Low-Temperature Direct Bonding of SiC to Si via Plasma Activation
Abstract
:1. Introduction
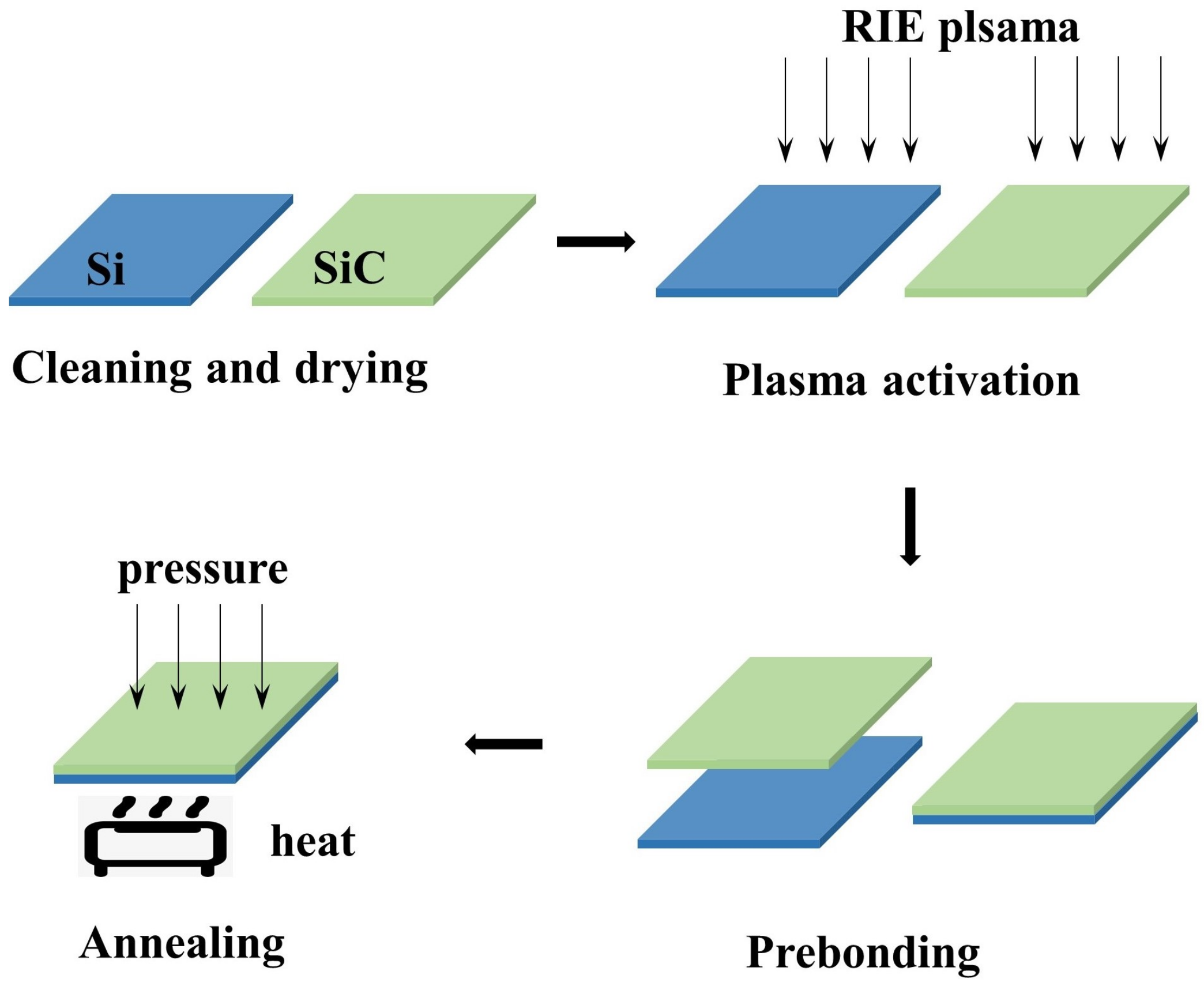
2. Experiments
3. Results and Discussion
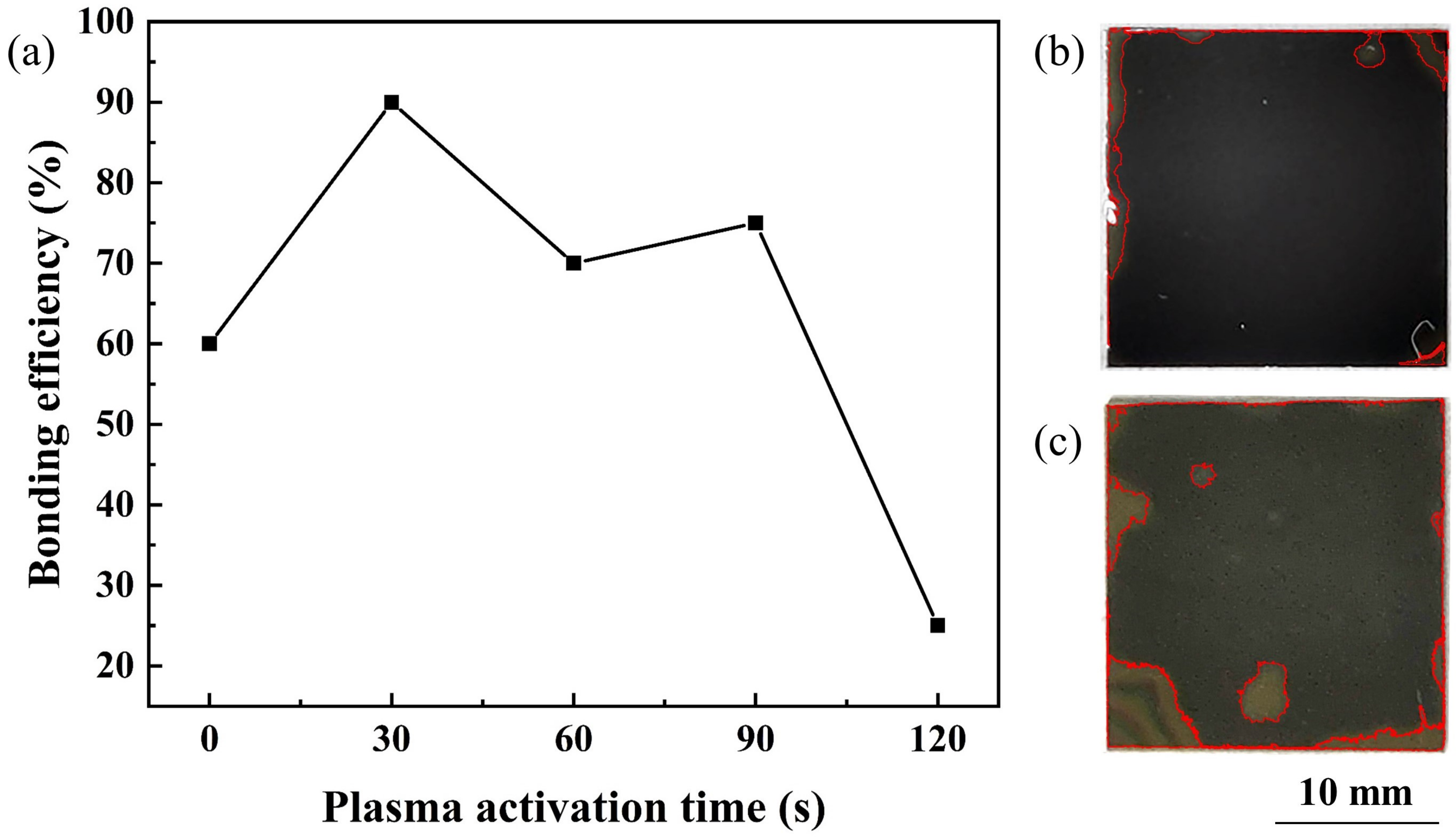
3.1. Effects of Bonding Parameters
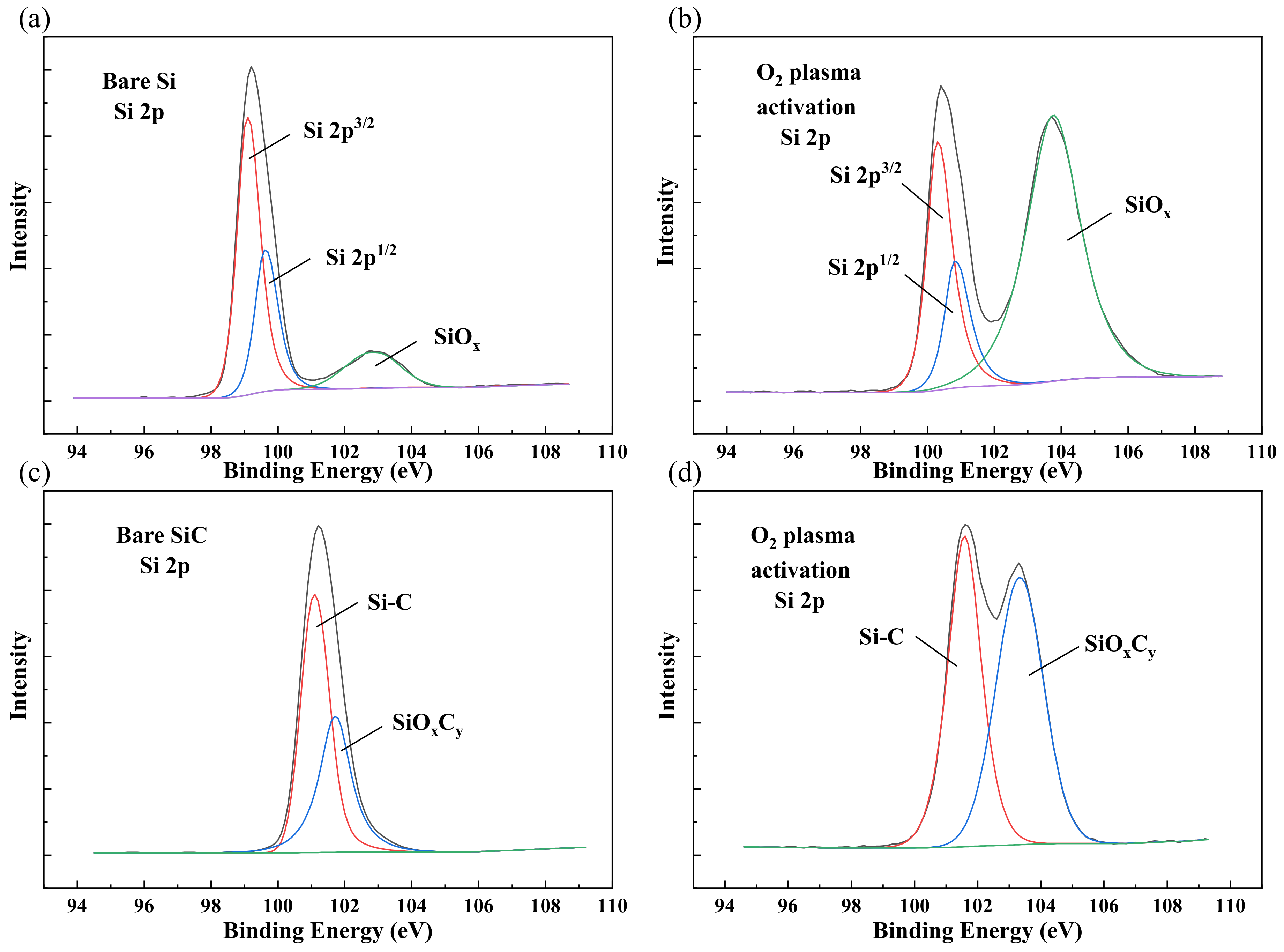
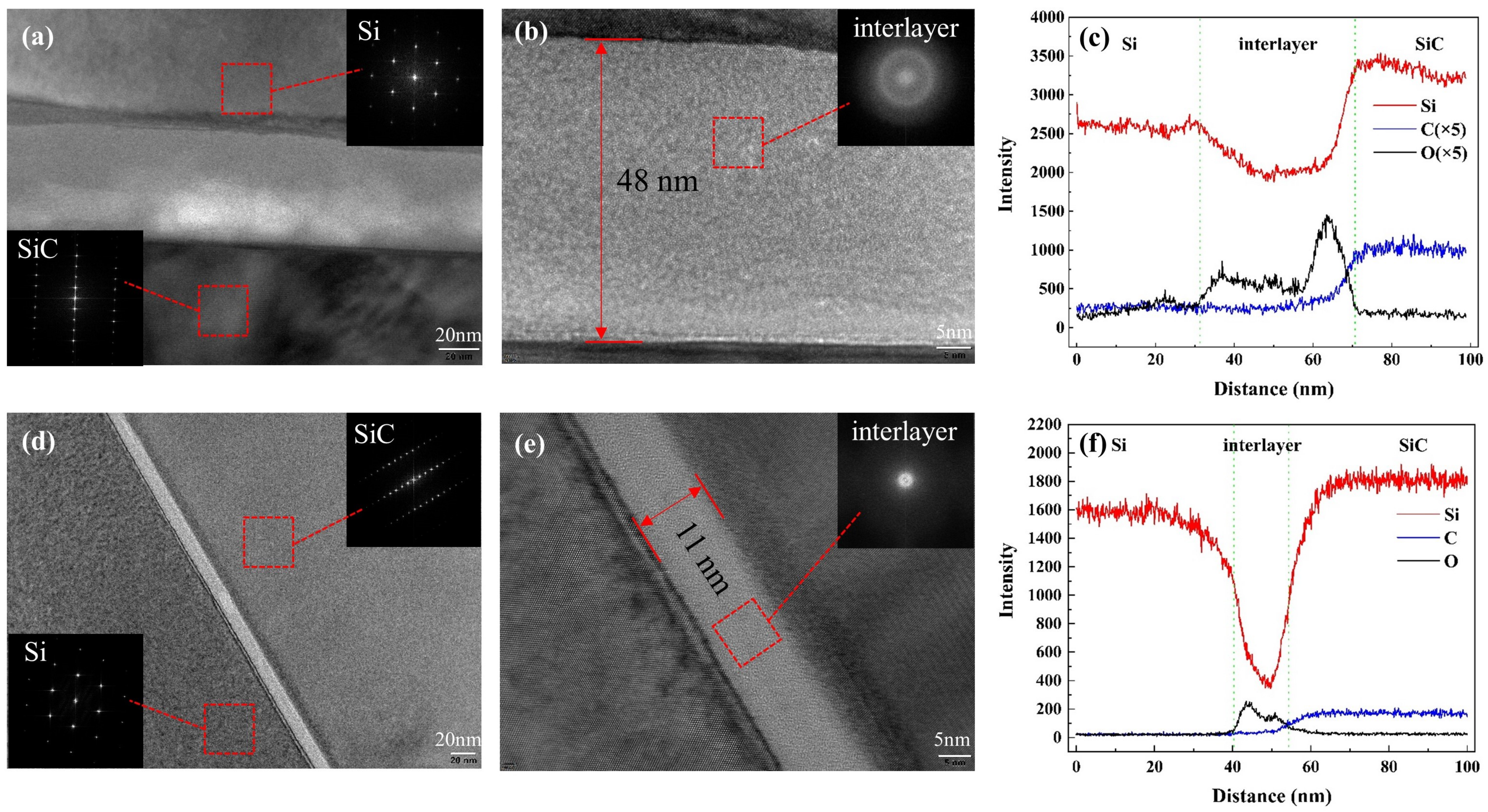
3.2. Surface and Interface Characterizations
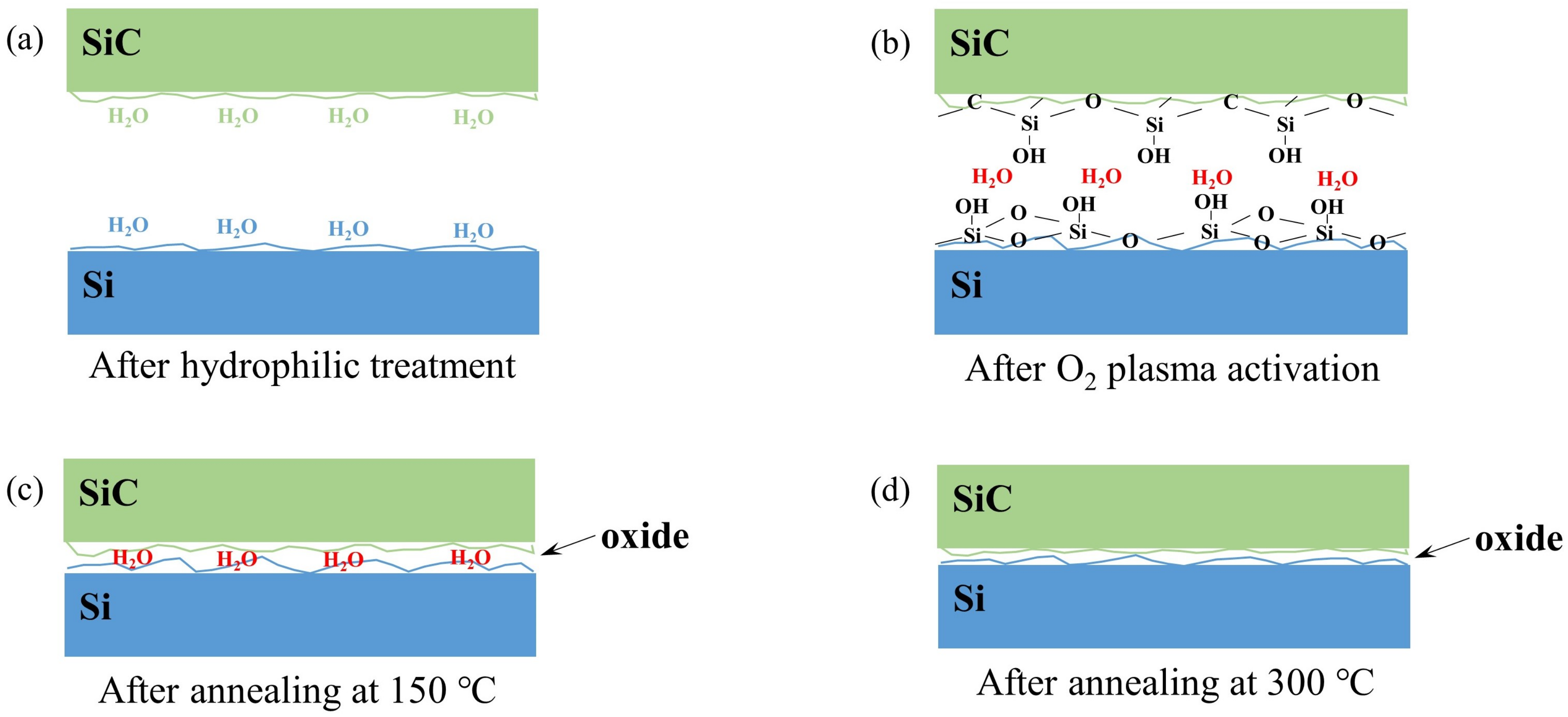
3.3. Bonding Mechanism for SiC/Si PAB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, M.; Baliga, B.J. Comparison of 6H-SiC, 3C-SiC, and Si for power devices. IEEE Trans. Electron Devices 1993, 40, 645–655. [Google Scholar] [CrossRef]
- Liang, J.; Nishida, S.; Arai, M.; Shigekawa, N. Effects of thermal annealing process on the electrical properties of p+-Si/n-SiC heterojunctions. Appl. Phys. Lett. 2014, 104, 161604. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Tomás, A.; Jennings, M.; Davis, M.; Covington, J.; Mawby, P.; Shah, V.; Grasby, T. Characterization and modeling of n-n Si/ SiC heterojunction diodes. J. Appl. Phys. 2007, 102, 014505. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.Y.; Cao, F.; Wang, H.Y. High performance of polysilicon/4H-SiC dual-heterojunction trench diode. IEEE Trans. Electron Devices 2017, 64, 1653–1659. [Google Scholar] [CrossRef]
- Guy, O.J.; Jenkins, T.; Lodzinski, M.; Castaing, A.; Wilks, S.; Bailey, P.; Noakes, T. Ellipsometric and MEIS studies of 4H-SiC/Si/SiO2 and 4H-SiC/SiO2 interfaces for MOS devices. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2007; Volume 556, pp. 509–512. [Google Scholar]
- Chan, C.; Mawby, P.A.; Gammon, P.M. Analysis of linear-doped Si/SiC power LDMOSFETs based on device simulation. IEEE Trans. Electron Devices 2016, 63, 2442–2448. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Tomás, A.; Lodzinski, M.; Guy, O.; Jennings, M.; Placidi, M.; Llobet, J.; Gammon, P.; Davis, M.; Covington, J.; Burrows, S.; et al. Si/SiC bonded wafer: A route to carbon free SiO2 on SiC. Appl. Phys. Lett. 2009, 94, 103510. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, Y.C.; Hao, Y.; Hu, Y.; Wang, G.; Cao, F. Simulation study of 4H-SiC UMOSFET structure with p+-polySi/SiC shielded region. IEEE Trans. Electron Devices 2017, 64, 3719–3724. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Zang, Y.; Song, L.; Han, Y.; Chu, Q. Epitaxial growth of Si/SiC heterostructures with different preferred orientations on 6H-SiC (0001) by LPCVD. CrystEngComm 2016, 18, 5681–5685. [Google Scholar] [CrossRef]
- Feng, Z.; Zhi-Ming, C.; Lian-Bi, L.; Shun-Feng, Z.; Tao, L. SiC based Si/SiC heterojunction and its rectifying characteristics. Chin. Phys. B 2009, 18, 4966. [Google Scholar] [CrossRef]
- Lianbi, L.; Zhiming, C.; Ying, Y. Hetero-epitaxy and structure characterization of Si films on 6H-SiC substrates. Mater. Lett. 2011, 65, 1257–1260. [Google Scholar] [CrossRef]
- Alexe, M.; Gösele, U. Wafer Bonding: Applications and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 75. [Google Scholar]
- Liang, J.; Nishida, S.; Hayashi, T.; Arai, M.; Shigekawa, N. Effects of interface state charges on the electrical properties of Si/SiC heterojunctions. Appl. Phys. Lett. 2014, 105, 151607. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Nishida, S.; Arai, M.; Shigekawa, N. Improved electrical properties of nn and pn Si/SiC junctions with thermal annealing treatment. J. Appl. Phys. 2016, 120, 034504. [Google Scholar] [CrossRef] [Green Version]
- Yeo, C.; Xu, D.; Yoon, S.; Fitzgerald, E. Low temperature direct wafer bonding of GaAs to Si via plasma activation. Appl. Phys. Lett. 2013, 102, 054107. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, C.; Niu, F.; Zhou, S.; Xu, J.; Tian, Y. Single-crystalline SiC integrated onto Si-based substrates via plasma-activated direct bonding. Ceram. Int. 2020, 46, 22718–22726. [Google Scholar] [CrossRef]
- Ramm, P.; Lu, J.J.Q.; Taklo, M.M. Handbook of Wafer Bonding; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Plach, T.; Hingerl, K.; Tollabimazraehno, S.; Hesser, G.; Dragoi, V.; Wimplinger, M. Mechanisms for room temperature direct wafer bonding. J. Appl. Phys. 2013, 113, 094905. [Google Scholar] [CrossRef]
- Fournel, F.; Martin-Cocher, C.; Radisson, D.; Larrey, V.; Beche, E.; Morales, C.; Delean, P.; Rieutord, F.; Moriceau, H. Water stress corrosion in bonded structures. ECS J. Solid State Sci. Technol. 2015, 4, P124. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Yang, X.; Zhao, Y.; Wu, J.; Guo, Z.; He, Z.; Fan, Z.; Yang, F. Low-Temperature Direct Bonding of SiC to Si via Plasma Activation. Appl. Sci. 2022, 12, 3261. https://doi.org/10.3390/app12073261
Wang F, Yang X, Zhao Y, Wu J, Guo Z, He Z, Fan Z, Yang F. Low-Temperature Direct Bonding of SiC to Si via Plasma Activation. Applied Sciences. 2022; 12(7):3261. https://doi.org/10.3390/app12073261
Chicago/Turabian StyleWang, Fengxuan, Xiang Yang, Yongqiang Zhao, Jingmin Wu, Zhiyu Guo, Zhi He, Zhongchao Fan, and Fuhua Yang. 2022. "Low-Temperature Direct Bonding of SiC to Si via Plasma Activation" Applied Sciences 12, no. 7: 3261. https://doi.org/10.3390/app12073261
APA StyleWang, F., Yang, X., Zhao, Y., Wu, J., Guo, Z., He, Z., Fan, Z., & Yang, F. (2022). Low-Temperature Direct Bonding of SiC to Si via Plasma Activation. Applied Sciences, 12(7), 3261. https://doi.org/10.3390/app12073261





