Bacterial Diversity and Dominant Spoilage Microorganisms in Fresh-Cut Broccoli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Pretreatment of Sample
2.3. Isolation of Bacteria
2.4. DNA Extraction
2.5. PCR Amplification
2.6. 16S rRNA Gene Phylogeny
2.7. Spoilage Characteristics of Representative Strains in Spoilage of Broccoli
2.7.1. Chlorophyll Content Determination
2.7.2. Nitrite Content Determination
2.7.3. MDA Content Assay
2.7.4. Soluble Sugar Content Analysis
2.7.5. Statistical Analysis for Spoilage Characteristic of Microorganisms in Broccoli
3. Results
3.1. Changes in Bacterial Counts and Isolates Separated from Broccoli
3.2. Identification of Bacterial Isolation
3.3. Effects of Spoilage Microorganisms on the Quality of Broccoli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; González-Gómez, D. Different postharvest strategies to preserve broccoli quality during storage and shelf life: Controlled atmosphere and 1-MCP. Food Chem. 2013, 138, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, L.; Cao, L.; Zhang, Q.; Song, Q.; Meng, Z.; Wu, X.; Xu, K. Inhibition of autophagy potentiates the anti-metastasis effect of phenethyl isothiocyanate through JAK2/STAT3 pathway in lung cancer cells. Mol. Carcinog. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Moreira, M.; Roura, S.I.; Ayala-Zavala, J.F.; González Aguilar, G.A. Using natural antimicrobials to enhance the safety and quality of fresh and processed fruits and vegetables: Types of antimicrobials—ScienceDirect. In Handbook of Natural Antimicrobials for Food Safety and Quality; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–313. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixid, N. Application of Modified Atmosphere Packaging as a safety approach to fresh-cut fruits and vegetables—A review. Trends Food Sci. Tech. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, Y. First report of postharvest fruit rot caused by Fusarium equiseti on stored cerasus Pseudocerasus in China. Plant. Dis. 2017, 101, 1451. [Google Scholar] [CrossRef]
- Dakwa, V.; Powell, S.; Eyles, A.; Gracie, A.; Tamplin, M.; Ross, S. Effect of peroxyacetic acid treatment and bruising on the bacterial community and shelf-life of baby spinach. Int. J. Food Microbiol. 2021, 343, 109086. [Google Scholar] [CrossRef]
- Bah, A.; Ferjani, R.; Fhoula, I.; Gharbi, Y.; Najjari, A.; Boudabous, A.; Ouzari, H.I. Microbial community dynamic in tomato fruit during spontaneous fermentation and biotechnological characterization of indigenous lactic acid bacteria. Ann. Microbiol. 2019, 69, 41–49. [Google Scholar] [CrossRef]
- Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of bacteriocin-producing lactic acid bacteria and bifidobacterium isolated from honeycombs against spoilage microorganisms and pathogens isolated from fruits and vegetables. Appl. Sci. 2020, 10, 7309. [Google Scholar] [CrossRef]
- Maffei, D.F.; Batalha, E.Y.; Landgraf, M.; Schaffner, D.W.; Franco, B.D. Microbiology of organic and conventionally grown fresh produce. Braz. J. Microbiol. 2016, 47, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Stoll, D.A.; Müller, A.; Meinhardt, A.K.; Dötsch, A.; Greiner, R.; Kulling, S.E.; Huch, M. Influence of salt concentration and iodized table salt on the microbiota of fermented cucumbers. Food Microbiol. 2020, 92, 103552. [Google Scholar] [CrossRef] [PubMed]
- Nahidul-Islam, S.M.; Takashi, K.; Hajime, T.; Bon, K. Bacterial and fungal microbiota in traditional Bangladeshi fermented milk products analysed by culture-dependent and culture-independent methods. Food Res. Int. 2018, 111, 431–437. [Google Scholar] [CrossRef]
- Rocha, K.E.M.; Milani, C.; Ventura, M.; Valencia, F.E.; Madiedo, R.; Rusa-Madiedo, P.; Delgado, S. Bacterial diversity of the Colombian fermented milk “Suero Costeño” assessed by culturing and high-throughput sequencing and DGGE analysis of 16S rRNA gene amplicons. Food Microbiol. 2017, 68, 129–136. [Google Scholar]
- Liang, H.; Yin, L.; Zhang, Y.; Chang, C.; Zhang, W. Dynamics and diversity of a microbial community during the fermentation of industrialized Qingcai paocai, a traditional Chinese fermented vegetable food, as assessed by Illumina MiSeq sequencing, DGGE and qPCR assay. Ann. Microbiol. 2018, 68, 111–122. [Google Scholar] [CrossRef]
- Li, R.; Cai, L.; Gao, T.; Li, C.; Ye, K. Comparing the quality characteristics and bacterial communities in meatballs with or without blown pack spoilage. LWT Food Sci. Technol. 2020, 130, 109529. [Google Scholar] [CrossRef]
- Li, W.J.; Xu, P.; Schumann, P.; Zhang, Y.Q.; Pukall, R.; Xu, L.H.; Stackebrandt, E.; Jiang, C.L. Georgenia ruanii sp. nov. a novel actinobacterium isolated from forest soil in Yunnan (China), and emended description of the genus Georgenia. Int. J. Syst. Evol. Microbiol. 2007, 57, 1424–1428. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 38, 14–26. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, H.; Ji, S. Potential of jasmonic acid (JA) in accelerating postharvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chem. 2020, 309, 125737. [Google Scholar] [CrossRef] [PubMed]
- Nerdy, N.; Putra, E. Spectrophotometric method for determination of nitrite and nitrate levels in broccoli and cauliflower with different fertilization treatment. Orient J Chem. 2018, 34, 2983–2991. [Google Scholar] [CrossRef]
- An, F.; Sun, H.; Wu, J.; Zhao, C.; Li, T.; Huang, H.; Fang, Q.; Mu, E.; Wu, R. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res. Int. 2021, 147, 110543. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Ma, L. A combination of 1-methylcyclopropene treatment and intermittent warming alleviates chilling injury and affects phenolics and antioxidant activity of peach fruit during storage. Sci. Hortic. 2018, 229, 175–181. [Google Scholar] [CrossRef]
- Fairbairn, N.J. A modified anthrone reagent. Chem. and Ind. 1953, 4, 86. [Google Scholar]
- Rezaei, M.; Fani, A.; Moini, A.L.; Mirzajani, P.; Malekirad, A.A.; Rafiei, M. Determining Nitrate and Nitrite content in beverages, fruits, vegetables, and stews marketed in Arak, Iran. Int. Sch. Res. Not. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Devleesschauwer, B.; Marvasi, M.; Giurcanu, M.C.; Hochmuth, G.J.; Speybroeck, N.; Havelaar, A.H.; Teplitski, M. High relative humidity pre-harvest reduces post-harvest proliferation of Salmonella in tomatoes. Food Microbiol. 2017, 66, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Rao, J.; Wu, Q.; Wu, D.; Chen, K. The effect of indirect plasma-processed air pretreatment on the microbial loads, decay, and metabolites of Chinese bayberries. LWT Food Sci. Technol. 2021, 150, 111998. [Google Scholar] [CrossRef]
- Pinto, L.; Ippolito, A.; Baruzzi, F. Control of spoiler Pseudomonas spp. on fresh cut vegetables by neutral electrolyzed water. Food Microbiol. 2015, 50, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Kim, J.B.; Kim, M.; Roh, E.; Jung, K.; Choi, M.; Changsik, O.H.; Choi, J.; Yun, J.; Heu, S. Microbiota on spoiled vegetables and their characterization. J. Food Prot. 2013, 76, 1350–1358. [Google Scholar]
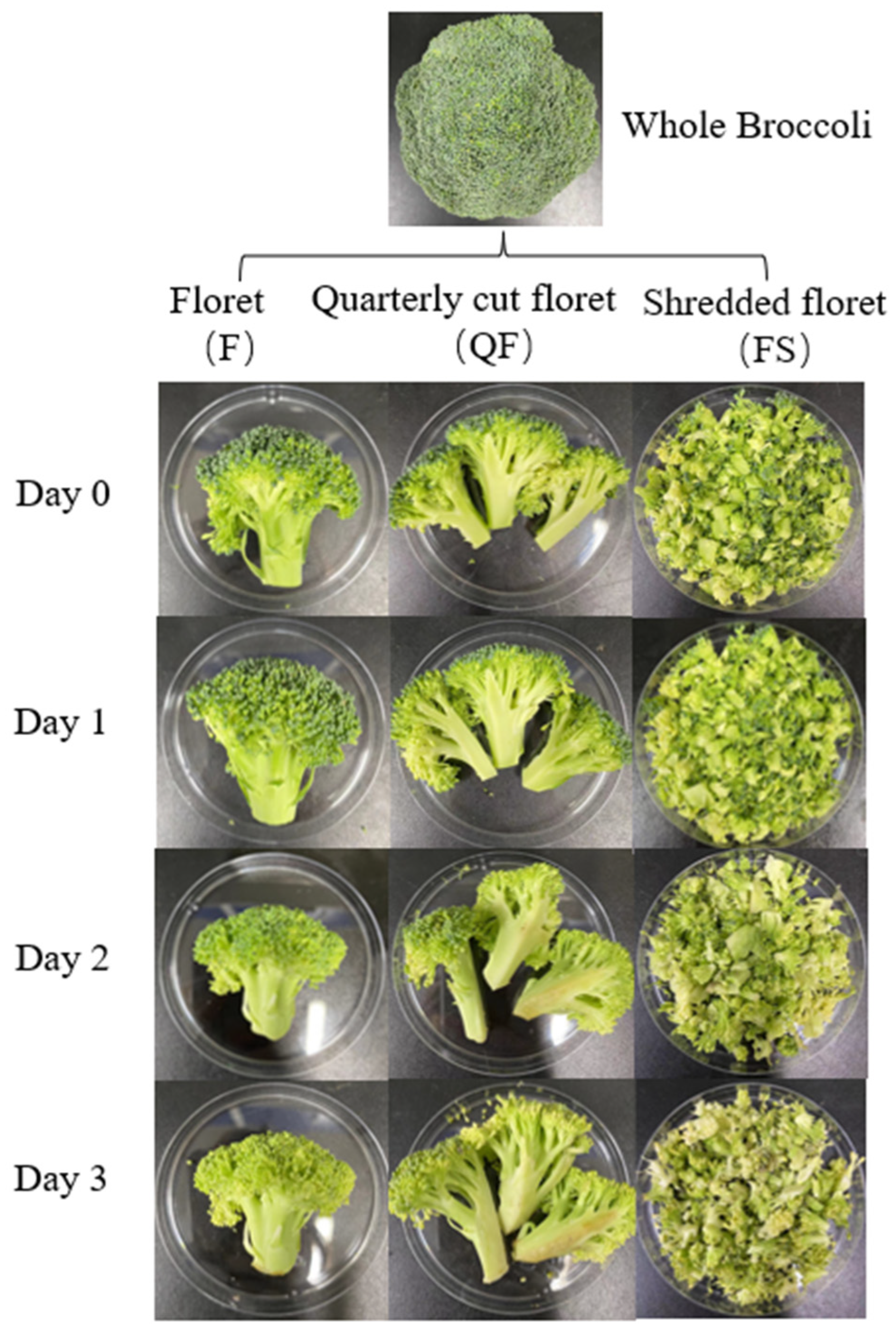





| Sample | Strain Number | Cutting Type | Color of Broccoli | Peculiar Smell | Storage Time (d) |
|---|---|---|---|---|---|
| A | BRO-A1~BRO-A20 | Floret | Green | No | 0 |
| B | BRO-B1~BRO-B21 | Floret | Green | No | 1 |
| C | BRO-C1~BRO-C12 | Floret | Light green | Minor | 2 |
| D | BRO-D1~BRO-D13 | Floret | Light yellow | Minor | 3 |
| E | BRO-E1~BRO-E9 | Quarterly cut floret | Light yellow | Minor | 3 |
| F | BRO-F1~BRO-F6 | Shredded floret | Yellow | Intense | 3 |
| G | BRO-G1~BRO-G16 | Shredded floret | Yellow | Intense | 3 |
| Phylum | Species | Strain No. | Similarity Range (%) | Reference Strain | Percentage (%) |
|---|---|---|---|---|---|
| Proteobacteria | Pseudomonas capeferrum | BRO-B1 BRO-B7 BRO-B3 BRO-D9 BRO-C1 BRO-B18 BRO-C9 BRO-C3 BRO-C11 | 99.29–99.60 | Pseudomonas capeferrum WCS358T | 9.28 |
| Pseudomonas alloputida | BRO-A11 BRO-E2 BRO-E3 BRO-G13 BRO-F2 BRO-E1 BRO-G12 | 99.86–100.00 | Pseudomonas alloputida Kh7T | 7.22 | |
| Pseudomonas rhizoryzae | BRO-A19 | 99.47 | Pseudomonas rhizoryzae RY24T | 1.03 | |
| Pseudomonas qingdaonensis | BRO-D11 | 100.00 | Pseudomonas qingdaonensis JJ3T | 1.03 | |
| Pseudomonas baetica | BRO-C4 | 99.58 | Pseudomonas baetica a390T | 1.03 | |
| Pseudomonas rhodesiae | BRO-C6 | 99.72 | Pseudomonas rhodesiae CIP 104664T | 1.03 | |
| Pseudomonas mosselii | BRO-D8 | 100.00 | Pseudomonas mosselii CIP 105259T | 1.03 | |
| Pseudomonas juntendi | BRO-B16 | 99.64 | Pseudomonas juntendi BML3T | 1.03 | |
| Pseudomonas entomophila | BRO-B19 | 99.83 | Pseudomonas entomophila L48T | 1.03 | |
| Pseudomonas atacamensis | BRO-E4 BRO-A1 BRO-A7 BRO-B2 | 99.84–100.00 | Pseudomonas atacamensis M7D1T | 4.12 | |
| Pseudomonas sichuanensis | BRO-B8 | 100.00 | Pseudomonas sichuanensis WCHPs060039T | 1.03 | |
| Pseudomonas oleovorans subsp. oleovorans | BRO-B14 BRO-G9 BRO-G10 | 98.87–99.71 | Pseudomonas oleovorans subsp. oleovorans DSM 1045T | 3.09 | |
| Acinetobacter johnsonii | BRO-B5 BRO-B10 BRO-D10 BRO-F3 BRO-B21 | 98.67–99.30 | Acinetobacter johnsonii CIP 64.6T | 5.15 | |
| Acinetobacter pittii | BRO-B11 BRO-D3 BRO-B4 BRO-G7 BRO-A5 BRO-G6 BRO-G1 | 99.86–100.00 | Acinetobacter pittii CIP 70.29T | 7.22 | |
| Acinetobacter oryzae | BRO-B12 BRO-B15 BRO-D5 BRO-F1 BRO-B20 BRO-C12 BRO-E6 | 98.98–99.36 | Acinetobacter oryzae B23T | 7.22 | |
| Acinetobacter guillouiae | BRO-D13 BRO-G16 | 98.75–98.86 | Acinetobacter guillouiae CIP 63.46T | 2.06 | |
| Acinetobacter junii | BRO-A17 BRO-D6 | 99.33 | Acinetobacter junii CIP 64.5T | 2.06 | |
| Acinetobacter proteolyticus | BRO-A13 | 100.00 | Acinetobacter proteolyticus NIPH 809T | 1.03 | |
| Acinetobacter bereziniae | BRO-C8 BRO-D7 | 99.85–100.00 | Acinetobacter bereziniae LMG 1003T | 2.06 | |
| Acinetobacter calcoaceticus | BRO-G4 | 100.00 | Acinetobacter calcoaceticus DSM 30006T | 1.03 | |
| Comamonas aquatica | BRO-B6 BRO-D12 BRO-G3 | 99.85–100.00 | Comamonas aquatica NBRC 14918T | 3.09 | |
| Comamonas koreensis | BRO-A12 | 98.86 | Comamonas koreensis KCTC 12005T | 1.03 | |
| Comamonas testosteroni | BRO-A14 BRO-A16 BRO-G8 BRO-C5 | 99.85–100.00 | Comamonas testosteroni ATCC 11996T | 4.12 | |
| Stenotrophomonas maltophilia | BRO-A2 BRO-F5 | 99.21–99.34 | Stenotrophomonas maltophilia MTCC 434T | 2.06 | |
| Stenotrophomonas pavanii | BRO-F4 | 99.86 | Stenotrophomonas pavanii DSM 25135T | 1.03 | |
| Stenotrophomonas rhizophila | BRO-A9 | 99.86 | Stenotrophomonas rhizophila DSM 14405T | 1.03 | |
| Stenotrophomonas terrae | BRO-A10 | 99.59 | Stenotrophomonas terrae DSM 18941T | 1.03 | |
| Stenotrophomonas lactitubi | BRO-C10 | 99.37 | Stenotrophomonas lactitubi M15T | 1.03 | |
| Stenotrophomonas indicatrix | BRO-F6 | 99.72 | Stenotrophomonas indicatrix WS40T | 1.03 | |
| Brevundimonas vesicularis | BRO-A8 | 100.00 | Brevundimonas vesicularis NBRC 12165T | 1.03 | |
| Brevundimonas diminuta | BRO-A18 | 99.35 | Brevundimonas diminuta ATCC 11568T | 1.03 | |
| Agrobacterium arsenijevicii | BRO-A4 | 98.13 | Agrobacterium arsenijevicii KFB 330T | 1.03 | |
| Alcaligenes faecalis subsp. phenolicus | BRO-G2 BRO-G5 | 99.72–99.73 | Alcaligenes faecalis subsp. phenolicus DSM 16503T | 2.06 | |
| Escherichia hermannii | BRO-A3 | 99.87 | Escherichia hermannii CIP 103176T | 1.03 | |
| Leclercia adecarboxylata | BRO-B13 | 100.00 | Leclercia adecarboxylata NBRC 102595T | 1.03 | |
| Lelliottia jeotgali | BRO-C7 | 99.28 | Lelliottia Jeotgali PFL01T | 1.03 | |
| Enterobacter chengduensis | BRO-G14 | 99.85 | Enterobacter chengduensis WCHECl-C4T | 1.03 | |
| Delftia tsuruhatensis | BRO-G11 | 100.00 | Delftia tsuruhatensis NBRC 16741T | 1.03 | |
| Proteus mirabilis | BRO-E8 | 99.86 | Proteus mirabilis ATCC 29906T | 1.03 | |
| Pectobacterium carotovorum | BRO-D2 | 99.72 | Pectobacterium carotovorum NCPPB 312T | 1.03 | |
| Actinobacteria | Glutamicibacter arilaitensis | BRO-D4 | 99.13 | Glutamicibacter arilaitensis Re117T | 1.03 |
| Glutamicibacter nicotianae | BRO-C2 | 99.55 | Glutamicibacter nicotianae NBRC 14234T | 1.03 | |
| Microbacterium algeriense | BRO-B9 | 99.47 | Microbacterium algeriense G1T | 1.03 | |
| Rothia marina | BRO-D1 | 99.75 | Rothia marina JSM 078151T | 1.03 | |
| Brevibacterium iodinum | BRO-A6 | 99.13 | Brevibacterium iodinum NCDO 613T | 1.03 | |
| Bacteroidetes | Sphingobacterium faecium | BRO-E5 BRO-A15 | 99.26–99.43 | Sphingobacterium faecium DSM 11690T | 2.06 |
| Myroides odoratus | BRO-E9 BRO-E7 BRO-B17 BRO-G15 | 99.17–100.00 | Myroides odoratus DSM 2801T | 4.12 | |
| Firmicutes | Lysinibacillus fusiformis | BRO-A20 | 99.59 | Lysinibacillus fusiformis NBRC 15717T | 1.03 |
| Storage Time (d) | Chlorophyll (mg/g(FW)) | Nitrite (mg/kg) | MDA (nmol/g(FW)) | Soluble Sugar (mg/g(FW)) | |
|---|---|---|---|---|---|
| 0 | 0.39 ± 0.01 A | 0.31 ± 0.03 BCD | 1.62 ± 0.07 CD | 2.66 ± 0.03 BCD | |
| control | 1 | 0.33 ± 0.02 aB | 0.35 ± 0.02 cA | 4.65 ± 0.04 aB | 2.70 ± 0.04 bB |
| 2 | 0.29 ± 0.00 aC | 0.11 ± 0.02 cD | 7.39 ± 0.22 cA | 2.83 ± 0.01 dA | |
| 3 | 0.27 ± 0.01 aC | 0.16 ± 0.01 eC | 4.79 ± 0.07 aB | 2.86 ± 0.06 bA | |
| Pseudomonas sp. BRO-C11 | 1 | 0.19 ± 0.01 dB | 1.61 ± 0.09 aA | 3.85 ± 0.15 bB | 3.17 ± 0.02 aB |
| 2 | 0.18 ± 0.01 bB | 0.77 ± 0.02 aB | 11.67 ± 0.04 aA | 3.54 ± 0.05 aA | |
| 3 | 0.13 ± 0.01 dC | 0.66 ± 0.00 aC | 3.20 ± 0.15 bC | 2.94 ± 0.05 bC | |
| Pseudomonas sp. BRO-G12 | 1 | 0.23 ± 0.01 cB | 0.71 ± 0.04 bA | 3.25 ± 0.03 cB | 3.16 ± 0.03 aB |
| 2 | 0.17 ± 0.01 bC | 0.23 ± 0.04 bC | 8.72 ± 0.03 bA | 3.17 ± 0.02 bB | |
| 3 | 0.16 ± 0.01 cC | 0.34 ± 0.01 cB | 3.03 ± 0.11 bC | 3.25 ± 0.03 aA | |
| Acinetobacter sp. BRO-F1 | 1 | 0.32 ± 0.01 aB | 0.36 ± 0.02 cA | 3.59 ± 0.06 bB | 2.69 ± 0.03 bB |
| 2 | 0.27 ± 0.02 aC | 0.12 ± 0.01 cD | 7.23 ± 0.02 cA | 2.85 ± 0.04 dA | |
| 3 | 0.24 ± 0.00 bD | 0.24 ± 0.02 dC | 3.24 ± 0.15 bC | 2.90 ± 0.05 bA | |
| Comamonas sp. BRO-C5 | 1 | 0.27 ± 0.01 bB | 0.35 ± 0.02 cB | 2.77 ± 0.11 cB | 2.73 ± 0.06 bB |
| 2 | 0.17 ± 0.02 bC | 0.23 ± 0.04 bC | 6.59 ± 0.04 dA | 2.96 ± 0.03 cA | |
| 3 | 0.14 ± 0.01 dD | 0.38 ± 0.01 bA | 2.47 ± 0.19 cC | 2.66 ± 0.02 cB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Yu, X.; Chen, Y.; Zhang, J.; Liu, G. Bacterial Diversity and Dominant Spoilage Microorganisms in Fresh-Cut Broccoli. Appl. Sci. 2022, 12, 3370. https://doi.org/10.3390/app12073370
Cui S, Yu X, Chen Y, Zhang J, Liu G. Bacterial Diversity and Dominant Spoilage Microorganisms in Fresh-Cut Broccoli. Applied Sciences. 2022; 12(7):3370. https://doi.org/10.3390/app12073370
Chicago/Turabian StyleCui, Siqi, Xiaolu Yu, Ya Chen, Jianli Zhang, and Guangmin Liu. 2022. "Bacterial Diversity and Dominant Spoilage Microorganisms in Fresh-Cut Broccoli" Applied Sciences 12, no. 7: 3370. https://doi.org/10.3390/app12073370






