Application of Light-Emitting Diode Lights to Bluefin Sea Robin (Chelidonichthys spinosus) Catch in Pot Fishery in the South Sea, Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. LED Lights
2.2. Experimental Fishing Gear
2.3. Field Experiments
2.4. Stomach Contents
2.5. Data Analysis
3. Results
3.1. Field Experiments
3.2. Stomach Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamada, U. Fishes of the East China Sea and the Yellow Sea; Seikai Regional Fisheries Research Laboratory: Nagasaki, Japan, 1986; pp. 68–69. [Google Scholar]
- Kim, I.S.; Choi, Y.; Lee, C.L.; Lee, Y.J.; Kim, B.J.; Kim, J.H. Illustrated Book of Korean Fishes; Kyohak Publishing: Seoul, Korea, 2005; pp. 228–229. [Google Scholar]
- KOSIS (Korean Statistical Information Service). 2022, Volume 2022. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1EW0005&vw_cd=MT_ZTITLE&list_id=K2_7&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=MT_ZTITLE&path=%252FstatisticsList%252FstatisticsListIndex.do (accessed on 10 July 2021).
- Kunishige, N. On the age and growth of the gurnard, Chelidonichthys spinousus, in the East China and the Yellow Seas. Bull. Seikai Reg. Fish. Res. Lab. 1965, 34, 133–147. [Google Scholar]
- Kozo, S.; Tomiko, S.; Kunishige, N.; Junko, N. On feeding habit of gunard, Chelidonichthys spinousus, in the East China Sea and the Yellow Sea. Bull. Seikai Reg. Fish Res. Lab. 1965, 33, 47–59. [Google Scholar]
- Huh, S.-H.; Park, J.M.; Baeck, G.W. Feeding Habits of Bluefin Searobin (Chelidonichthys spinosus) in the Coastal Waters off Busan. Korean J. Ichthyol. 2007, 19, 51–56. [Google Scholar]
- Kim, J.-B.; Kim, J.-Y.; Lee, D.-W.; Choi, J.-H. Feeding Habits of Bluefin Searobin (Chelidonichthys spinosus) around Jeju Island. Korean J. Fish. Aquat. Sci. 2011, 44, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.J. Effectiveness of Crab and Lobster Traps. Can. J. Fish. Aquat. Sci. 1990, 47, 1228–1251. [Google Scholar] [CrossRef]
- Suuronen, P.; Chopin, F.; Glass, C.; Løkkeborg, S.; Matsushita, Y.; Queirolo, D.; Rihan, D. Low impact and fuel efficient fishing—Looking beyond the horizon. Fish. Res. 2012, 119–120, 135–146. [Google Scholar] [CrossRef]
- Tran, P.D.; Nguyen, L.T.; To, P.V.; Nguyen, K.Q. Effects of the trap entrance designs on the catch efficiency of swimming crab Charybdis feriata fishery. Fish. Res. 2020, 232, 105730. [Google Scholar] [CrossRef]
- Major, R.N.; Taylor, D.I.; Connor, S.; Connor, G.; Jeffs, A. Factors affecting bycatch in a developing New Zealand scampi potting fishery. Fish. Res. 2017, 186, 55–64. [Google Scholar] [CrossRef]
- Hébert, M.; Miron, G.; Moriyasu, M.; Vienneau, R.; DeGrâce, P. Efficiency and ghost fishing of snow crab (Chionoecetes opilio) traps in the Gulf of St. Lawrence. Fish. Res. 2001, 52, 143–153. [Google Scholar] [CrossRef]
- Winger, P.D.; Walsh, P.J. The feasibility of escape mechanisms in conical snow crab traps. ICES J. Mar. Sci. 2007, 64, 1587–1591. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.-C.; Park, C.-D.; Park, S.-W.; Lee, J.-H.; Tokai, T. Size selectivity of trap for male red queen crab Chionoecetes japonicus with the extended SELECT model. Fish. Sci. 2000, 66, 494–501. [Google Scholar] [CrossRef]
- Olsen, L.; Herrmann, B.; Grimaldo, E.; Sistiaga, M. Effect of pot design on the catch efficiency of snow crabs (Chionoecetes opilio) in the Barents Sea fishery. PLoS ONE 2019, 14, e0219858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petetta, A.; Vasapollo, C.; Virgili, M.; Bargione, G.; Lucchetti, A. Pots vs trammel nets: A catch comparison study in a Mediterranean small-scale fishery. PeerJ 2020, 8, e9287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.Q.; Humborstad, O.-B.; Løkkeborg, S.; Winger, P.; Bayse, S.M. Effect of light-emitting diodes (LEDs) on snow crab catch rates in the Barents Sea pot fishery. ICES J. Mar. Sci. 2019, 76, 1893–1901. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.Q.; Winger, P. A trap with light-emitting diode (LED) lights: Evaluating the effect of location and orientation of lights on the catch rate of snow crab (Chionoecetes opilio). Aquac. Fish. 2019, 4, 255–260. [Google Scholar] [CrossRef]
- Matsushita, Y.; Azuno, T.; Yamashita, Y. Fuel reduction in coastal squid jigging boats equipped with various combinations of conventional metal halide lamps and low-energy LED panels. Fish. Res. 2012, 125–126, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Matsushita, Y.; Azuno, T. Catch performance of coastal squid jigging boats using LED panels in combination with metal halide lamps. Fish. Res. 2012, 113, 182–189. [Google Scholar] [CrossRef] [Green Version]
- An, Y.-I.; Jeong, H.-G. Catching efficiency of LED fishing lamp and behavioral reaction of common squid Todarodes pacificus to the shadow section of color LED light. Bull. Korean Soc. Fish. Technol. 2011, 47, 183–193. [Google Scholar] [CrossRef]
- An, Y.-I.; He, P.; Arimoto, T.; Jang, U.-J. Catch performance and fuel consumption of LED fishing lamps in the Korea hairtail angling fishery. Fish. Sci. 2017, 83, 343–352. [Google Scholar] [CrossRef]
- Hannah, R.W.; Lomeli, M.J.; Jones, S.A. Tests of artificial light for bycatch reduction in an ocean shrimp (Pandalus jordani) trawl: Strong but opposite effects at the footrope and near the bycatch reduction device. Fish. Res. 2015, 170, 60–67. [Google Scholar] [CrossRef]
- Ortiz, N.; Mangel, J.C.; Wang, J.; Shigueto, J.A.; Pingo, S.; Jimenez, A.; Suarez-Yana, T.; Swimmer, Y.; Carvalho, F.; Godley, B. Reducing green turtle bycatch in small-scale fisheries using illuminated gillnets: The cost of saving a sea turtle. Mar. Ecol. Prog. Ser. 2016, 545, 251–259. [Google Scholar] [CrossRef]
- Lomeli, M.J.M.; Groth, S.D.; Blume, M.T.O.; Herrmann, B.; Wakefield, W.W. Effects on the bycatch of eulachon and juvenile groundfish by altering the level of artificial illumination along an ocean shrimp trawl fishing line. ICES J. Mar. Sci. 2018, 75, 2224–2234. [Google Scholar] [CrossRef]
- Virgili, M.; Vasapollo, C.; Lucchetti, A. Can ultraviolet illumination reduce sea turtle bycatch in Mediterranean set net fisheries? Fish. Res. 2018, 199, 1–7. [Google Scholar] [CrossRef]
- Bryhn, A.C.; Königson, S.J.; Lunneryd, S.-G.; Bergenius, M.A. Green lamps as visual stimuli affect the catch efficiency of floating cod (Gadus morhua) pots in the Baltic Sea. Fish. Res. 2014, 157, 187–192. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Winger, P.D.; Morris, C.; Grant, S.M. Artificial lights improve the catchability of snow crab (Chionoecetes opilio) traps. Aquac. Fish. 2017, 2, 124–133. [Google Scholar] [CrossRef]
- Humborstad, O.-B.; Utne-Palm, A.C.; Breen, M.; Løkkeborg, S. Artificial light in baited pots substantially increases the catch of cod (Gadus morhua) by attracting active bait, krill (Thysanoessa inermis). ICES J. Mar. Sci. 2018, 75, 2257–2264. [Google Scholar] [CrossRef]
- Marchesan, M.; Spoto, M.; Verginella, L.; Ferrero, E.A. Behavioural effects of artificial light on fish species of commercial interest. Fish. Res. 2005, 73, 171–185. [Google Scholar] [CrossRef]
- An, Y.-I.; Jeong, H.-G.; Jung, B.-M. Behavioral reaction of common squid Todarodes pacificus to different colors of LED Light. Bull. Korean Soc. Fish. Technol. 2009, 45, 135–143. [Google Scholar] [CrossRef]
- Larsen, R.B.; Herrmann, B.; Sistiaga, M.; Brinkhof, J.; Tatone, I.; Langård, L. Performance of the Nordmøre Grid in Shrimp Trawling and Potential Effects of Guiding Funnel Length and Light Stimulation. Mar. Coast. Fish. 2017, 9, 479–492. [Google Scholar] [CrossRef]
- Larsen, R.B.; Herrmann, B.; Sistiaga, M.; Brčić, J.; Brinkhof, J.; Tatone, I. Could green artificial light reduce bycatch during Barents Sea Deep-water shrimp trawling? Fish. Res. 2018, 204, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Grimaldo, E.; Sistiaga, M.; Herrmann, B.; Larsen, R.B.; Brinkhof, J.; Tatone, I. Improving release efficiency of cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) in the Barents Sea demersal trawl fishery by stimulating escape behaviour. Can. J. Fish. Aquat. Sci. 2018, 75, 402–416. [Google Scholar] [CrossRef] [Green Version]
- Melli, V.; Krag, L.A.; Herrmann, B.; Karlsen, J.D. Investigating fish behavioural responses to LED lights in trawls and potential applications for bycatch reduction in the Nephrops-directed fishery. ICES J. Mar. Sci. 2018, 75, 1682–1692. [Google Scholar] [CrossRef] [Green Version]
- Baeck, G.W.; Kim, H.J.; Jeong, J.M. Diet Composition of Bullet Mackerel, Auxis rochei (Risso, 1810) in the Coastal Waters of Iloilo, Philippines. Korean J. Ichthyol. 2014, 26, 349–354. [Google Scholar]
- Pinkas, L. Food habits of albacore, bluefin tuna and bonito in California waters. Calif. Dept. Fish Game Fish Bull. 1971, 152, 1–105. [Google Scholar]
- Ciriaco, S.; Marchesan, M.; Verginella, L.; Vinzi, E.; Ferrero, E.A.; Spoto, M. Preliminary observations on the effects of artificial light on the marine environment, with special reference to three fish species of commercial value protected by Miramare Marine Reserve. Boll. Geof. Teor. App. 2003, 44, 19–26. [Google Scholar]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Vázquez, F.J.S. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Kehayias, G.; Bouliopoulos, D.; Chiotis, N.; Koutra, P. A photovoltaic-battery-LED lamp raft design for purse seine fishery: Application in a large Mediterranean lake. Fish. Res. 2011, 109, 107–113. [Google Scholar] [CrossRef]
- Utne-Palm, A.C.; Breen, M.; Løkkeborg, S.; Humborstad, O.-B. Behavioural responses of krill and cod to artificial light in laboratory experiments. PLoS ONE 2018, 13, e0190918. [Google Scholar] [CrossRef] [Green Version]
- Winger, P.D.; Walsh, P.J. Selectivity, efficiency, and underwater observations of modified trap designs for the snow crab (Chionoecetes opilio) fishery in Newfoundland and Labrador. Fish. Res. 2011, 109, 107–113. [Google Scholar] [CrossRef]
- Terrats, A.; Petrakis, G.; Papaconstantinou, C. Feeding habits of Aspitrigla cuculus (L., 1758) (red gurnard), Lepidotrigla cavillone (Lac., 1802) (large scale gurnard) and Trigloporus lastoviza (Brunn., 1768) (rock gurnard) around Cyclades and Dodecanese Islands (E. Mediterranean). Mediterr. Mar. Sci. 2000, 1, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Forward, R.B. Behavioral responses of a sand-beach amphipod to light and pressure. J. Exp. Mar. Biol. Ecol. 1986, 102, 55–74. [Google Scholar] [CrossRef]
- Forward, R.B. Phototaxis of a sand-beach amphipod: Physiology and tidal rhythms. J. Comp. Physiol. A Sensory Neural Behav. Physiol. 1980, 135, 243–250. [Google Scholar] [CrossRef]
- Crisp, D.J.; Ritz, D.A. Responses of cirripede larvae to light. I. Experiments with white light. Mar. Biol. 1973, 23, 327–335. [Google Scholar] [CrossRef]
- Forward, R.B., Jr. Diel vertical migration: Zooplankton photobiology and behaviour. Oceanogr. Mar. Biol. Annu. Rev. 1988, 26, 1–393. [Google Scholar]
- Batty, R.S.; Blaxter, J.H.S.; Richard, J.M. Light intensity and the feeding behaviour of herring, Clupea harengus. Mar. Biol. 1990, 107, 383–388. [Google Scholar] [CrossRef]
- Blaxter, J.; Batty, R. Herring behaviour in the light and dark. In Light and Life in the Sea; Cambridge University Press: Cambridge, UK, 1990; pp. 209–220. [Google Scholar]
- Nightingale, B.; Longcore, T.; Simenstad, C.A. Artificial night lighting and fishes. In Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; pp. 257–276. [Google Scholar]
- McConnell, A.; Routledge, R.; Connors, B. Effect of artificial light on marine invertebrate and fish abundance in an area of salmon farming. Mar. Ecol. Prog. Ser. 2010, 419, 147–156. [Google Scholar] [CrossRef] [Green Version]
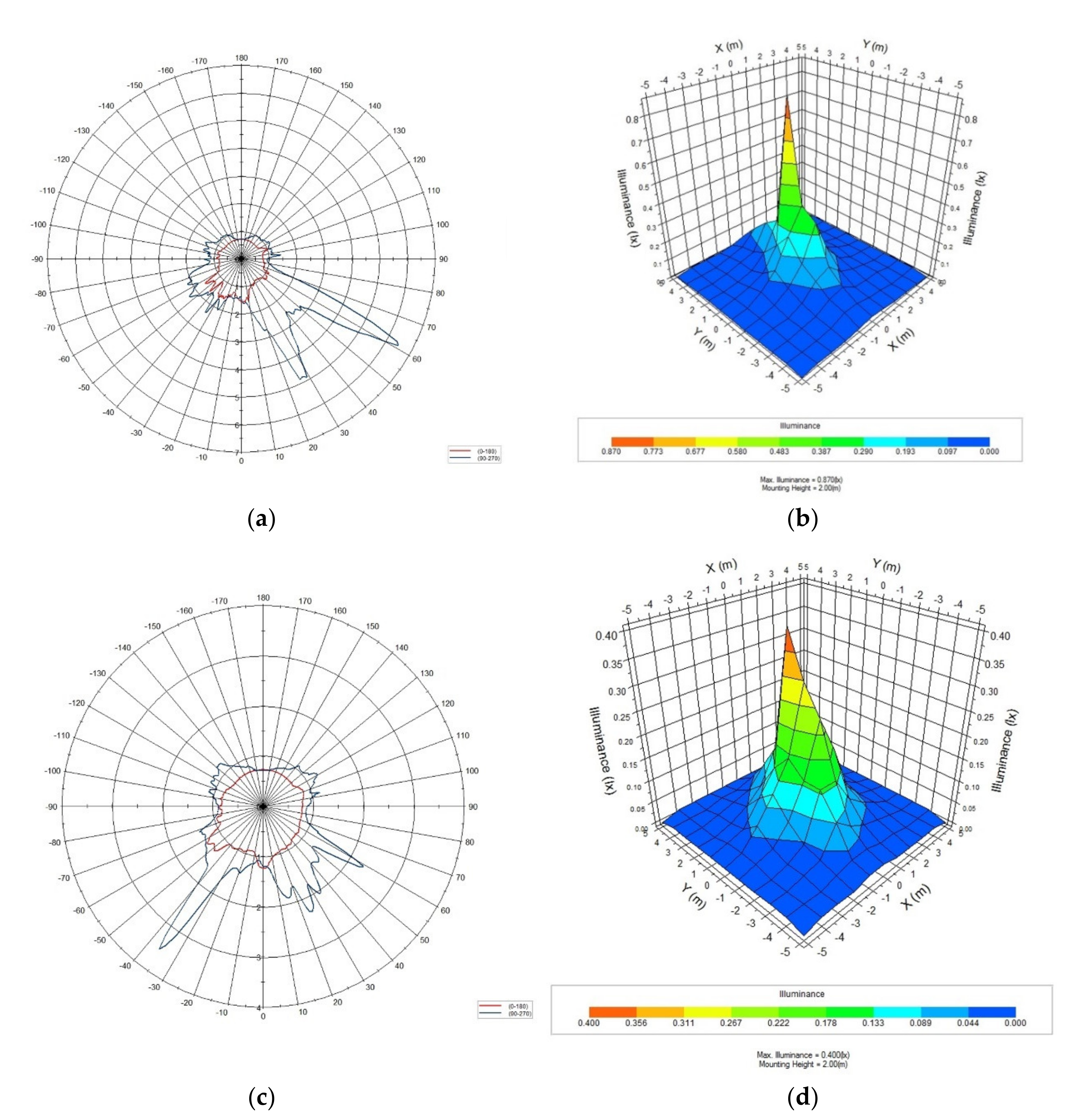

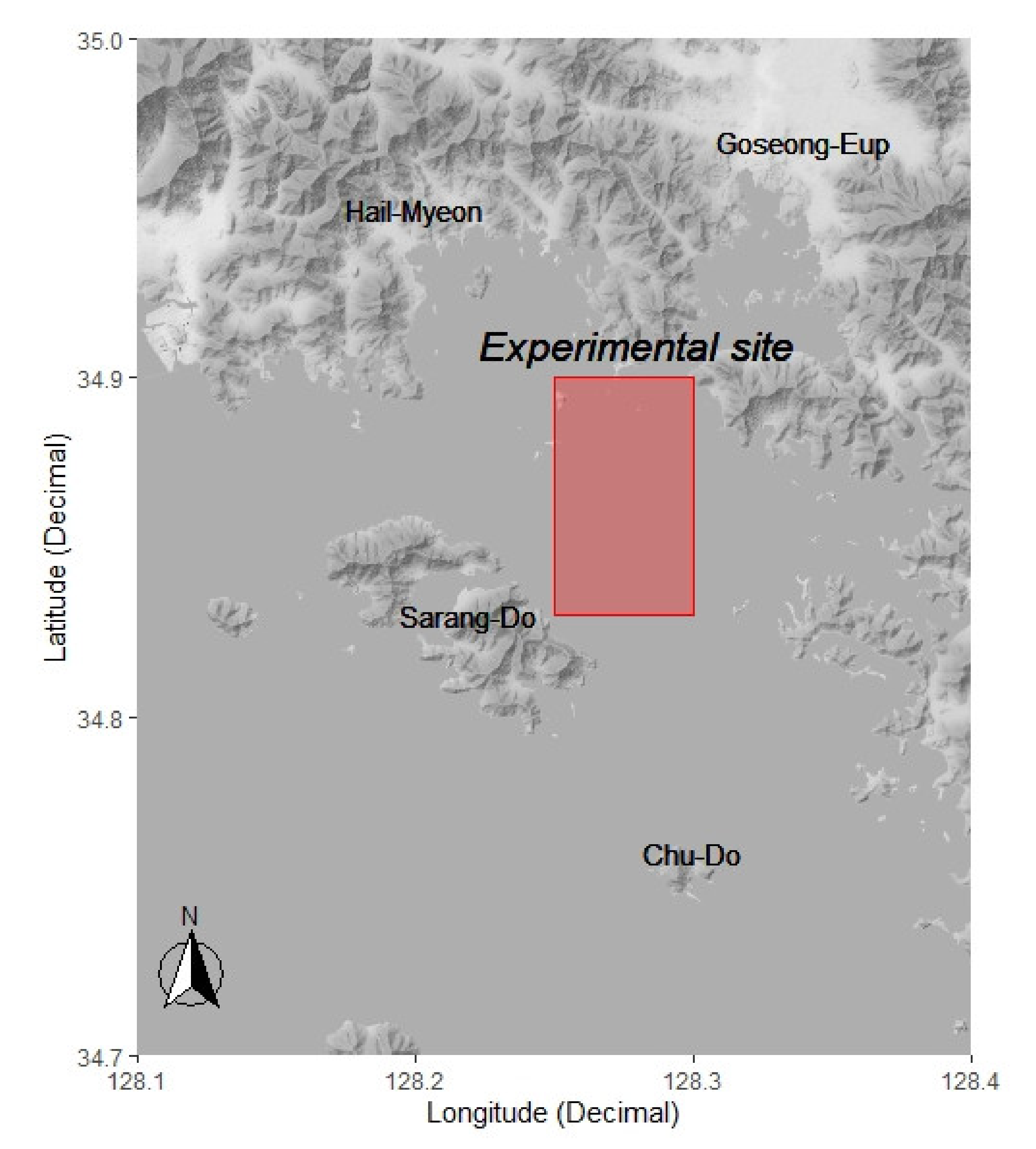
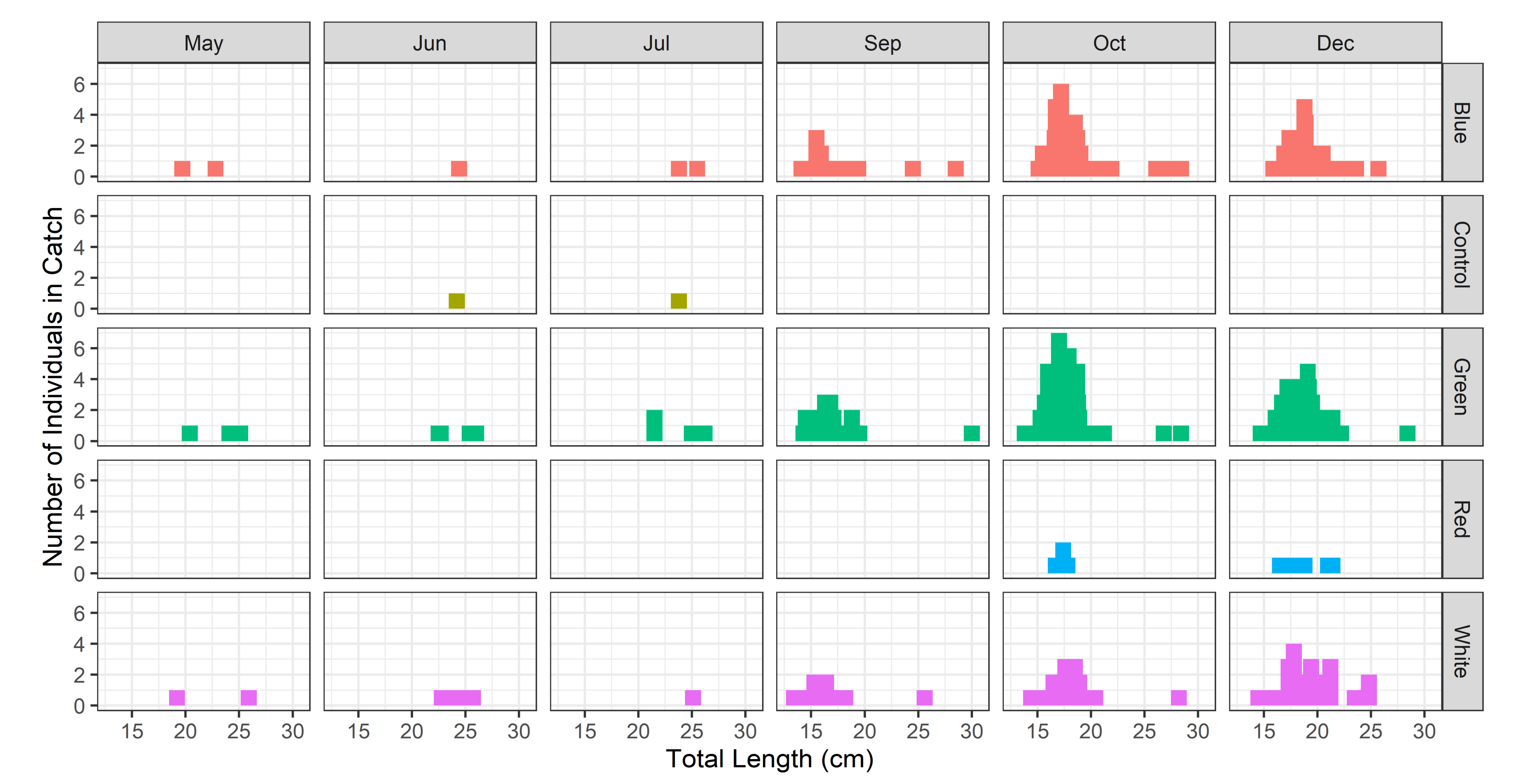

| Color of LED Light | Peak Wave Length (nm) | Color Coordinate (×) | Color Coordinate (Y) |
|---|---|---|---|
| Green | 522 | 0.1880 | 0.7171 |
| Blue | 463 | 0.1362 | 0.0556 |
| Red | 629 | 0.6886 | 0.3119 |
| White | 451 | 0.2474 | 0.2395 |
| Gear Type | Catch Number of Bluefin Sea Robin | Mean Catch (±Standard Deviation) | Total Weight (g) |
|---|---|---|---|
| Control | 2 | 0.33 ± 0.52 | 246 |
| Red LED | 10 | 1.67 ± 2.58 | 527 |
| Green LED | 273 | 45.50 ± 51.32 | 15,114 |
| White LED | 133 | 22.17 ± 23.30 | 7593 |
| Blue LED | 157 | 26.17 ± 32.22 | 9582 |
| Total | 575 | 33,062 |
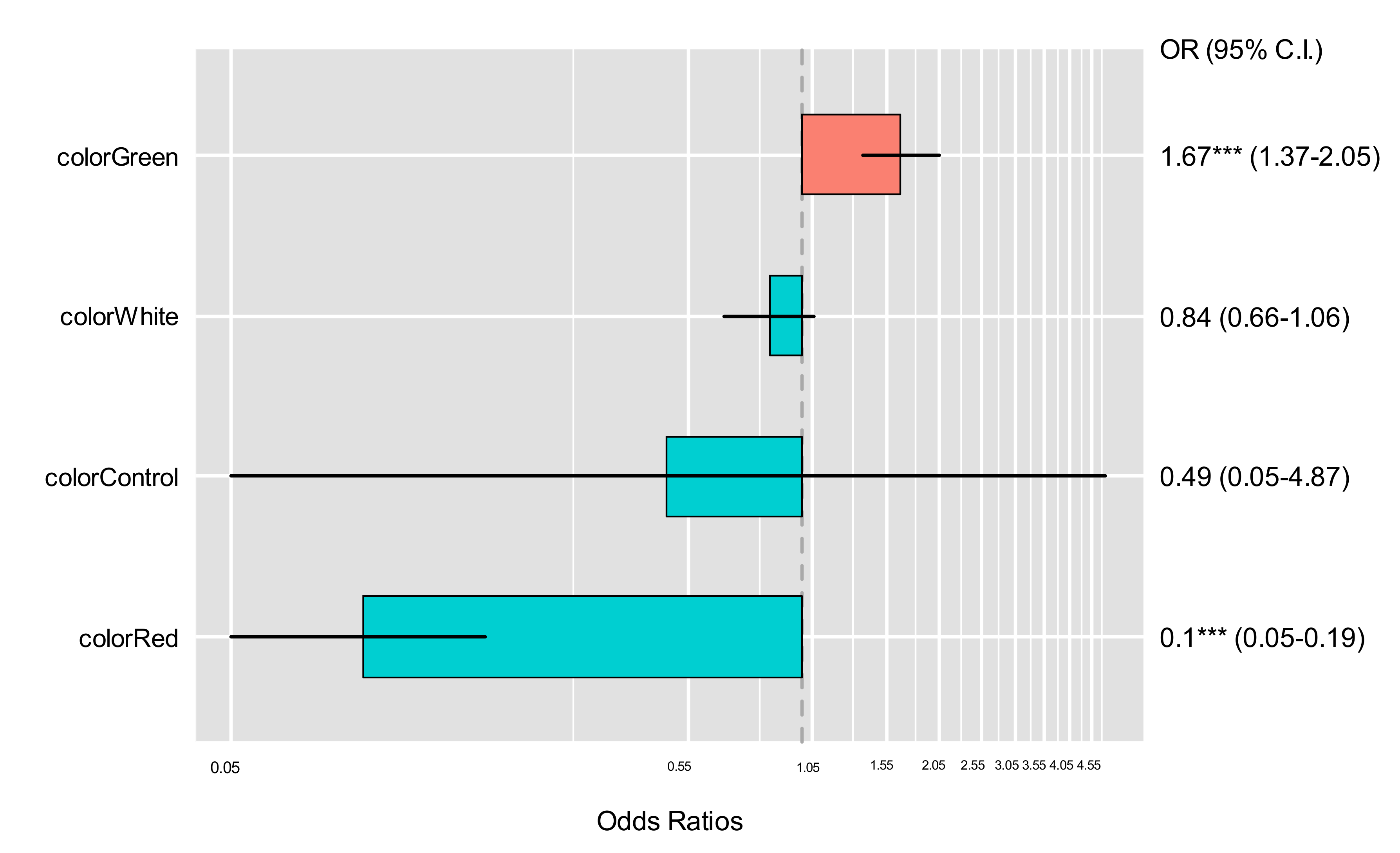
| Treatment | Estimate | S.E. | z-Value | 95% CI | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 0.41 | 0.37 | 1.13 | 0.74–3.09 | 0.26 |
| Control | −0.72 | 1.18 | −0.61 | 0.05–4.87 | 0.54 |
| Green | 0.52 | 0.10 | 4.95 | 1.37–2.05 | 0.00 |
| Red | −2.28 | 0.33 | −6.93 | 0.05–0.19 | 0.00 |
| White | −0.17 | 0.12 | −1.44 | 0.66–1.06 | 0.15 |
| Random effect | Variable | Variance | S.D. | ||
| Trial no. | Intercept | 2.28 | 2.279 |
| Prey Organisms | %N | %W | %F | IRI | %IRI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrura | Blue | Green | White | Blue | Green | White | Blue | Green | White | Blue | Green | White | Blue | Green | White | |
| Caridea | Gammaridae sp. | 81.7 | 88.4 | 84.8 | 6.5 | 21.9 | 5.0 | 6.9 | 23.3 | 21.1 | 608.5 | 2456.3 | 1890.6 | 27.2 | 68.8 | 58.4 |
| Crangon sp. 2 | 8.7 | 6.3 | 3.2 | 18.5 | 10.8 | 12.2 | 31.0 | 39.5 | 21.1 | 842.6 | 674.5 | 323.2 | 37.6 | 18.9 | 10.0 | |
| Hippolyte sp. | 1.6 | 1.8 | 3.2 | 15.0 | 19.9 | 12.7 | 10.3 | 11.6 | 15.8 | 172.6 | 252.4 | 251.5 | 7.7 | 7.1 | 7.8 | |
| Eualus sp. | 2.6 | 0.8 | 0.8 | 10.6 | 26.2 | 3.6 | 10.3 | 2.3 | 5.3 | 136.7 | 62.8 | 23.1 | 6.1 | 1.8 | 0.7 | |
| Crangon sp. 1 | 2.6 | 1.2 | 1.6 | 16.3 | 4.4 | 10.2 | 13.8 | 7.0 | 5.3 | 261.0 | 39.1 | 62.1 | 11.6 | 1.1 | 1.9 | |
| Latreutes anoplonyx | 1.4 | 0.0 | 0.0 | 12.7 | 0.0 | 0.0 | 6.9 | 0.0 | 0.0 | 98.0 | 0.0 | 0.0 | 4.4 | 0.0 | 0.0 | |
| Caprella spp. | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.3 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Brachyura | Portunidae sp. | 0.2 | 0.2 | 0.0 | 1.3 | 4.1 | 0.2 | 6.9 | 2.3 | 0.0 | 10.9 | 10.1 | 0.0 | 0.5 | 0.3 | 0.0 |
| Tritodynamia horvathi | 0.5 | 0.0 | 0.0 | 11.3 | 0.0 | 0.0 | 6.9 | 0.0 | 0.0 | 81.0 | 0.0 | 0.0 | 3.6 | 0.0 | 0.0 | |
| Pinnotheridae sp. | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 3.2 | 0.0 | 0.0 | 5.3 | 0.0 | 0.0 | 21.3 | 0.0 | 0.0 | 0.7 | |
| Pisces | Unidentifed | 0.2 | 0.8 | 0.8 | 5.8 | 8.6 | 17.8 | 3.4 | 7.0 | 5.3 | 20.9 | 65.2 | 97.6 | 0.9 | 1.8 | 3.0 |
| Polycheata | Perinereis sp. | 0.0 | 0.2 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 2.3 | 0.0 | 0.0 | 2.8 | 0.0 | 0.0 | 0.1 | 0.0 |
| Decapoda | Areopaguristes sp. | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 5.4 | 0.0 | 0.0 | 5.3 | 0.0 | 0.0 | 32.6 | 0.0 | 0.0 | 1.0 |
| Isopoda | Isopoda sp. | 0.5 | 0.2 | 4.0 | 1.8 | 3.0 | 29.7 | 3.4 | 2.3 | 15.8 | 7.9 | 7.5 | 532.9 | 0.4 | 0.2 | 16.5 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, P.; Kim, H.; Kim, S. Application of Light-Emitting Diode Lights to Bluefin Sea Robin (Chelidonichthys spinosus) Catch in Pot Fishery in the South Sea, Korea. Appl. Sci. 2022, 12, 4149. https://doi.org/10.3390/app12094149
Kim P, Kim H, Kim S. Application of Light-Emitting Diode Lights to Bluefin Sea Robin (Chelidonichthys spinosus) Catch in Pot Fishery in the South Sea, Korea. Applied Sciences. 2022; 12(9):4149. https://doi.org/10.3390/app12094149
Chicago/Turabian StyleKim, Pyungkwan, Hyungseok Kim, and Seonghun Kim. 2022. "Application of Light-Emitting Diode Lights to Bluefin Sea Robin (Chelidonichthys spinosus) Catch in Pot Fishery in the South Sea, Korea" Applied Sciences 12, no. 9: 4149. https://doi.org/10.3390/app12094149





