12, 3044. https://doi.org/10.3390/app12063044
Benitez AR, Margalit D, Ryskin M, Dor M, Shuali U, Nir S, Polubesova T, Ben-Ari J, Kertsnus-Banchik J, Undabeytia T. Modified Compositions of Micelle–Clay and Liposome–Clay Composites for Optimal Removal from Water of Bacteria and Hydrophobic Neutral Chemicals. Applied Sciences. 2022; 12(6):3044. https://doi.org/10.3390/app12063044
Chicago/Turabian StyleBenitez, Ana R., Dani Margalit, Marklen Ryskin, Maoz Dor, Uri Shuali, Shlomo Nir, Tamara Polubesova, Julius Ben-Ari, Jeny Kertsnus-Banchik, and Tomas Undabeytia. 2022. "Modified Compositions of Micelle–Clay and Liposome–Clay Composites for Optimal Removal from Water of Bacteria and Hydrophobic Neutral Chemicals" Applied Sciences 12, no. 6: 3044. https://doi.org/10.3390/app12063044








