Raman Spectroscopic Investigation of Osteoclastic Activity under the Influence of Bisphosphonate
Abstract
:1. Introduction
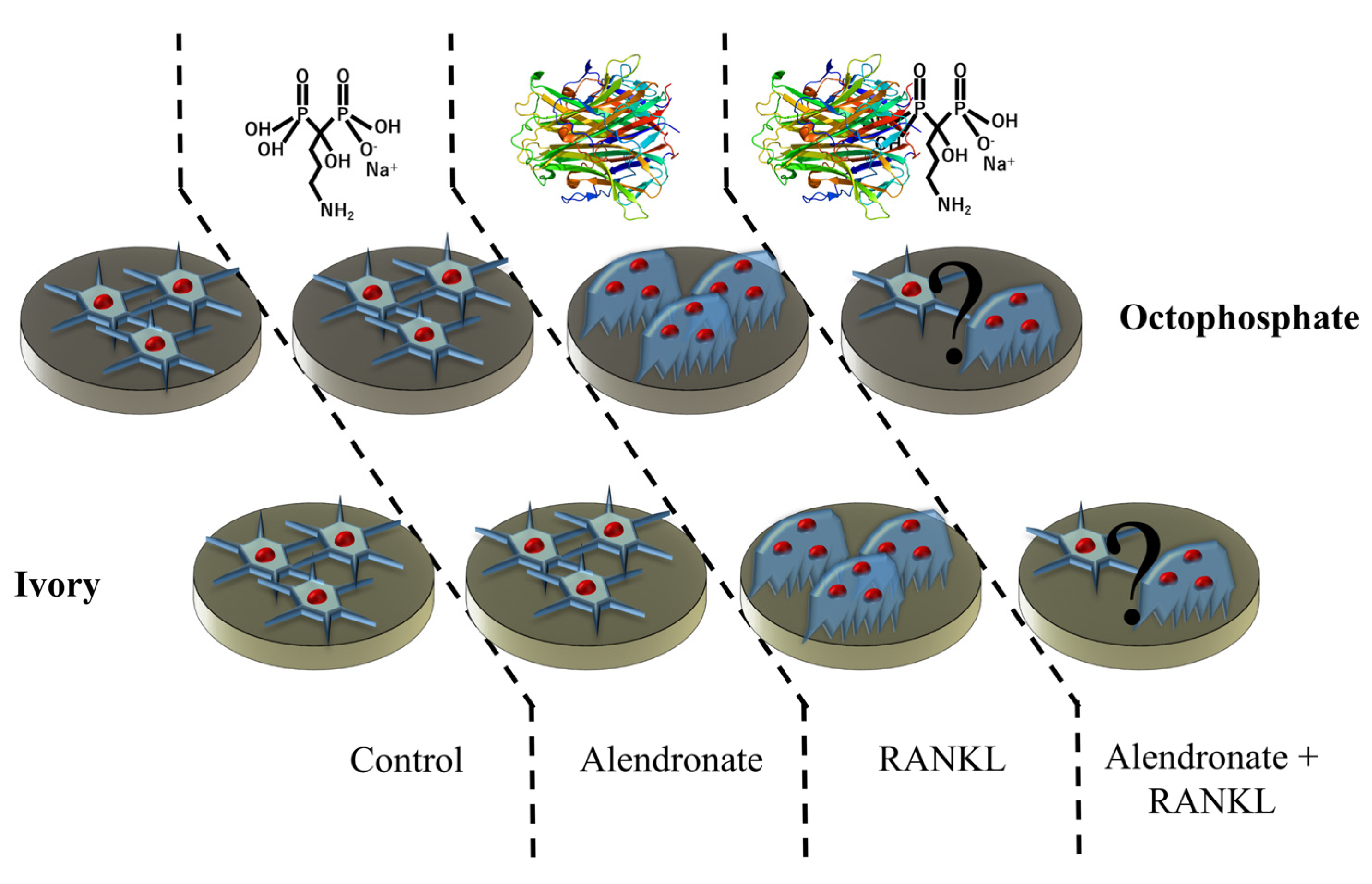
2. Materials and Methods
2.1. Samples Preparation
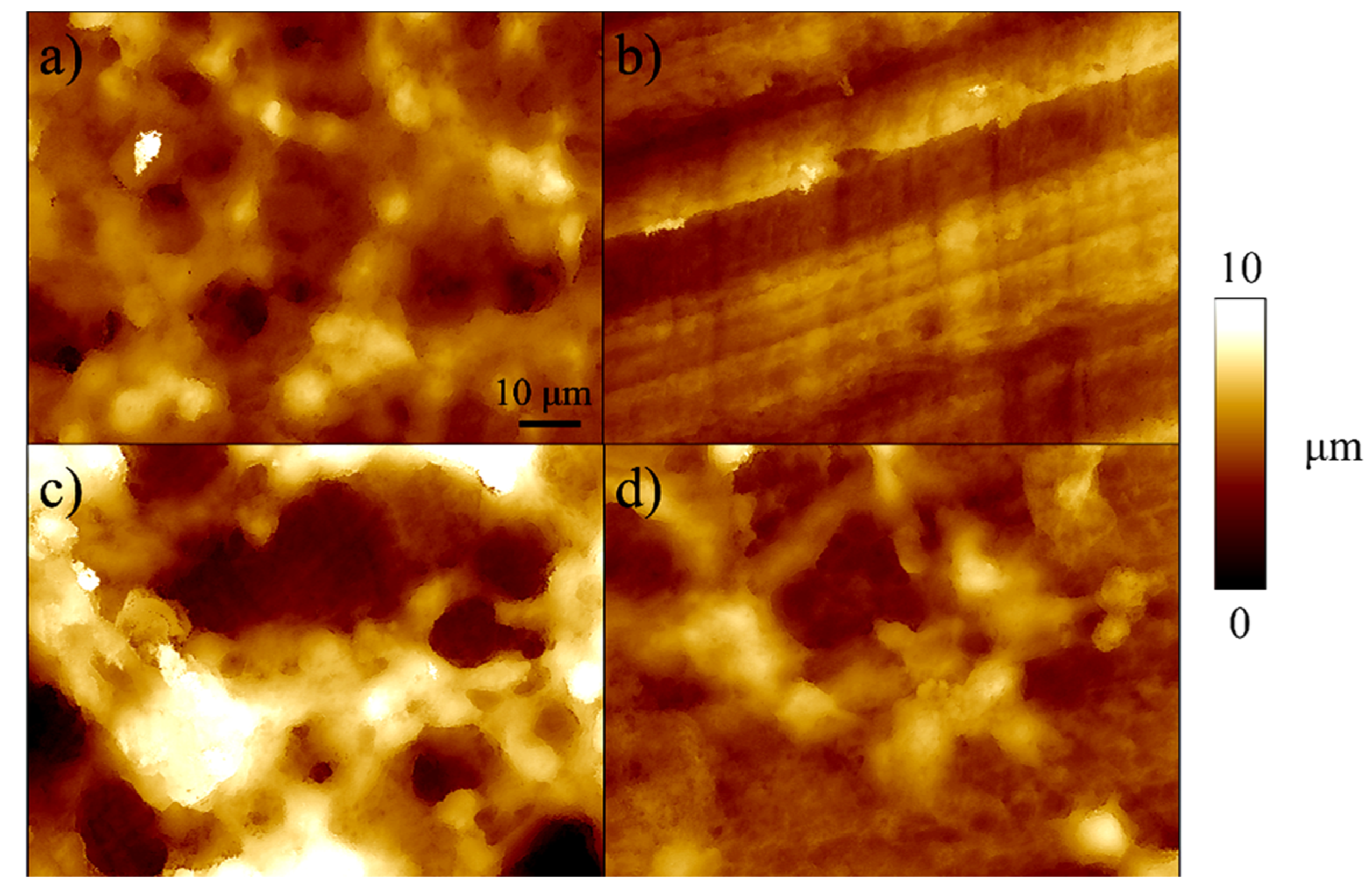
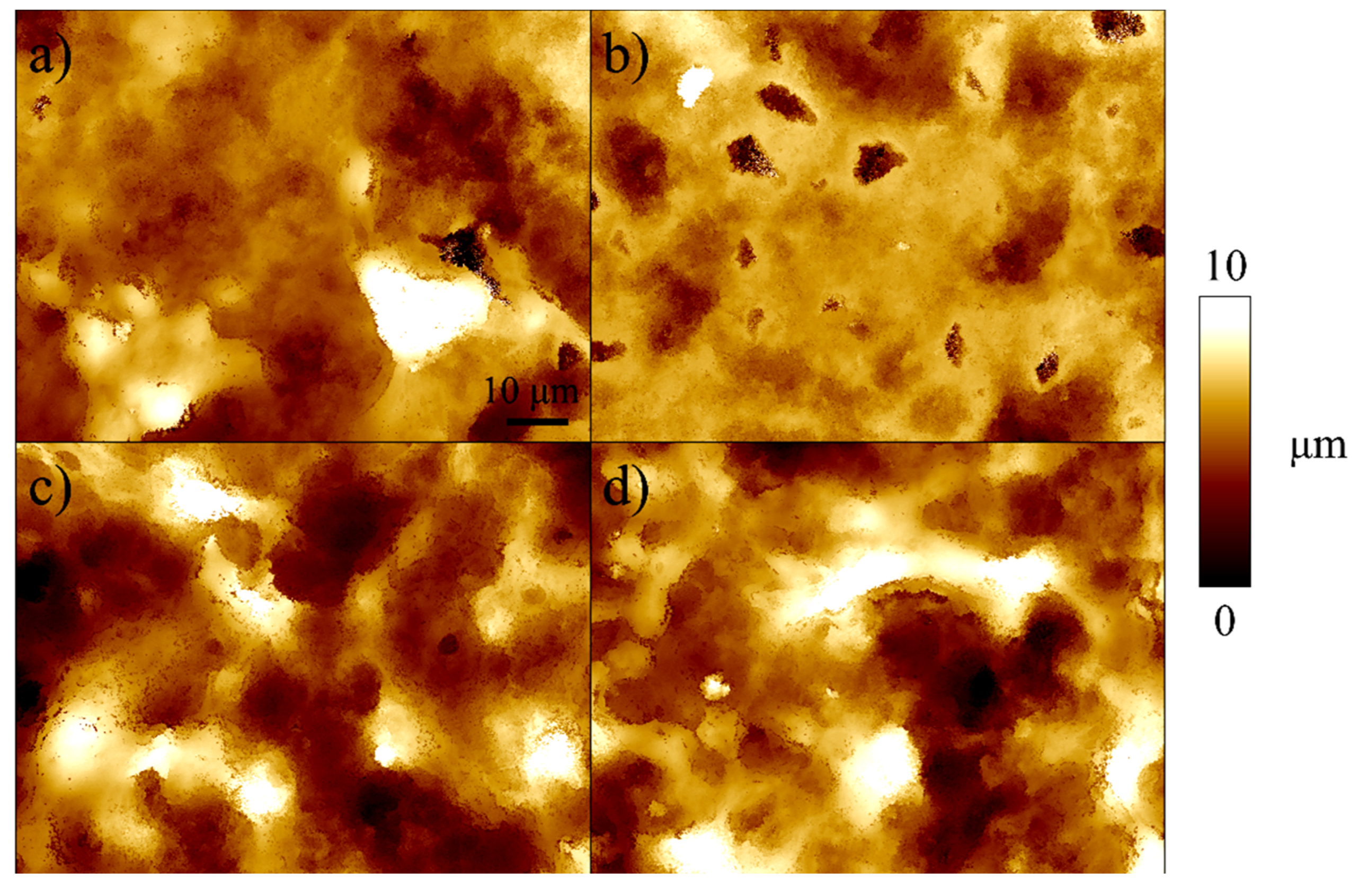
2.2. Sample Characterization
2.3. Cell Culture
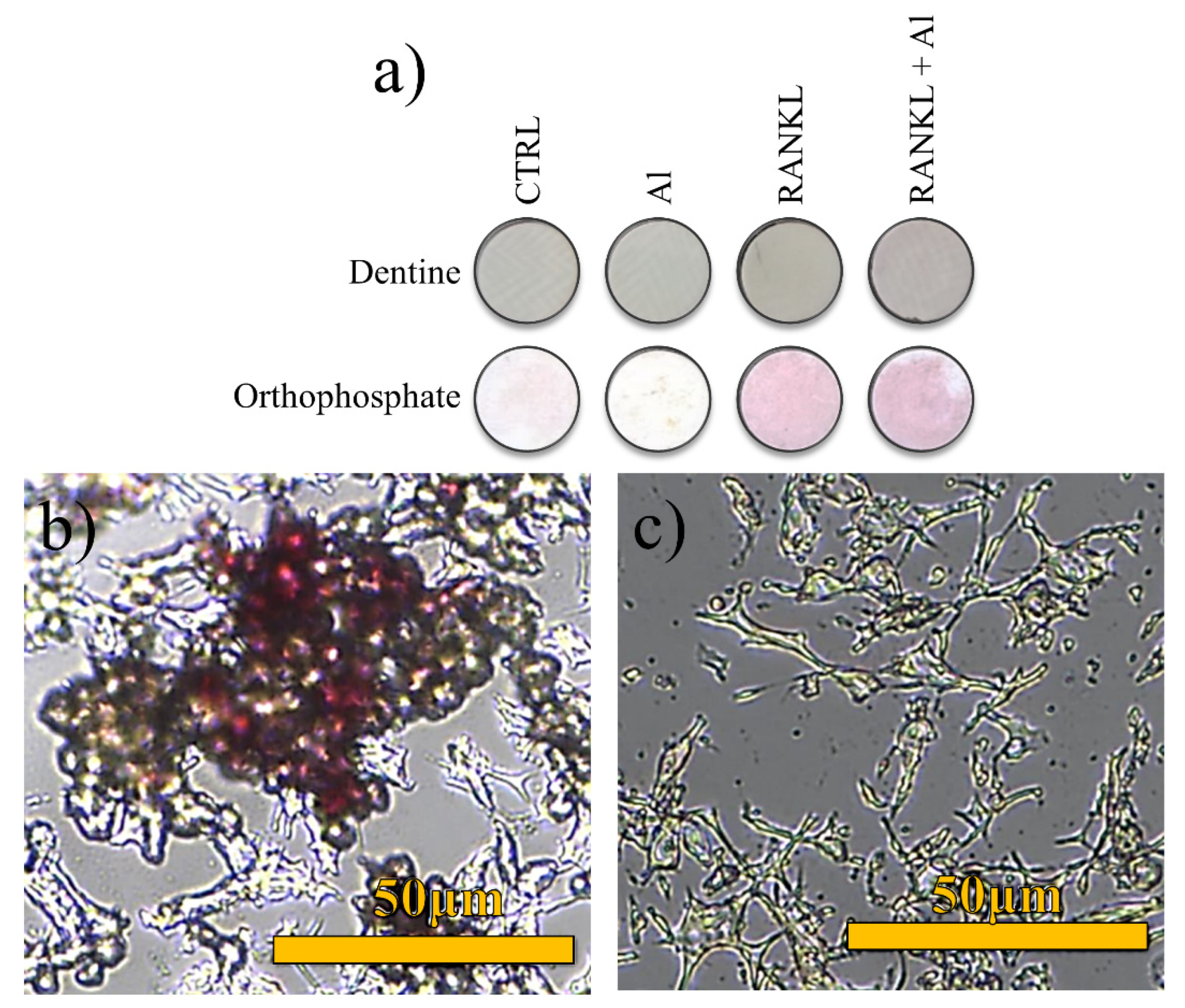
2.4. Biological Testing
2.5. Statistical Analysis
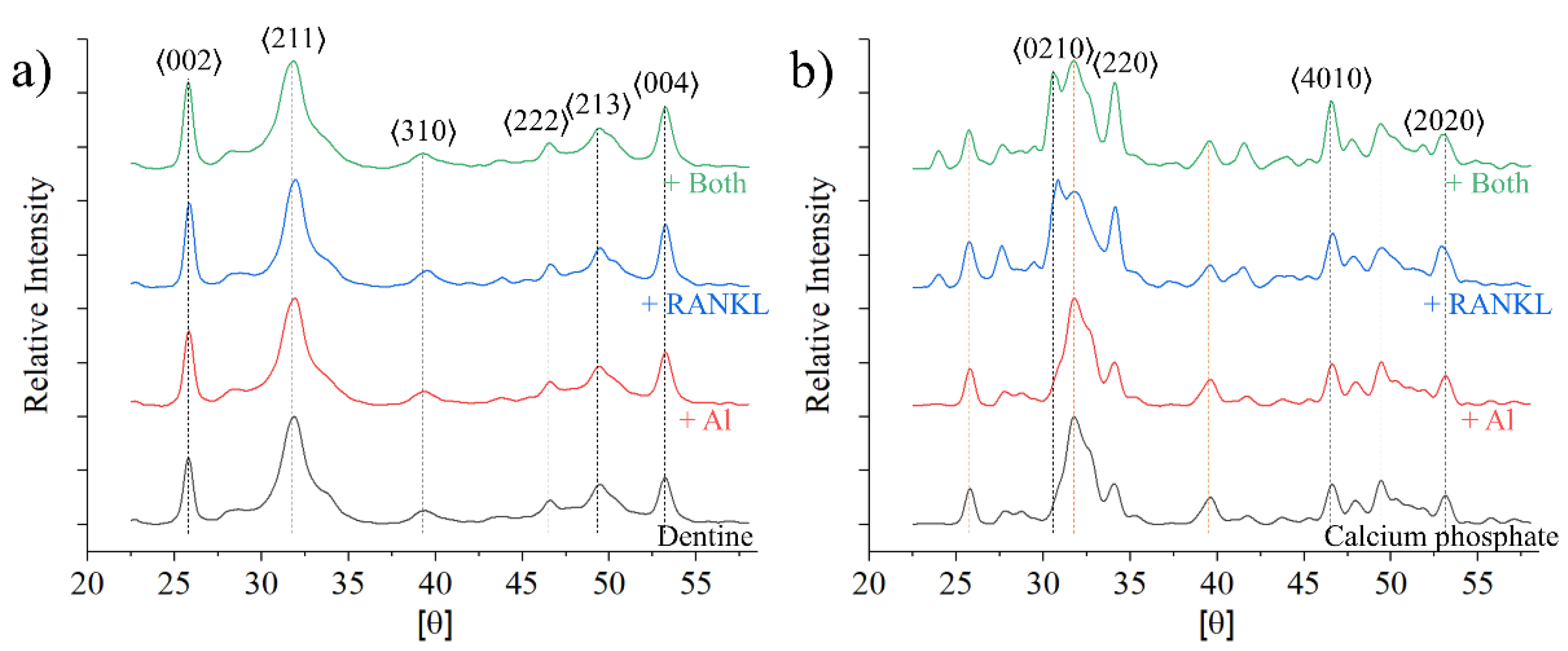
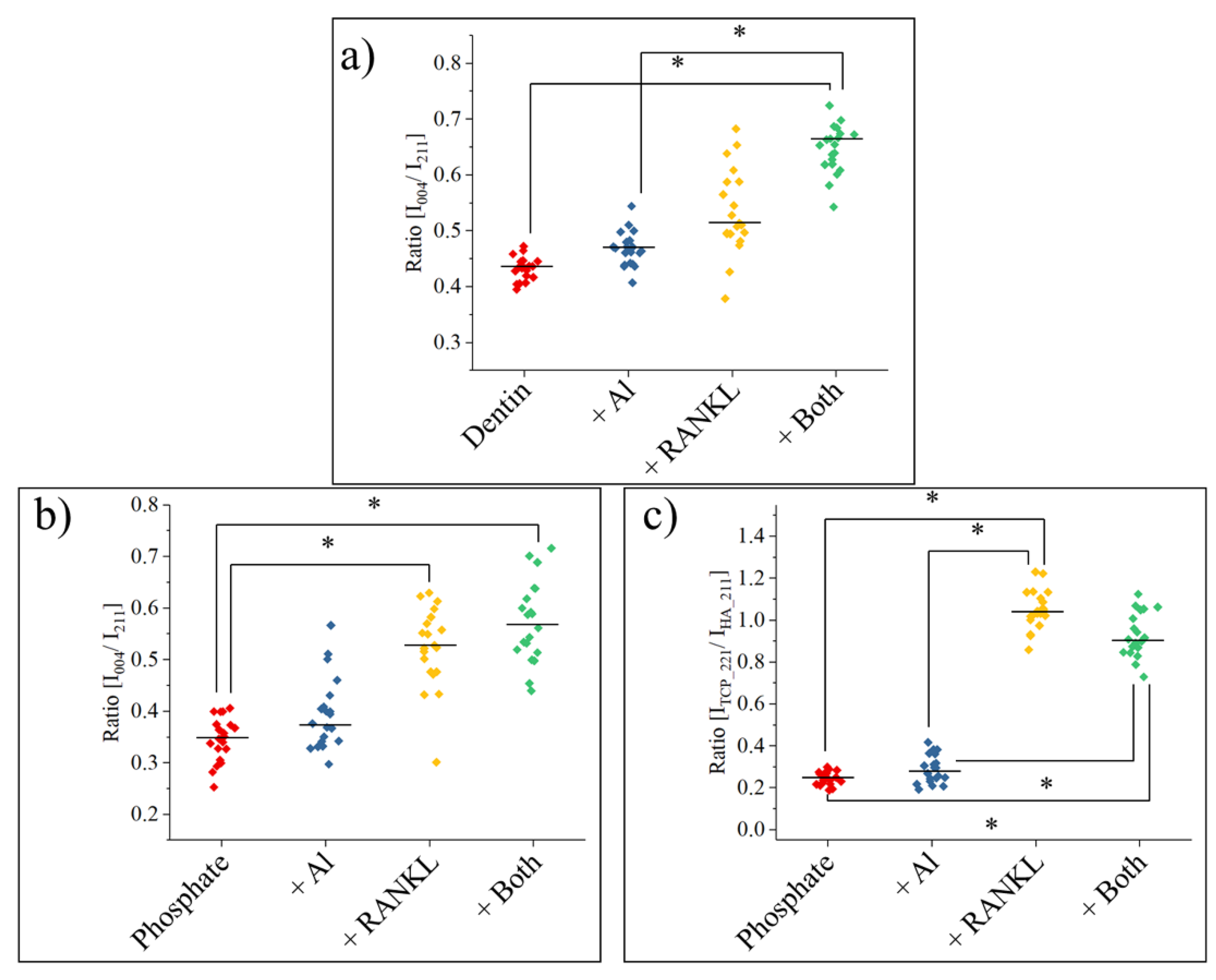
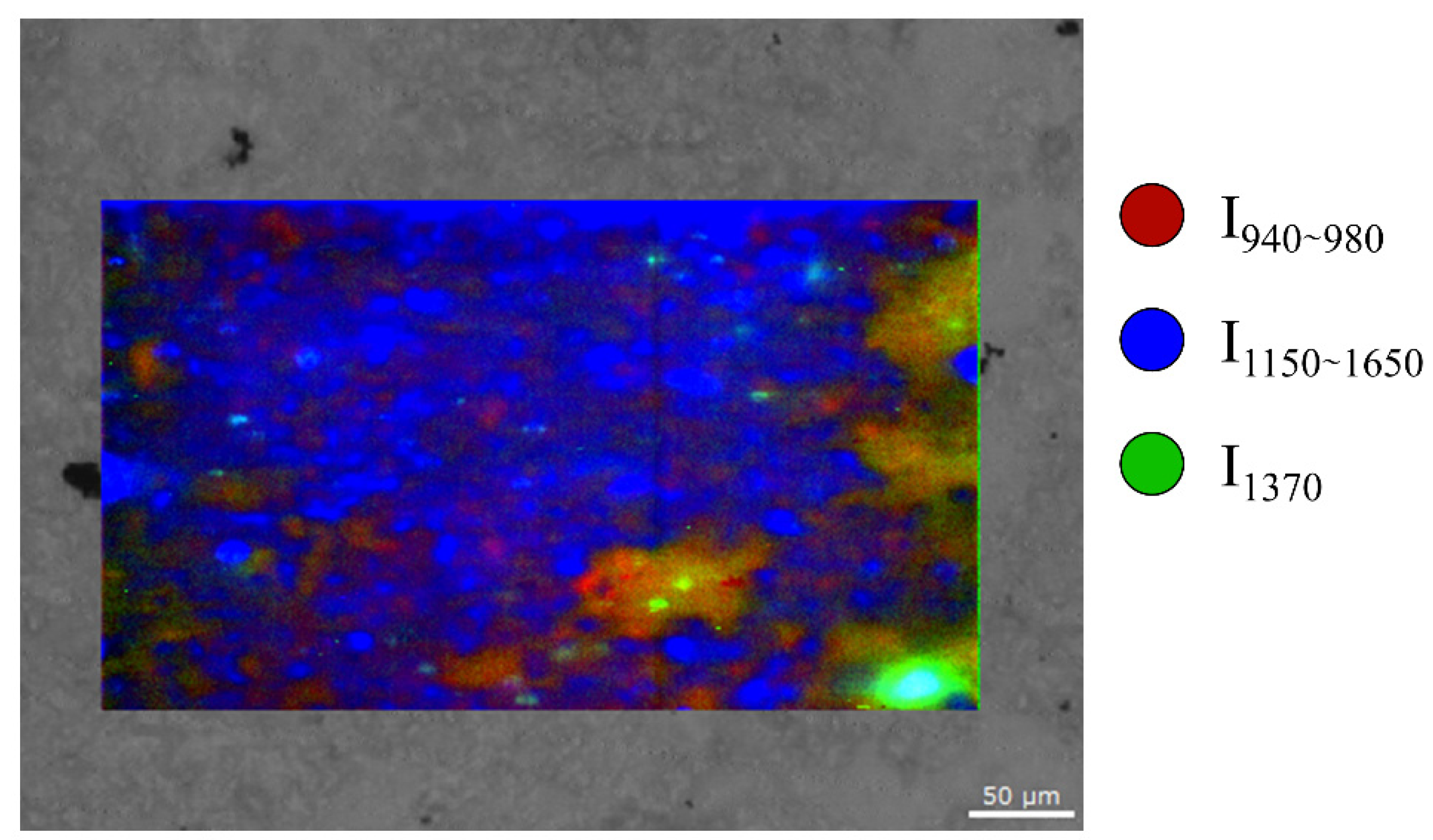
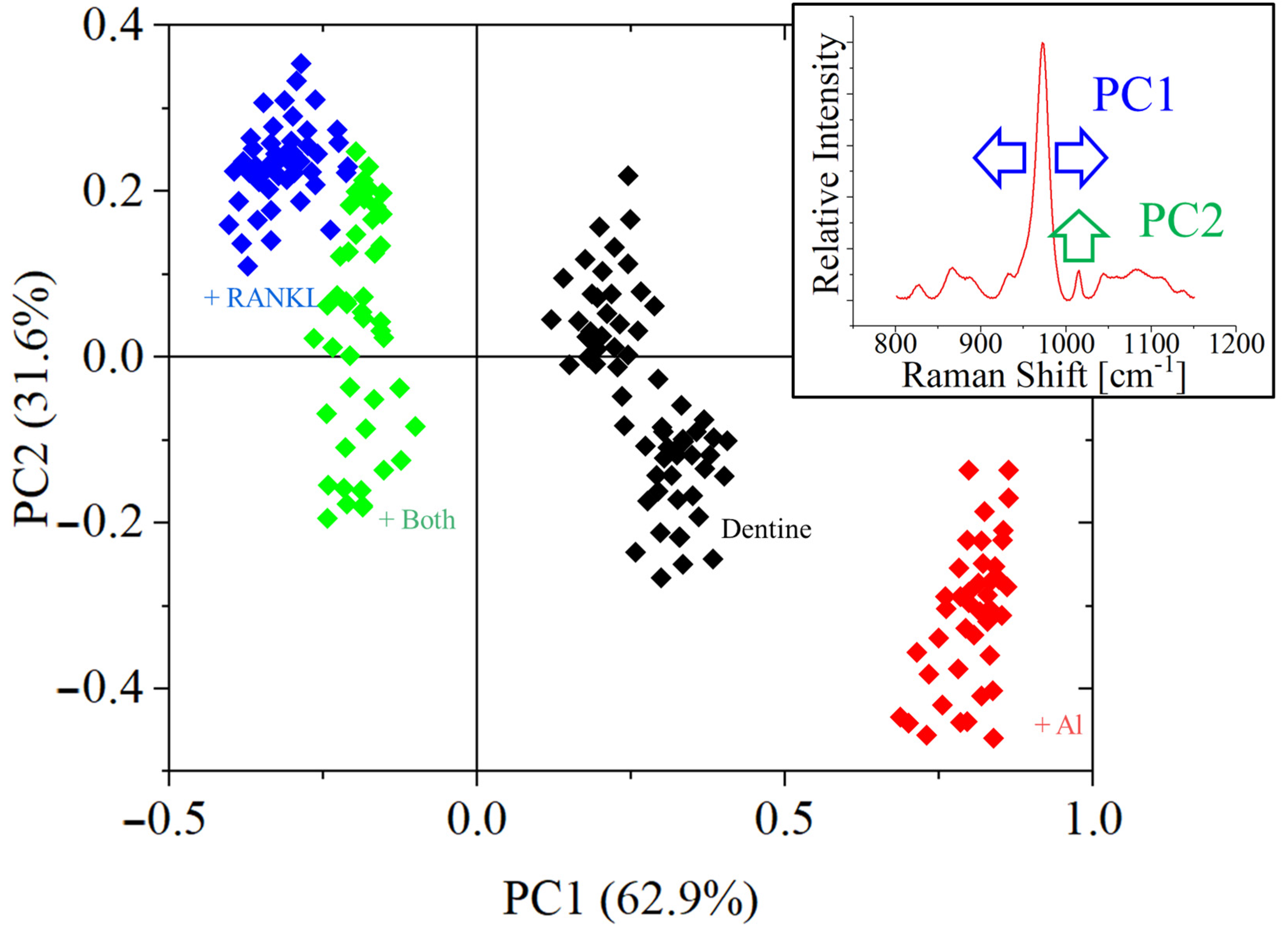
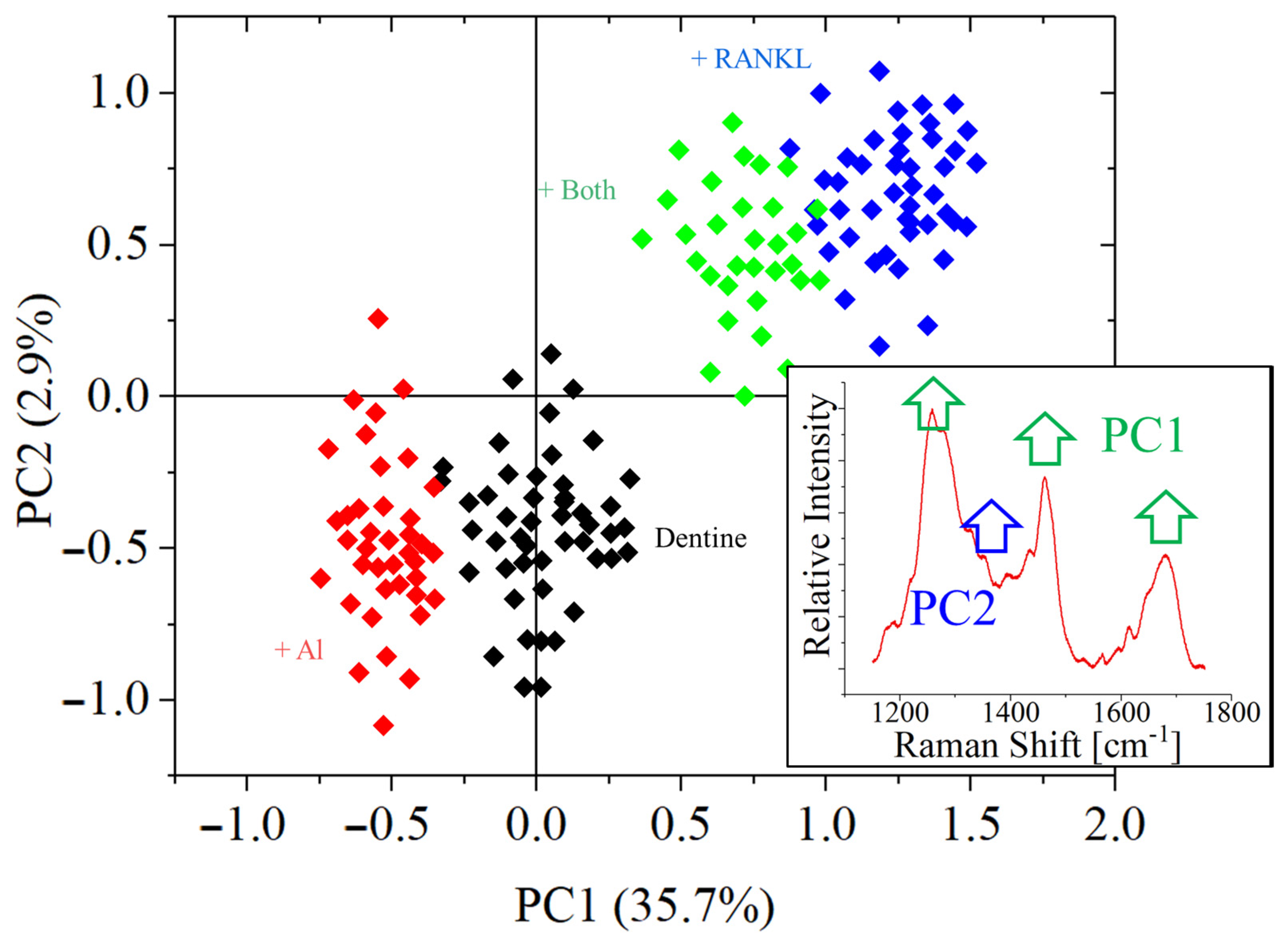
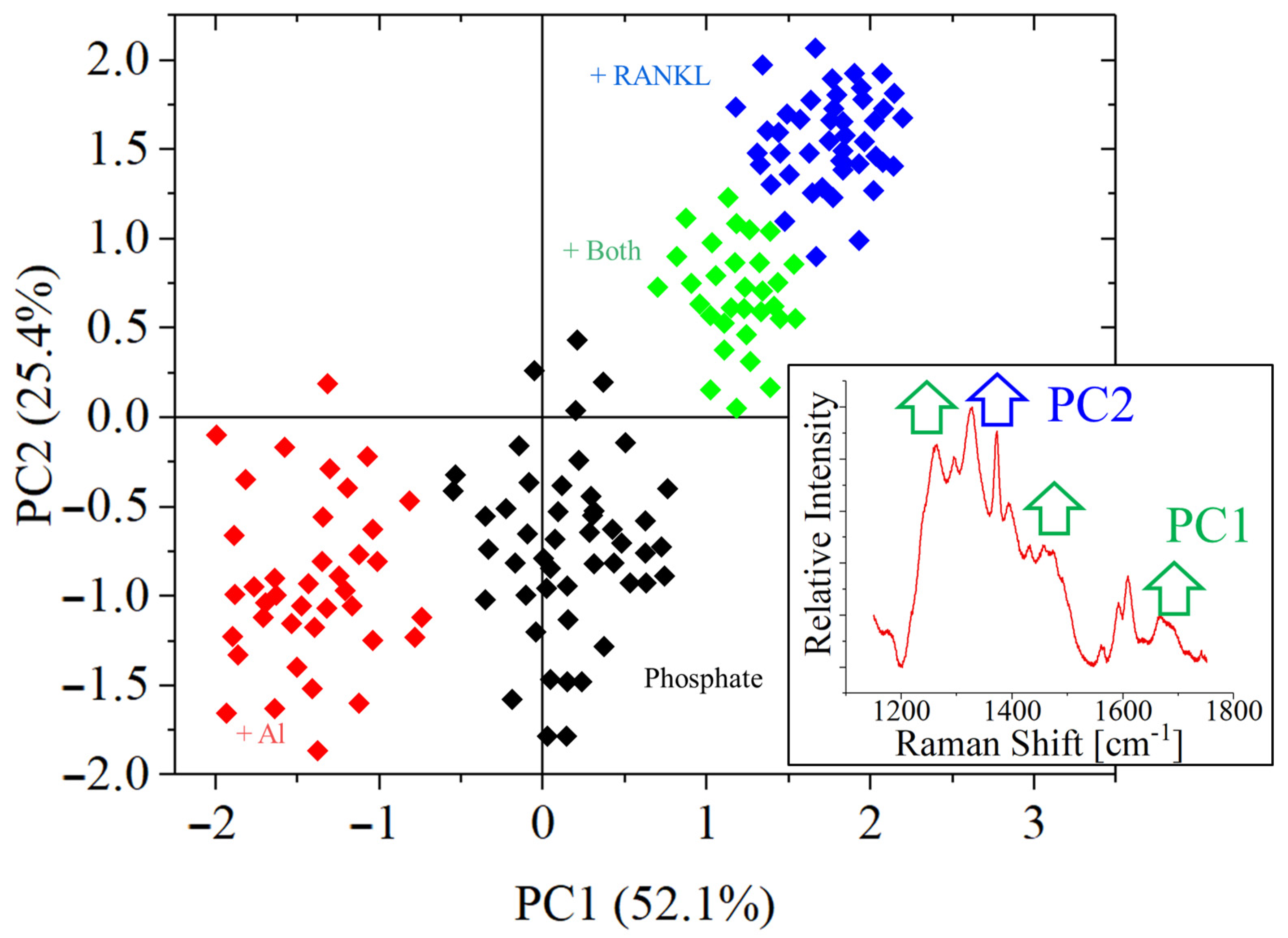
3. Results
4. Discussion
5. Conclusions
- -
- The width of the phosphate band at about 960 cm−1 reduces due to osteoclast bone remodeling;
- -
- The relative intensity of the amide bands increases due to bone remodeling, for dentine substrates;
- -
- The bands related to thymidine phosphorylase appeared after differentiation on both substrates.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, I.; Branco, J.C. Rank/Rankl/opg: Literature review. Acta Reumatol. Port. 2011, 36, 209–218. [Google Scholar] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbar, S.; Drury, J.; Fordham, J.N.; Datta, H.K.; Francis, R.M.; Tuck, S.P. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J. Clin. Pathol. 2011, 64, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, M.; Kurokawa, K. Renal osteodystrophy and secondary hyperparathyroidism. Clin. Calcium. 2002, 12, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Geusens, P.P.; Landewé, R.B.M.; Garnero, P.; Chen, D.; Dunstan, C.R.; Lems, W.F.; Stinissen, P.; ven der Heijde, D.M.F.M.; van der Linder, S.; Boers, M. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006, 54, 1772–1777. [Google Scholar] [CrossRef]
- Bargman, R.; Posham, R.; Boskey, A.; Carter, E.; Dicarlo, E.; Verdelis, K.; Raggio, C.; Pleshko, N. High-and low-dose oPg-Fc cause osteopetrosis-like changes in infant mice. Pediatric Res. 2012, 72, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Sircar, K.; Aprikian, A.; Potti, A.; Goltzman, D.; Rabbani, S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 2006, 107, 289–298. [Google Scholar] [CrossRef]
- Lipton, A.; Jun, S. RANKL inhibition in the treatment of bone metastases. Curr. Opin. Support. Palliat. Care 2008, 2, 197–203. [Google Scholar] [CrossRef]
- Russell, R.G.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Fleisch, H. Bisphosphonates--history and experimental basis. Bone 1987, 8 (Suppl. S1), S23–S28. [Google Scholar] [PubMed]
- Ebetino, F.H.; Hogan, A.-M.L.; Sun, S.; Tsoumpra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U.; et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Reszka, A.; Rodan, G.; Rogers, M. Bisphosphonates: Mechanisms of Action. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; Chapter 78; p. 1361-XLIII. [Google Scholar]
- Lin, T.-J. Using Molecular Dynamics to Model the Binding Affinities of Nitrogen-Containing Bisphosphonates on Hydroxyapatite Surface. arXiv 2018, arXiv:1810.13268. [Google Scholar]
- Baron, R. Molecular mechanisms of bone resorption by the osteoclast. Anat. Rec. 1989, 224, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Grasser, W. Effects of bisphosphonates on isolated rat osteoclasts as examined by reflected light microscopy. J. Bone Miner. Res. 1990, 5, 31–40. [Google Scholar] [CrossRef]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, G.D.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar] [CrossRef]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kandler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef]
- Molvik, H.; Khan, W. Bisphosphonates and their influence on fracture healing: A systematic review. Osteoporos. Int. 2015, 26, 1251–1260. [Google Scholar] [CrossRef]
- Singh, T.; Kaur, V.; Kumar, M.; Kaur, P.; Murthy, R.S.R.; Rawal, R.K. The critical role of bisphosphonates to target bone cancer metastasis: An overview. J. Drug Target. 2015, 23, 1–15. [Google Scholar] [CrossRef]
- Clézardin, P. Insights into the antitumor effects of bisphosphonates from preclinical models and potential clinical implications. IBMS BoneKEy 2009, 6, 210–217. [Google Scholar] [CrossRef]
- Hanlie, H.; Liyun, T.; Tao, J. The crystal characteristics of enamel and dentin by XRD method. J. Wuhan Univ. Technol. (Mater. Sci. Ed.) 2006, 21, 9–12. [Google Scholar] [CrossRef]
- Tavares D dos, S.; Castro L de, O.; Soares GD de, A.; Alves, G.G.; Granjeiro, J.M. Synthesis and cytotoxicity evaluation of granular magnesium substituted β-tricalcium phosphate. J. Appl. Oral. Sci. 2013, 21, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsad, M.S.M.; Lee, P.M.; Hung, L.K. Synthesis and Characterization of Hydroxyapatite Nanoparticles and β-TCP Particles. In 2011 2nd International Conference on Biotechnology and Food Science; IACSIT Press: Singapore, 2011. [Google Scholar]
- Kekkonen, J.; Finnilä, M.A.J.; Heikkilä, J.; Anttonen, V.; Nissinen, I. Chemical imaging of human teeth by a time-resolved Raman spectrometer based on a CMOS single-photon avalanche diode line sensor. Analyst 2019, 144, 6089–6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, A.; Ozaki, Y.; Itoh, K.; Matsushima, S.; Iriyama, K. Raman spectroscopic evidence for the microenvironmental change of some tyrosine residues of lens proteins in cold cataract. Biochem. Biophys. Res. Commun. 1984, 119, 989–994. [Google Scholar] [CrossRef]
- Farlay, D.; Panczer, G.; Rey, C.; Delmas, P.D.; Boivin, G. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J. Bone Miner. Metab. 2010, 28, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Arancibia, S.; Rodríguez-Martínez, E.; Badillo-Ramírez, I.; López-González, U.; Saniger, J.M. Structural changes of amyloid beta in hippocampus of rats exposed to ozone: A Raman spectroscopy study. Front Mol. Neurosci. 2017, 10, 137. [Google Scholar] [CrossRef]
- Jung, G.B.; Kang, I.S.; Lee, Y.J.; Kim, D.; Park, H.K.; Lee, G.J.; Kim, C. Label-free Noninvasive Characterization of Osteoclast Differentiation Using Raman Spectroscopy Coupled with Multivariate Analysis. Curr. Opt. Photon. 2017, 1, 412–420. [Google Scholar]
- Rosiek, E. Metabolic Characterization of Cellular Health Using a Multimodal Spectroscopy Technique [Internet]. 2019. Available online: https://deepblue.lib.umich.edu/handle/2027.42/148875 (accessed on 30 March 2022).
- Michalowska, M.; Znorko, B.; Kaminski, T.; Oksztulska-Kolanek, E.; Pawlak, D. New insights into tryptophan and its metabolites in the regulation of bone metabolism. J. Physiol. Pharmacol. 2015, 66, 779–791. [Google Scholar]
- Otto, C.; van den Tweel, T.J.J.; de Mul, F.F.M.; Greve, J. Surface-enhanced Raman spectroscopy of DNA bases. J. Raman Spectrosc. 1986, 17, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V.; Janković-Častvan, I.; Wu, V.M. Bone mineral crystallinity governs the orchestration of ossification and resorption during bone remodeling. ACS Biomater. Sci. Eng. 2019, 5, 3483–3498. [Google Scholar] [CrossRef]
- Lassus, J.; Salo, J.; Jiranek, W.A.; Santavirta, S.; Nevalainen, J.; Matucci-Cerinic, M.; Horák, P.; Konttinen, Y. Macrophage activation results in bone resorption. Clin. Orthop. Relat. Res. 1998, 352, 7–15. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Translat. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zambuzzi, W.F.; Oliveira RC de Pereira, F.L.; Cestari, T.M.; Taga, R.; Granjeiro, J.M. Rat subcutaneous tissue response to macrogranular porous anorganic bovine bone graft. Braz. Dent. J. 2006, 17, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, S. Adenosine as a Neuromodulator. Annu. Rev. Neurosci. 1985, 8, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Seuvre, A.-M.; Koenig, J.L. FT-IR and laser-Raman spectra of thymine and thymidine. Carbohydr. Res. 1984, 134, 23–38. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, D.; Cai, C.; Xiong, Y.; Li, S.; Su, Y.; Si, M. NIR-SERS studies of DNA and DNA bases attached on polyvinyl alcohol (PVA) protected silver grass-like nanostructures. Vib. Spectrosc. 2013, 67, 71–79. [Google Scholar] [CrossRef]
- Tripon, C.; Muntean, C.M.; Bratu, I.; Nalpantidis, K.; Deckert, V. (Sub)picosecond processes in DNA and RNA constituents: A Raman spectroscopic assessment. Polym. Bull 2017, 74, 4087–4100. [Google Scholar] [CrossRef]
- Matsumae, G.; Shimizu, T.; Tian, Y.; Takahashi, D.; Ebata, T.; Alhasan, H.; Yokota, S.; Kadoya, K.; Terkawi, M.A.; Iwasaki, N. Identification of thymidine phosphorylase as a potential therapeutic target for bone loss associated periprosthetic osteolysis. SSRN Electron J. 2021. [Google Scholar] [CrossRef]
- Matsumae, G.; Shimizu, T.; Tian, Y.; Takahashi, D.; Ebata, T.; Alhasan, H.; Yokota, S.; Kadoya, K.; Terkawi, M.A.; Iwasaki, N. Targeting thymidine phosphorylase as a potential therapy for bone loss associated with periprosthetic osteolysis. Bioeng. Transl. Med. 2021, 6, e10232. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Du, J.; He, J.; Lin, P.; Amini, B.; Starbuck, M.W.; Novane, N.; Shah, J.J.; Davis, R.E.; et al. Thymidine phosphorylase exerts complex effects on bone resorption and formation in myeloma. Sci. Transl. Med. 2016, 8, 353ra113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salduz, A.; Eralp, L. The local management of bone metastases. In Breast Disease; Springer International Publishing: Cham, Switzerland, 2019; pp. 619–633. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, E.; Adachi, T.; Boschetto, F.; Zhu, W.; Adachi, K.; Kanamura, N.; Yamamoto, T.; Pezzotti, G. Raman Spectroscopic Investigation of Osteoclastic Activity under the Influence of Bisphosphonate. Appl. Sci. 2022, 12, 3757. https://doi.org/10.3390/app12083757
Marin E, Adachi T, Boschetto F, Zhu W, Adachi K, Kanamura N, Yamamoto T, Pezzotti G. Raman Spectroscopic Investigation of Osteoclastic Activity under the Influence of Bisphosphonate. Applied Sciences. 2022; 12(8):3757. https://doi.org/10.3390/app12083757
Chicago/Turabian StyleMarin, Elia, Tetsuya Adachi, Francesco Boschetto, Wenliang Zhu, Keiji Adachi, Narisato Kanamura, Toshiro Yamamoto, and Giuseppe Pezzotti. 2022. "Raman Spectroscopic Investigation of Osteoclastic Activity under the Influence of Bisphosphonate" Applied Sciences 12, no. 8: 3757. https://doi.org/10.3390/app12083757





