Comparative Study of Physiological Changes in Turbot Scophthalmus maximus in Different Living Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling Site
2.2. Blood Collection and Tissue Sampling
2.3. Plasma Biochemical and Hematological Analyses
2.4. Molecular Analyses
2.5. Histology
2.6. Statistical Analysis
3. Results
3.1. Plasma Biochemistry and Leukocyte Profile
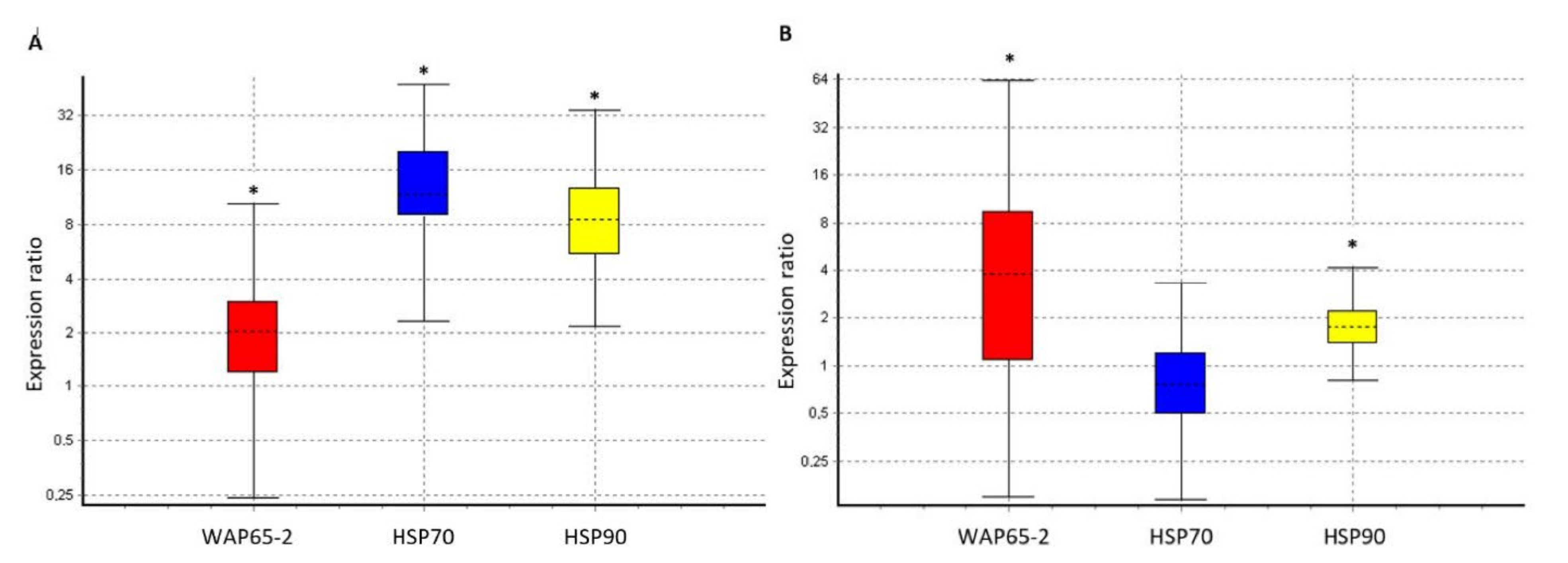
3.2. Molecular Analyses
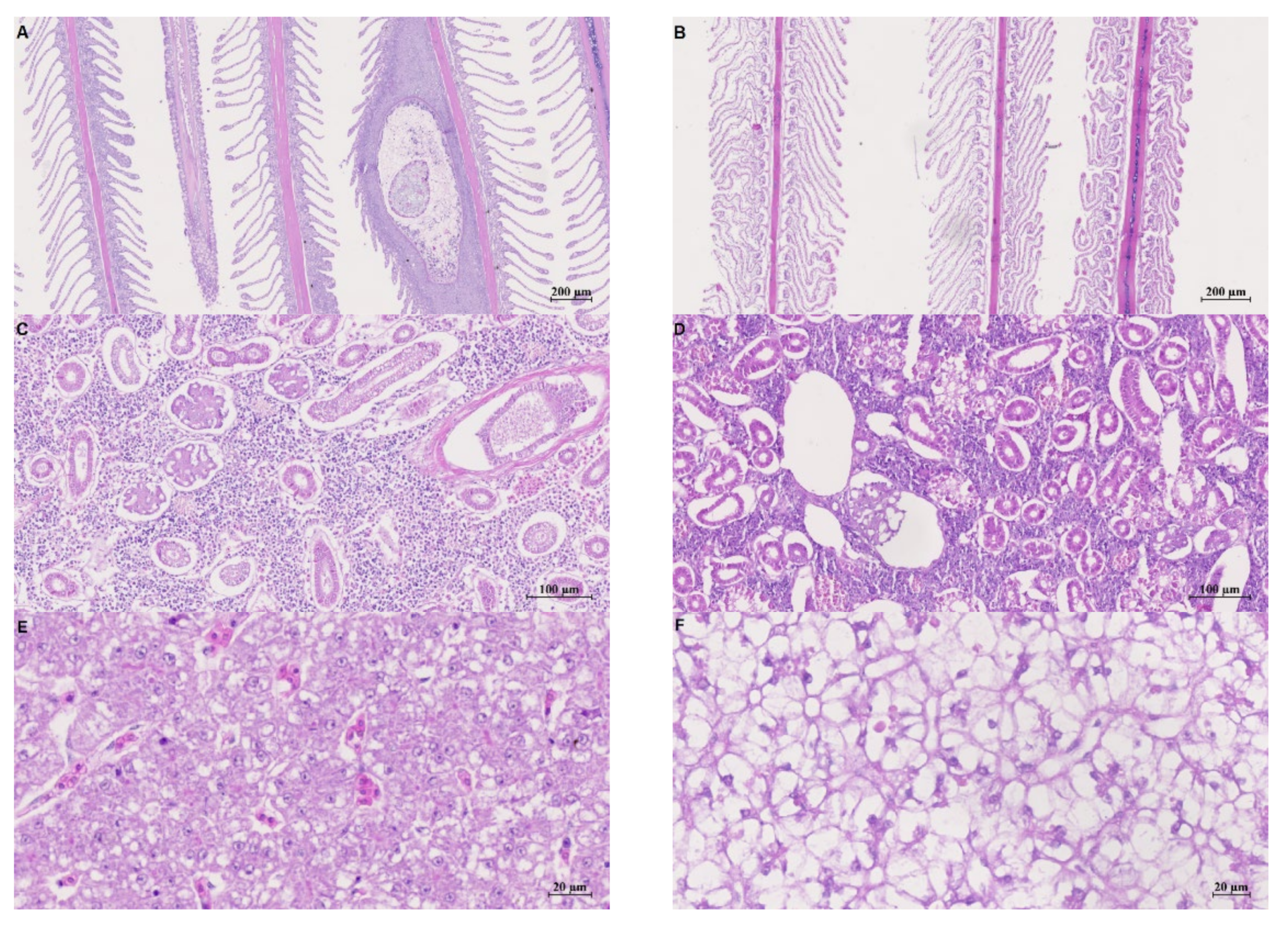
3.3. Histology Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Izquierdo, M.; Tort, L.; Robaina, L.; Vergara, J. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Harper, C.; Wolf, J.C. Morphologic Effects of the Stress Response in Fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.C. Stress management and welfare. In Biology of Stress in Fish—Fish Physiology 35; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 463–539. [Google Scholar]
- Čož-Rakovac, R.; Strunjak-Perović, I.; Topić Popović, N.; Hacmanjek, M.; Šmuc, T.; Jadan, M.; Lipej, Z.; Homen, Z. Cage culture effects on fish mullets (Mugilidae) liver histology and blood chemistry profile. J. Fish Biol. 2008, 72, 2557–2569. [Google Scholar] [CrossRef]
- Fazio, F.; Marafioti, S.; Arfuso, F.; Piccione, G.; Faggio, C. Comparative study of the biochemical and haematological parameters of four wild Tyrrhenian fish species. Veterinární Med. 2013, 58, 576–581. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress Indicators in Fish. Fish Physiol. 2016, 35, 405–462. [Google Scholar] [CrossRef]
- Jardas, I. Jadranska Ihtiofauna; Školska Knjiga: Zagreb, Croatia, 1996; Volume 533. [Google Scholar]
- Person-Le Ruyet, J. Turbot (Scophthalmus maximus) Grow-out in Europe: Practices, Results, and Prospects. Turk. J. Fish. Aquat. Sci. 2002, 2, 29–39. [Google Scholar]
- Labatut, R.A.; Olivares, J.F. Culture of turbot (Scophthalmus maximus) juveniles using shallow raceways tanks and recirculation. Aquac. Eng. 2004, 32, 113–127. [Google Scholar] [CrossRef]
- Danancher, D.; Garcia-Vazquez, E. Turbot—Scophthalmus maximus. In Genetic Impact of Aquaculture Activities on Native Populations; Svåsand, T., Crosetti, D., García-Vázquez, E., Verspoor, E., Eds.; 2007; pp. 55–61. Available online: https://www.vliz.be/imisdocs/publications/318461.pdf (accessed on 23 November 2021).
- Bouza, C.; Vandamme, S.; Hermida, M.; Cabaleiro, S.; Volckaert, F.; Martinez, M. AquaTrace species Turbot (Scophthalmus maximus). Aquatrace.eu. World Wide Web Electronic Publication. 2014. Available online: https://fishreg.jrc.ec.europa.eu/web/aquatrace/leaflets/turbot (accessed on 23 November 2021).
- FAO. Scophthalmus maximus. Cultured Aquatic Species Information Programme. Text by Rodríguez Villanueva, J.L., Fernández Souto, B. Fisheries and Aquaculture Division [Online]. Rome. 2022. Updated 6 June 2005. Available online: https://www.fao.org/fishery/en/culturedspecies/psetta_maxima/ (accessed on 9 February 2022).
- Foss, A.; Imsland, A.K.D.; Van De Vis, H.; Abbink, W.; Lambooij, B.; Roth, B. Physiological response of temperature shocks in turbot and sole. J. Appl. Aquac. 2019, 31, 34–47. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, X.; Wang, Z.; Meng, Z.; Huang, B.; Guan, C. Physiological response of juvenile turbot (Scophthalmus maximus. L.) during hyperthermal stress. Aquaculture 2020, 529, 735645. [Google Scholar] [CrossRef]
- Liu, B.; Jia, R.; Han, C.; Huang, B.; Lei, J.-L. Effects of stocking density on antioxidant status, metabolism and immune response in juvenile turbot (Scophthalmus maximus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, K.; Lei, J.; Huang, B.; Jia, R.; Wang, G. Stocking density effects on growth and stress response of juvenile turbot (Scophthalmus maximus) reared in land-based recirculating aquaculture system. Acta Oceanol. Sin. 2016, 36, 31–38. [Google Scholar] [CrossRef]
- Person-le Ruyet, J.; Lamers, A.; Le Roux, A.; Sévère, A.; Boeuf, G.; Mayer-Gostan, N. Long-term ammonia exposure of turbot: Effects on plasma parameters. J. Fish Biol. 2003, 62, 879–894. [Google Scholar] [CrossRef]
- Mota, V.C.; Hop, J.; Sampaio, L.A.; Heinsbroek, L.T.N.; Verdegem, M.C.J.; Eding, E.H.; Verreth, J.A.J. The effect of low pH on physiology, stress status and growth performance of turbot (Psetta maxima L.) cultured in recirculating aquaculture systems. Aquac. Res. 2018, 49, 3456–3467. [Google Scholar] [CrossRef]
- Jia, R.; Han, C.; Lei, J.-L.; Liu, B.-L.; Huang, B.; Huo, H.-H.; Yin, S.-T. Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat. Toxicol. 2015, 169, 1–9. [Google Scholar] [CrossRef]
- Mumford, S.; Heidel, J.; Smith, C.; Morrison, J.; MacConnell, B.; Blazer, V. Fish Histology and Histopathology, 4th ed.; US Fish & Wildlife Service: Elkins, WV, USA, 2007. [Google Scholar]
- Švob, M. Histološke i Histokemijske Metode; Svjetlost: Sarajevo, Bosnia and Herzegovina, 1974; pp. 142–205. [Google Scholar]
- Barton, B.A.; Morgan, J.D.; Vijayan, M.M. Physiological and condition-related indicators of environmental stress in fish. In Biological Indicators of Aquatic Ecosystem Stress; Adams, S.M., Ed.; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 111–148. [Google Scholar]
- Wedemeyer, G.H.A.; Barton, B.A.; McLeay, D.J. Stress and acclimation. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- Zhou, X.; Li, M.; Abbas, K. Comparison of haemtology and serum biochemistry of cultured and wild Dojo loach Misgurnus anguillicaudatus. Fish Physiol. Biochem. 2009, 35, 435–441. [Google Scholar] [CrossRef]
- Congleton, J.L.; Wagner, T. Blood biochemistry indicators of nutritional status in juvenile salmonids. J. Fish Biol. 2006, 69, 473–490. [Google Scholar] [CrossRef]
- Jia, Y.; Gao, Y.; Chen, X.; Huang, B. Determination of optimal fasting time before blood sampling to get baseline data on serum biochemical characteristics in juvenile turbot (Scophthalmus maximus). Aquaculture 2018, 487, 83–88. [Google Scholar] [CrossRef]
- Waring, C.P.; Stagg, R.M.; Poxton, M.G. Physiological responses to handling in the turbot. J. Fish Biol. 1996, 48, 161–173. [Google Scholar] [CrossRef]
- Martínez, B.; Miranda, J.; Nebot, C.; Rodriguez, J.; Cepeda, A.; Franco, C. Differentiation of Farmed and Wild Turbot (Psetta maxima): Proximate Chemical Composition, Fatty Acid Profile, Trace Minerals and Antimicrobial Resistance of Contaminant Bacteria. Food Sci. Technol. Int. 2010, 16, 435–441. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Rolbiecki, L. Helmintofauna of turbot Scophthalmus maximus (Linneaues, 1758) from the southern Baltic Sea including new data. Pol. J. Vet. Sci. 2015, 18, 599–605. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Estensoro, I.; Sánchez, J.P. Immunity to gastrointestinal microparasites of fish. Dev. Comp. Immunol. 2016, 64, 187–201. [Google Scholar] [CrossRef]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Aldrin, J.; Messager, J.; Saleun, S. Analyses sanguines de turbots d’elevage immatures (Scophthalmus maximus L.). Aquaculture 1984, 40, 17–25. [Google Scholar] [CrossRef]
- Hille, S. A literature review of the blood chemistry of rainbow trout, Salmo gairdneri Rich. J. Fish Biol. 1982, 20, 535–569. [Google Scholar] [CrossRef]
- Wood, C.M.; McDonald, M.D.; Sundin, L.; Laurent, P.; Walsh, P.J. Pulsatile urea excretion in the gulf toadfish: Mechanisms and controls. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 667–684. [Google Scholar] [CrossRef]
- Kulkarni, R.S.; Barad, V.S. Effect of starvation on haematological and serum biochemical changes in the freshwater fish, Notopterus notopterus (Pallas). Int. J. Innov. Stud. Auat. Biol. Fish. 2015, 1, 24–29. [Google Scholar]
- Ajeniyi, S.A.; Solomon, R.J. Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat. Sci. 2014, 12, 124–138. [Google Scholar]
- Ziskowski, J.; Mercaldo-Allen, R.; Pereira, J.; Kuropat, C.; Goldberg, R. The effects of fin rot disease and sampling method on blood chemistry and hematocrit measurements of winter flounder, Pseudopleuronectes americanus from New Haven Harbor (1987–1990). Mar. Pollut. Bull. 2008, 56, 740–750. [Google Scholar] [CrossRef]
- Jee, J.-H.; Kim, S.-G.; Kang, J.-C. Effects of phenanthrene on growth and basic physiological functions of the olive flounder, Paralichthys olivaceus. J. Exp. Mar. Biol. Ecol. 2004, 304, 123–136. [Google Scholar] [CrossRef]
- Srivastav, A.; Rai, R.; Srivastav, S.K.; Suzuki, N.; Mishra, D. Effects of lead on the plasma electrolytes of a freshwater fish, Heteropneustes fossilis. Int. Aquat. Res. 2013, 5, 4. [Google Scholar] [CrossRef][Green Version]
- Henry, J.B. Book Review: Clinical Diagnosis and Management by Laboratory Methods. Lab. Med. 2001, 32, 700. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Mckelvie, D.H. Clinical Biochemistry. In Pathology of Laboratory Animal; Springer: New York, NY, USA, 1978; Volume II, pp. 1750–1815. [Google Scholar]
- Dick, T.A.; Chambers, C.; Isinguzo, I. Cestoidea (phylum Platyhelminthes). In Fish Diseases and Disorders; Woo, P.T.K., Ed.; CAB International: Wallingford, UK, 2006; pp. 391–416. [Google Scholar]
- Tocher, D.R.; Mourente, G.; Van der Eecken, A.; Evjemo, J.O.; Diaz, E.; Bell, J.G.; Geurden, I.; Lavens, P.; Olsen, Y. Effects of dietary vitamin E on antioxidant defense mechanisms of juvenile turbot (Schophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparus aurata L.). Aquac. Nutr. 2002, 8, 195–207. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione proxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Rueda-Jasso, R.; Conceição, L.; Dias, J.; De Coen, W.; Gomes, E.; Rees, J.; Soares, F.; Dinis, M.; Sorgeloos, P. Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 2004, 231, 417–433. [Google Scholar] [CrossRef]
- Morales, A.E.; Pérez-Jiménez, A.; Hidalgo, M.C.; Abellán, E.; Cardenete, G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 153–161. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L.; Maerz, J.C. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Zebral, Y.D.; Zafalon-Silva, B.; Wiegand Mascarenhas, M.; Berteaux Robaldo, R. Leucocyte profile and growth rate as indicators of crowding stress in pejerey fingerlings (Odontesthes bonariensis). Aquac. Res. 2015, 46, 2270–2276. [Google Scholar] [CrossRef]
- Davis, A.K.; Maney, D.L. The use of glucocorticoid hormones or leukocyte profiles to measure stress in vertebrates: What’s the difference? Methods Ecol. Evol. 2017, 9, 1556–1568. [Google Scholar] [CrossRef]
- Eissa, N.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Han, M.; Song, Z.; Song, C.; Xu, S.; Li, J.; Wang, Y.; Li, X.; Yue, X. Growth, stress and non-specific immune responses of turbot (Scophthalmus maximus) larvae exposed to different light spectra. Aquaculture 2020, 520, 734950. [Google Scholar] [CrossRef]
- Huang, J.-F.; Xu, Q.-Y.; Chang, Y.-M. Effects of temperature and dietary protein on gene expression ofHsp70andWap65and immunity of juvenile mirror carp (Cyprinus carpio). Aquac. Res. 2014, 46, 2776–2788. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, A.; Wang, X.; Lei, J.; Li, W.; Wang, T.; Yang, Z.; Qu, J. Interaction of temperature and salinity on the expression of immunity factors in different tissues of juvenile turbot Scophthalmus maximus based on response surface methodology. Chin. J. Oceanol. Limnol. 2014, 33, 28–36. [Google Scholar] [CrossRef]
- Forsyth, R.B.; Candido, E.P.M.; Babich, S.L.; Iwama, G.K. Stress Protein Expression in Coho Salmon with Bacterial Kidney Disease. J. Aquat. Anim. Heal. 1997, 9, 18–25. [Google Scholar] [CrossRef]
- Diaz-Rosales, P.; Pereiro, P.; Figueras, A.; Novoa, B.; Dios, S. The warm temperature acclimation protein (Wap65) has an important role in the inflammatory response of turbot (Schophthalmus maximus). Fish Shellfish Immunol. 2014, 14, 80–92. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Pal, A.; Sahu, N.; Jain, K.; Dalvi, R.; Misra, S.; Debnath, D. Metabolic modulation in Labeo rohita fingerlings during starvation: Hsp70 expression and oxygen consumption. Aquaculture 2008, 285, 234–237. [Google Scholar] [CrossRef]
- Steinel, N.; Bolnick, D.I. Melanomacrophage Centers As a Histological Indicator of Immune Function in Fish and Other Poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Kurtović, B.; Teskeredžić, E.; Teskeredžić, Z. Histological comparison of spleen and kidney tissue from farmed and wild European sea bass (Dicentrarchus labrax L.). Acta Adriat. 2008, 49, 147–154. [Google Scholar]


| Genes | Primers Sequence (5′-3′) | Size (bp) | Reaction Condition |
|---|---|---|---|
| s18 | FW: ACTGAGGATGAGGTTGAGAG RV: TCCAGACCATTAGCAAGGA | 133 | 59 °C 3.5 mM MgCl2 |
| WAP65-2 | FW: GTTAGACGCCATCACCACTG RV: CGCATGTAGACTGGACCTGA | 87 | 63 °C 3 mM MgCl2 |
| HSP70 | FW: GTGTGCTCATTCAGGTCTATG RV: ACCCTTGTCGTTTGTGATG | 81 | 59 °C 3 mM MgCl2 |
| HSP90 | FW: GAAGTGTCTGGAGCTCTTTG RV: ATGCGGGACAGGTATTCT | 182 | 60 °C 3.5 mM MgCl2 |
| Plasma Parameter | Farmed Turbot | Wild-Caught Turbot | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | 25th–75th Percentile | Min–Max | Median | 25th–75th Percentile | Min–Max | ||
| Glucose (mmol/L) | 2.31 | 1.50–2.94 | 0.66–3.88 | 1.61 | 0.75–4.21 | 0.05–16.4 | 0.367 |
| Triglycerides (mmol/L) | 7.54 | 5.69–9.73 | 1.26–14.46 | 1.49 | 0.95–2.06 | 0.37–5.17 | <0.0001 |
| Cholesterol (mmol/L) | 3.85 | 3.37–4.77 | 2.64–5.44 | 4.21 | 3.42–5.99 | 0.87–12.32 | 0.1917 |
| Total protein (g/L) | 40.5 | 37–43 | 34–49 | 26 | 22.25–33.75 | 10–46 | <0.0001 |
| Albumin (g/L) | 11.5 | 11–12 | 9–14 | 7 | 6–10 | 3–14 | <0.0001 |
| Globulin (g/L) | 28.5 | 28–31 | 25–35 | 19 | 16.25–23.50 | 7–32 | <0.0001 |
| A/G ratio | 0.39 | 0.37–0.41 | 0.32–0.46 | 0.42 | 0.38–0.45 | 0.30–0.54 | <0.05 |
| Bilirubin (µmol/L) | 1.03 | 0.79–1.58 | 0.19–4.47 | 0.052 | 0.048–0.42 | 0.047–1.38 | <0.0001 |
| Urea (mmol/L) | 2.26 | 1.85–2.62 | 1.13–3.56 | 2.42 | 1.76–3.47 | 0.64–5.18 | 0.295 |
| Creatinine (µmol/L) | 107 | 68–122 | 12–173 | 27.40 | 23.75–32.375 | 14.5–58 | <0.0001 |
| Calcium (mmol/L) | 3.14 | 2.87–3.34 | 2.63–3.75 | 3.97 | 3.46–4.99 | 3.05–6.98 | <0.0001 |
| Phosphorus (mmol/L) | 5.25 | 4.30–5.98 | 3.1–7.4 | 6.91 | 6.16–9.08 | 3.59–11.28 | <0.0001 |
| Alkaline phosphatase (U/L) | 21.5 | 17–33 | 12–39 | 95 | 70.75–127 | 39–402 | <0.0001 |
| Creatine kinase (U/L) | 211.5 | 78–1158 | 18–4032 | 16 | 4–54.5 | 1–795 | <0.0001 |
| Superoxide dismutase (U/mL) | 0.881 | 0.423–1.792 | 0.179–3.579 | 0.232 | 0.003–0.334 | 0–0.649 | <0.0001 |
| Glutathione peroxidase (U/L) | 210 | 185.5–240.5 | 127–317 | 582 | 283–870 | 108–2914 | <0.0001 |
| Leucocyte profile | |||||||
| Lymphocytes (%) | 81.30 | 67.40–84.22 | 44.90–91.67 | 67.20 | 55.55–78.13 | 33.33–89.16 | 0.053 |
| Neutrophils (%) | 4.17 | 0.39–8.00 | 0–16.00 | 17.03 | 10.45–24.47 | 3.78–56.41 | <0.001 |
| Monocytes (%) | 15.75 | 10.87–28.31 | 8.33–39.79 | 16.39 | 8.29–20.93 | 4.42–27.82 | 0.334 |
| N:L ratio | 0.05 | 0.005–0.10 | 0–0.36 | 0.23 | 0.13–0.50 | 0.05–1.69 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Križanac, S.; Topić Popović, N.; Barišić, J.; Beer-Ljubić, B.; Bujak, M.; Babić, S.; Bojanić, K.; Čož-Rakovac, R.; Matulić, D.; Strunjak-Perović, I. Comparative Study of Physiological Changes in Turbot Scophthalmus maximus in Different Living Conditions. Appl. Sci. 2022, 12, 4201. https://doi.org/10.3390/app12094201
Križanac S, Topić Popović N, Barišić J, Beer-Ljubić B, Bujak M, Babić S, Bojanić K, Čož-Rakovac R, Matulić D, Strunjak-Perović I. Comparative Study of Physiological Changes in Turbot Scophthalmus maximus in Different Living Conditions. Applied Sciences. 2022; 12(9):4201. https://doi.org/10.3390/app12094201
Chicago/Turabian StyleKrižanac, Silvia, Natalija Topić Popović, Josip Barišić, Blanka Beer-Ljubić, Maro Bujak, Sanja Babić, Krunoslav Bojanić, Rozelindra Čož-Rakovac, Daniel Matulić, and Ivančica Strunjak-Perović. 2022. "Comparative Study of Physiological Changes in Turbot Scophthalmus maximus in Different Living Conditions" Applied Sciences 12, no. 9: 4201. https://doi.org/10.3390/app12094201
APA StyleKrižanac, S., Topić Popović, N., Barišić, J., Beer-Ljubić, B., Bujak, M., Babić, S., Bojanić, K., Čož-Rakovac, R., Matulić, D., & Strunjak-Perović, I. (2022). Comparative Study of Physiological Changes in Turbot Scophthalmus maximus in Different Living Conditions. Applied Sciences, 12(9), 4201. https://doi.org/10.3390/app12094201









