Exogenous Selenium Treatment Promotes Glucosinolate and Glucoraphanin Accumulation in Broccoli by Activating Their Biosynthesis and Transport Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Total Sulfur and Se Contents
2.3. Determination of GSLs Composition and Content
2.4. Determination of MYR Activity
2.5. Determination of Sulforaphane Level
2.6. RNA Extraction and RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Se on Total Sulfur and Se Contents
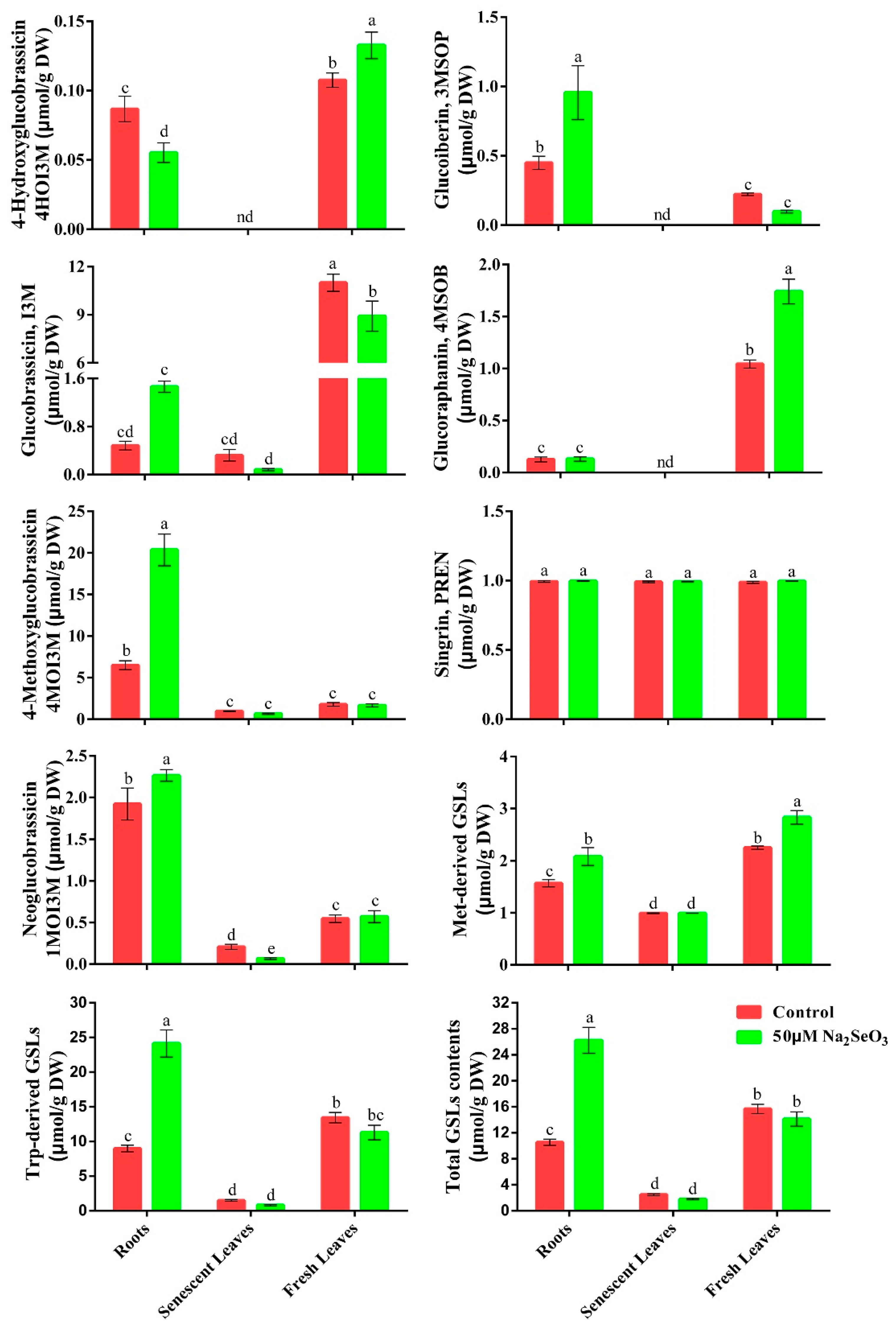
3.2. Effect of Se on GSLs Contents
3.3. Effects of Se on MYR Activity and Sulforaphane Level after Autolysis
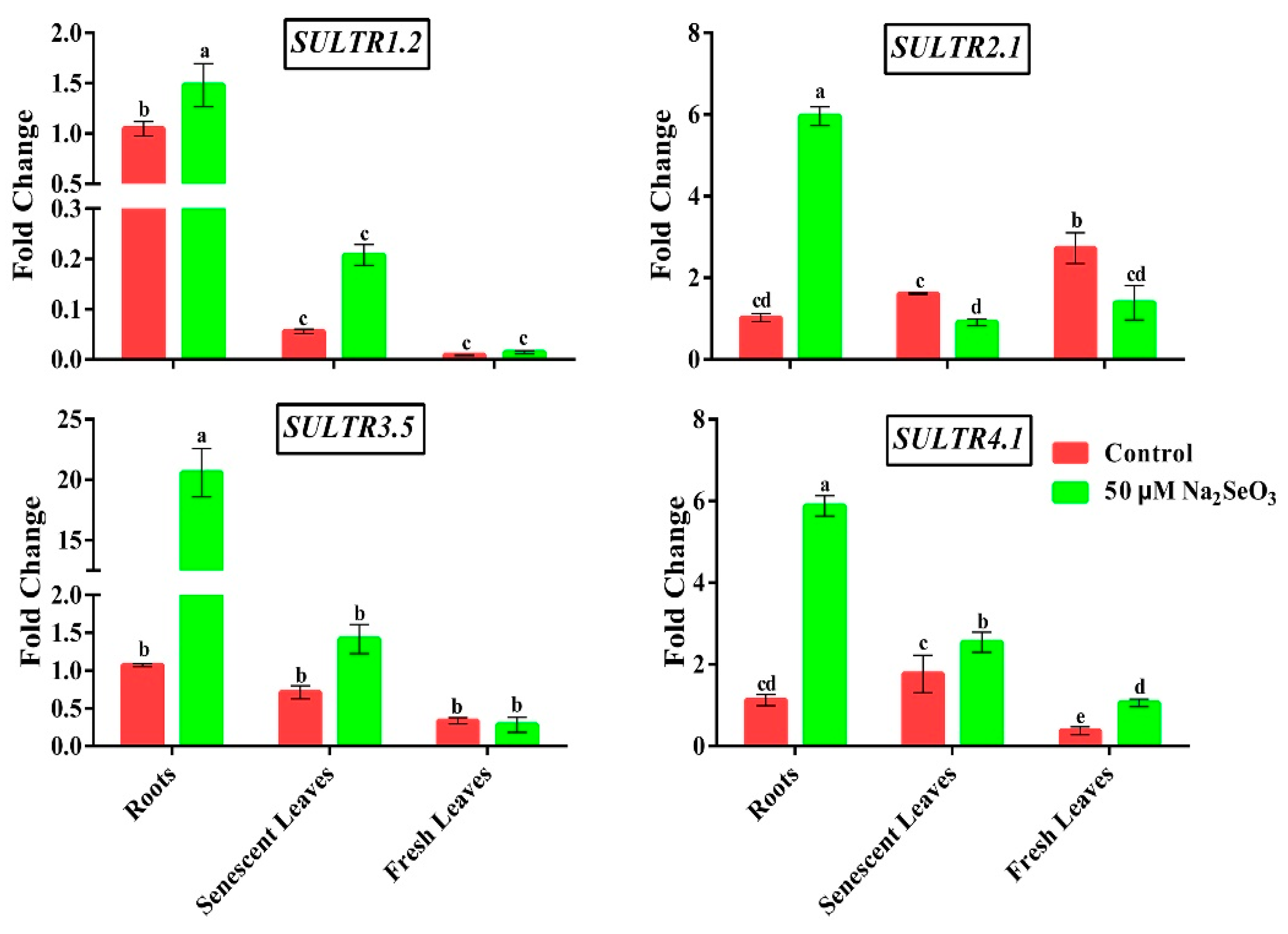
3.4. Effect of Se on the Transcript Levels of Genes Related to Sulfate Uptake and Transport
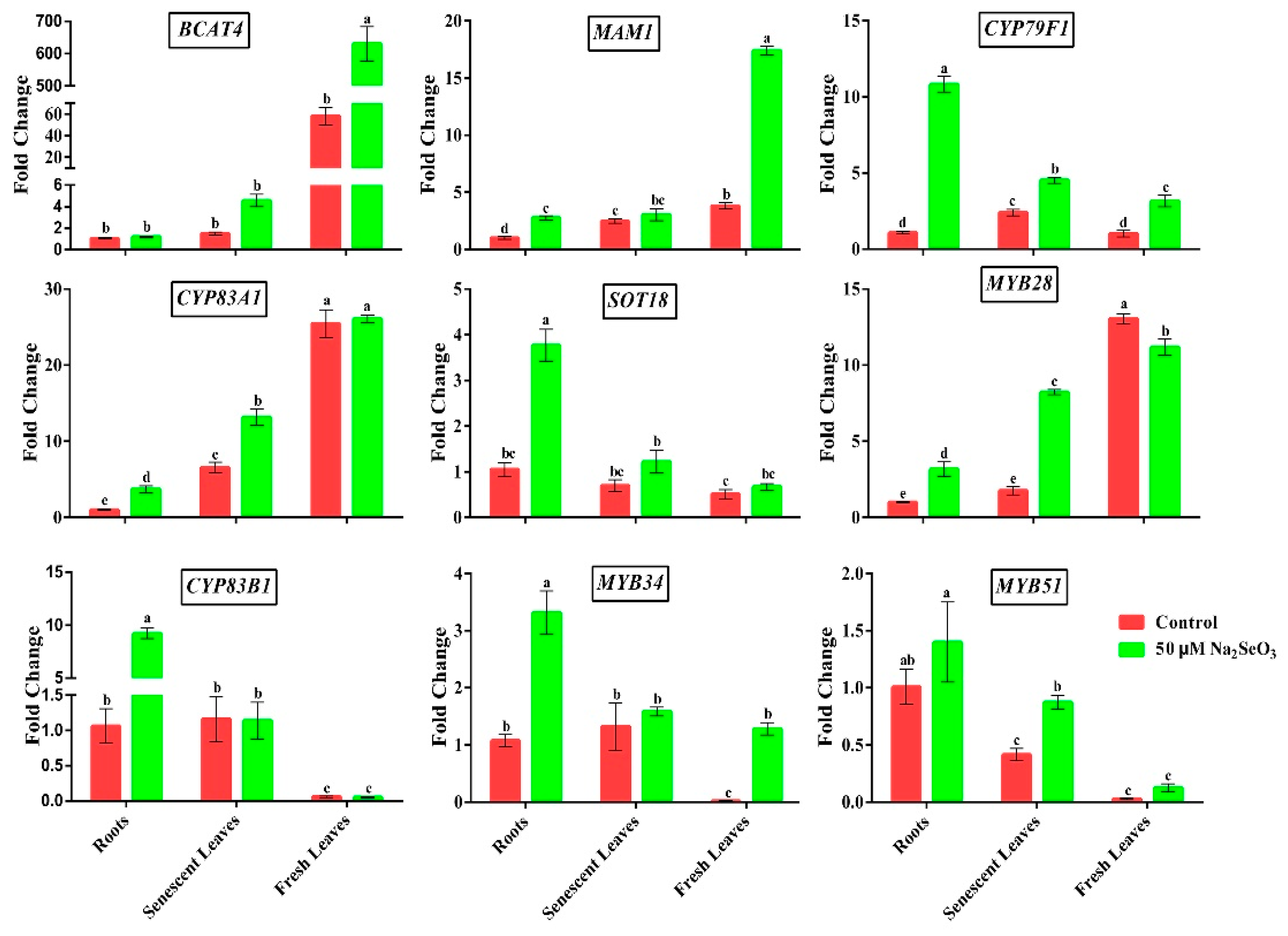
3.5. Effect of Se on the Transcript Levels of Genes Related to GSLs Biosynthesis
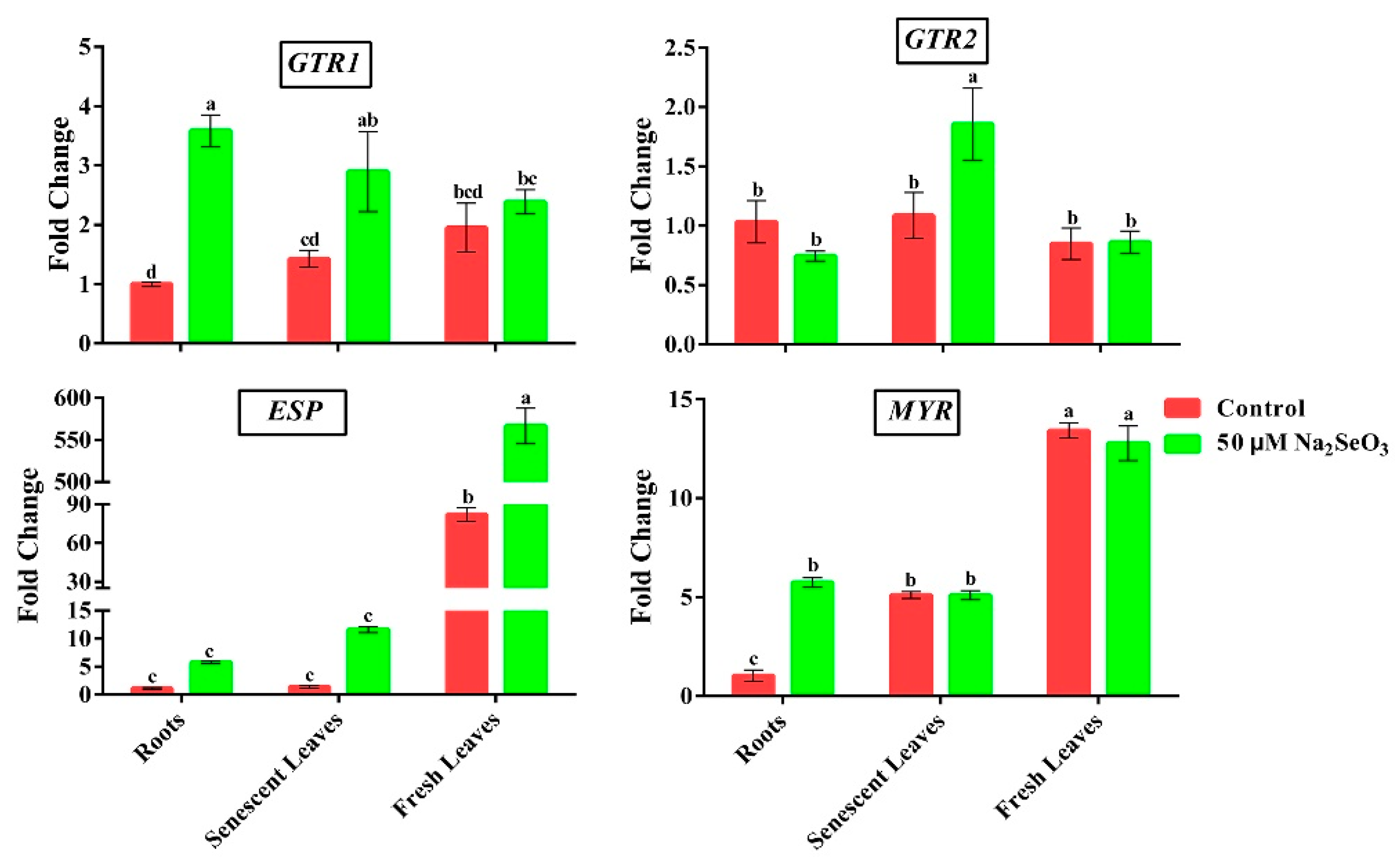
3.6. Effect of Se on the Transcript Levels of GSLs Transporters and 4MSOB Degradation Genes
4. Discussion
4.1. Se-Induced Total Sulfur and GSLs Accumulation in Broccoli Roots
4.2. Induction of 4MSOB Accumulation in Young Leaves by Se through the GSLs Transporters
4.3. Effect of Se Supplementation on Sulforaphane Content in Broccoli
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Xia, Y.; Liu, H.Y.; Gan, R.Y. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food. Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Winde, I.; Wittstock, U. Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry 2011, 72, 1566–1575. [Google Scholar] [CrossRef]
- Wei, L.Y.; Liu, C.H.; Zheng, H.H.; Zheng, L. Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chem. 2020, 307, 125562. [Google Scholar] [CrossRef]
- Sangkret, S.; Pongmalai, P.; Devahastin, S.; Chiewchan, N. Enhanced production of sulforaphane by exogenous glucoraphanin hydrolysis catalyzed by myrosinase extracted from Chinese flowering cabbage (Brassica rapa var. parachinensis). Sci. Rep. 2019, 9, 9882. [Google Scholar] [CrossRef]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane role in aging and neurodegeneration. GeroScience 2019, 41, 655–670. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Zhang, J.; Liu, Y.X.; Tian, C.P.; Zhou, J.M. An Arabidopsis Secondary Metabolite Directly Targets Expression of the Bacterial Type III Secretion System to Inhibit Bacterial Virulence. Cell Host Microbe 2020, 27, 601–613. [Google Scholar] [CrossRef]
- Stahl, E.; Bellwon, P.; Huber, S.; Schlaeppi, K.; Bernsdorff, F.; Zeier, J. Regulatory and Functional Aspects of Indolic Metabolism in Plant Systemic Acquired Resistance. Mol. Plant 2016, 9, 662–681. [Google Scholar] [CrossRef] [Green Version]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates-gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Li, Z.; Li, X.; Zhang, M. Genomic signatures of vegetable and oilseed allopolyploid Brassica juncea and genetic loci controlling the accumulation of glucosinolates. Plant Biotechnol. J. 2021, 19, 2619–2628. [Google Scholar] [CrossRef]
- Zuluaga, D.L.; Graham, N.S.; Klinder, A.; Marcotrigiano, A.R.; Aarts, M.G.M. Overexpression of the MYB29 transcription factor affects aliphatic glucosinolate synthesis in Brassica oleracea. Plant Mol. Biol. 2019, 101, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 Distinctly Regulate Indolic Glucosinolate Biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Huseby, S.; Koprivova, A.; Lee, B.R.; Saha, S.; Mithen, R.; Wold, A.B. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J. Exp. Bot. 2013, 64, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Esfandiari, A.; Saei, A.; McKenzie, M.J.; Matich, A.J.; Babalar, M.; Hunter, D.A. Preferentially enhancing anti-cancer isothiocyanates over glucosinolates in broccoli sprouts: How NaCl and salicylic acid affect their formation. Plant Physiol. Biochem. 2017, 115, 343–353. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; Avila, F.W.; Fish, T.; Yuan, Y.; Hui, M.X. Effects of selenium supplementation on glucosinolate biosynthesis in broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Ramos, S.J.; Yuan, Y.; Faquin, V.; Guilherme, L.R.; Li, L. Evaluation of Genotypic Variation of Broccoli (Brassica oleracea var. italic) in Response to Selenium Treatment. J. Agric. Food Chem. 2011, 5, 3657–3665. [Google Scholar] [CrossRef]
- Ávila, F.W.; Faquin, V.; Yang, Y.; Ramos, S.J.; Guilherme, L.R.G.; Thannhauser, T.W. Assessment of the Anticancer Compounds Se-Methylselenocysteine and Glucosinolates in Se-Biofortified Broccoli (Brassica oleracea L. var. italica) Sprouts and Florets. J. Agric. Food Chem. 2013, 61, 6216–6223. [Google Scholar] [CrossRef]
- Tsai, C.F.; Ou, B.R.; Liang, Y.C.; Yeh, J.Y. Growth inhibition and antioxidative status induced by selenium-enriched broccoli extract and selenocompounds in DNA mismatch repair-deficient human colon cancer cells. Food Chem. 2013, 139, 267–273. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Zhou, W. Selenium mitigates the chromium toxicity in Brassica napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Biochem. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Silva, V.M.; Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; Reis, A.R.D. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculate L. walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Han, D.; Tu, S.; Dai, Z.; Huang, W.; Jia, W.; Shao, H. Comparison of selenite and selenate in alleviation of drought stress in Nicotiana tabacum L. Chemosphere 2022, 287, 132136. [Google Scholar] [CrossRef]
- Johnson, L.J.; Meacham, S.L.; Kruskall, L.J. The antioxidants-vitamin C, vitamin E, selenium, and carotenoids. J. Agromed. 2003, 9, 65–82. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; McKenzie, M.J. Mechanisms of Selenium Enrichment and Measurement in Brassicaceous Vegetables, and Their Application to Human Health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Laing, A.M. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 4, 881. [Google Scholar] [CrossRef]
- Ren, G.X.; Ran, X.L.; Zeng, R.Y.; Chen, J.W.; Wang, Y.B.; Yang, G.H. Effects of sodium selenite spray on apple production, quality, and sucrose metabolism-related enzyme activity. Food Chem. 2020, 339, 127883. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Aro, A. Selenium Working Group. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Businelli, D. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 14, 4075–4097. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef]
- Ahmad, P.; Allah, E.F.; Hashem, A. Exogenous Application of Selenium Mitigates Cadmium Toxicity in Brassica juncea L. (Czern & Cross) by Up-Regulating Antioxidative System and Secondary Metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 44, 10542–10554. [Google Scholar] [CrossRef]
- Ávila, F.W.; Yang, Y.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.G.; Thannhauser, W.T. Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem. 2014, 165, 578–586. [Google Scholar] [CrossRef]
- Hsu, F.C.; Wirtz, M.; Heppel, S.C.; Bogs, J.; Krämer, U.; Khan, M.S. Generation of Se-fortified broccoli as functional food: Impact of Se fertilization on S metabolism. Plant Cell Environ. 2011, 34, 192–207. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Selenium Influences Glucosinolate and Isothiocyanates and Increases Sulfur Uptake in Arabidopsis thaliana and Rapid-Cycling Brassica oleracea. J. Agric. Food Chem. 2013, 61, 202–209. [Google Scholar] [CrossRef]
- Lyi, S.M.; Heller, L.I.; Rutzke, M.; Welch, R.M.; Kochian, L.V.; Li, L. Molecular and Biochemical Characterization of the Selenocysteine Se-Methyltransferase Gene and Se-Methylselenocysteine Synthesis in Broccoli. Plant Physiol. 2005, 138, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smits, E.A. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Charron, C.S.; Kopsell, D.A.; Randle, W.M.; Sams, C.E. Sodium selenate fertilization increases selenium accumulation and decreases glucosinolate concentration in rapid-cycling Brassica oleracea. J. Sci. Food Agric. 2011, 81, 962–966. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.Y.; Liu, F.X.; Fan, X.; Pan, S.Y. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018, 111, 205–211. [Google Scholar] [CrossRef]
- McKenzie, M.; Matich, A.; Hunter, D.; Esfandiari, A.; Trolove, S.; Lill, R. Selenium Application During Radish (Raphanus sativus) Plant Development Alters Glucosinolate Metabolic Gene Expression and Results in the Production of 4-(methylseleno)but-3-enyl glucosinolate. Plants 2019, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Pilon-Smits, E.A. Selenium Biofortification in Radish Enhances Nutritional Quality via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics, and Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.W.; Mao, S.X.; Xu, H.R.; Wu, Q.; Liang, M.T.; Wu, Q.Y. Effects of Sulfur and Selenium on Glucosinolate Biosynthesis in Cabbage. Plant Mol. Biol. Rep. 2019, 38, 62–74. [Google Scholar] [CrossRef]
- Gui, J.Y.; Rao, S.; Gou, Y.; Xu, F.; Cheng, S. Comparative study of the effects of selenium yeast and sodium selenite on selenium content and nutrient quality in broccoli florets (Brassica oleracea L. var. italica). J. Sci. Food Agric. 2022, 4, 1707–1718. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.A.; Hawkesford, M.; De Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant. Sci. 2014, 5, 704. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhao, Y.; Sun, J.; Yu, L.; Chen, P. Chemical profiling of glucosinolates in cruciferous vegetables-based dietary supplements using ultra-high performance liquid chromatography coupled to tandem high resolution mass spectrometry. J. Food Compos. Anal. 2017, 61, 67–72. [Google Scholar] [CrossRef]
- Wang, J.W.; Mao, S.X.; Wu, Q.; Yuan, Y.M.; Liang, M.T.; Wu, Q.Y. Effects of LED illumination spectra on glucosinolate and sulforaphane accumulation in broccoli seedlings. Food Chem. 2021, 356, 129550. [Google Scholar] [CrossRef]
- Mao, S.X.; Wang, J.Y.; Wu, Q.; Liang, M.T.; Yuan, Y.M.; Huang, K. Effect of selenium-sulfur interaction on the anabolism of sulforaphane in broccoli. Phytochemistry 2020, 179, 112499. [Google Scholar] [CrossRef]
- Zhuang, L.; Xua, K.X.; Zhua, Y.L.; Wang, F.W.; Xiao, J.X.; Guo, L.P. Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress. Food Chem. 2021, 334, 127520. [Google Scholar] [CrossRef]
- Lim, S.; Lee, E.J.; Kim, J. Decreased sulforaphane concentration and reduced myrosinase activity of radish (Raphanus sativus L.) root during cold storage. Postharvest Biol. Technol. 2015, 100, 219–225. [Google Scholar] [CrossRef]
- Huang, K.; Lin, J.C.; Wu, Q.Y.; Yan, J.Y.; Liu, M.Y.; Zhang, S.; Xiao, W.J. Changes in Sulforaphane and Selenocysteine Methyltransferase Transcript Levels in Broccoli Treated with Sodium Selenite. Plant Mol. Biol. Rep. 2016, 34, 807–814. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Honsel, A.; Kojima, M.; Haas, R. Sulphur limitation and early sulphur deficiency responses in poplar: Significance of gene expression, metabolites, and plant hormones. J. Exp. Bot. 2012, 63, 1873–1893. [Google Scholar] [CrossRef]
- Kim, Y.B.; Li, X.H.; Kim, S.J.; Kim, H.H.; Lee, J.; Kim, H.R. MYB Transcription Factors Regulate Glucosinolate Biosynthesis in Different Organs of Chinese Cabbage (Brassica rapa ssp. pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef] [Green Version]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta Gen. Subj. 2018, 11, 2333–2342. [Google Scholar] [CrossRef]
- Takahashi, H. Sulfate transport systems in plants: Functional diversity and molecular mechanisms underlying regulatory coordination. J. Exp. Bot. 2019, 19, 4075–4087. [Google Scholar] [CrossRef]
- Maruyama-Nakashita, A.; Watanabe-Takahashi, A.; Inoue, E.; Yamaya, T.; Saito, K.; Takahashib, H. Sulfur-Responsive Elements in the 3′-Nontranscribed Intergenic Region Are Essential for the Induction of SULFATE TRANSPORTER 2;1 Gene Expression in Arabidopsis Roots under Sulfur Deficiency. Plant Cell 2015, 27, 1279–1296. [Google Scholar] [CrossRef] [Green Version]
- Shroff, R.; Vergara, F.; Muck, A.; Svatos, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef] [Green Version]
- Matich, A.J.; McKenzie, M.J.; Lill, R.E.; McGhie, T.K.; Chen, R.K.-Y.; Rowan, D.D. Distribution of selenoglucosinolates and their metabolites in Brassica treated with sodium selenite. J. Agric. Food Chem. 2015, 63, 1896–1905. [Google Scholar] [CrossRef]
- Shanks, C.M.; Hecker, A.; Cheng, C.Y.; Brand, L.; Collani, S.; Kieber, J.J. Role of BASIC PENTACYSTEINE transcription factors in a subset of cytokinin signaling responses. Plant J. 2018, 95, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Nour-Eldin, H.H.; Andersen, T.H.; Burow, M.; Madsen, S.R.; Jørgensen, M.E.; Olsen, C.E. NRT/PTR transporters are essential for translocation of glucosinolate defense compounds to seeds. Nature 2013, 488, 531–534. [Google Scholar] [CrossRef]
- Madsen, S.R.; Olsen, C.E.; Nour-Eldin, H.H.; Halkier, B.A. Elucidating the role of transport processes in leaf glucosinolate distribution. Plant Physiol. 2014, 166, 1450–1462. [Google Scholar] [CrossRef]
- Andersen, T.G.; Nour-Eldin, H.H.; Fuller, V.L.; Olsen, C.E.; Burow, M.; Halkier, B.A. Integration of Biosynthesis and Long-Distance Transport Establish Organ-Specific Glucosinolate Profiles in Vegetative Arabidopsis. Plant Cell 2013, 25, 3133–3145. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.Y.; Hunziker, P.; Koroleva, O.; Blennow, A.; Crocoll, C.; Halkier, B.A. GTR-Mediated Radial Import Directs Accumulation of Defensive Glucosinolates to Sulfur-Rich Cells in the Phloem Cap of Arabidopsis Inflorescence Stem. Mol. Plant 2019, 12, 1474–1484. [Google Scholar] [CrossRef]
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jørgensen, M.E.; Olsen, C.E.; Andersen, J.S. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2017, 35, 377–382. [Google Scholar] [CrossRef]
- Jiang, D.; Lei, J.; Cao, B.; Wu, S.; Chen, G.; Chen, C. Molecular Cloning and Characterization of Three Glucosinolate Transporter (GTR) Genes from Chinese Kale. Genes 2019, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.F.; Lv, C.Z.; Zou, L.G.; Sun, J.; Song, X.J.; Mao, J.W. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Dosz, E.B.; Jeffery, E.H. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J. Funct. Foods 2013, 5, 987–990. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.Y.; Liu, Y.L.; Xie, L.; Pan, S.Y. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Kim, E.O.; Lim, S.J.; Nam, E.J.; Yun, J.H. Exposure of kale root to NaCl and Na2SeO3 increases isothiocyanate levels and Nrf2 signaling without reducing plant root growth. Sci. Rep. 2018, 8, 3999. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.; Carolina, E.; Pablo, V.; Jorge, P. Glucosinolates as an effective tool in plant-parasitic nematodes control: Exploiting natural plant defenses. Appl. Soil Ecol. 2022, 176, 104497. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef]
- Vanduchova, A.; Tomankova, V.; Anzenbacher, P.; Anzenbacherova, E. Influence of Sulforaphane Metabolites on Activities of Human Drug-Metabolizing Cytochrome P450 and Determination of Sulforaphane in Human Liver Cells. J. Med. Food 2016, 19, 1141–1146. [Google Scholar] [CrossRef]



| No. | Trivial Name | Precursor | Abbreviation | Chemical Name | Correction Coefficient |
|---|---|---|---|---|---|
| 1 | Glucoiberin | Met | 3MSOP | 3-(Methylsulfinyl)propyl GSL | 1 |
| 2 | Sinigrin | Met | PREN | 2-Propenyl GSL | 1 |
| 3 | Glucoraphanin | Met | 4MSOB | 4-(Methylsulfinyl)butyl GSL | 1 |
| 4 | 4-Hydroxyglucobrassicin | Trp | 4HOI3M | 4-Hydroxyindol-3-ylmethyl GSL | 0.28 |
| 5 | Glucobrassicin | Trp | I3M | Indol-3-ylmethyl GSL | 0.29 |
| 6 | 4-Methoxyglucobrassicin | Trp | 4MOI3M | 4-Methoxyindol-3-ylmethyl GSL | 0.25 |
| 7 | Neoglucobrassicin | Trp | 1MOI3M | 1-Methoxyindol-3-ylmethyl GSL | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Wang, J.; Huang, H.; Mao, S.; Wu, Q.; Huang, K. Exogenous Selenium Treatment Promotes Glucosinolate and Glucoraphanin Accumulation in Broccoli by Activating Their Biosynthesis and Transport Pathways. Appl. Sci. 2022, 12, 4101. https://doi.org/10.3390/app12094101
Wu Q, Wang J, Huang H, Mao S, Wu Q, Huang K. Exogenous Selenium Treatment Promotes Glucosinolate and Glucoraphanin Accumulation in Broccoli by Activating Their Biosynthesis and Transport Pathways. Applied Sciences. 2022; 12(9):4101. https://doi.org/10.3390/app12094101
Chicago/Turabian StyleWu, Qi, Junwei Wang, Huiping Huang, Shuxiang Mao, Qiuyun Wu, and Ke Huang. 2022. "Exogenous Selenium Treatment Promotes Glucosinolate and Glucoraphanin Accumulation in Broccoli by Activating Their Biosynthesis and Transport Pathways" Applied Sciences 12, no. 9: 4101. https://doi.org/10.3390/app12094101





