Tea Infusions as a Source of Phenolic Compounds in the Human Diet
Abstract
:1. Introduction
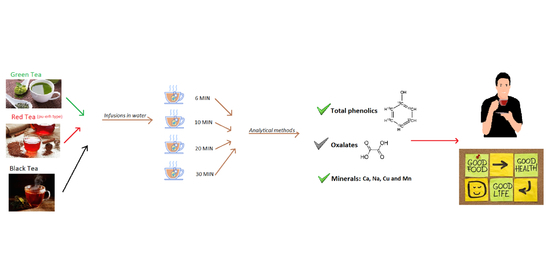
2. Materials and Methods
2.1. Food Samples
2.2. Determination of Total Content of Phenolic Compounds
2.3. Determination of Oxalate Content
2.4. Determination of Calcium, Copper, and Manganese
2.5. Determination of Sodium
2.6. Statistical Data Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.Y.; Lin, Y.Y.; Chang, Y.C.; Huang, W.Y.; Lin, W.S.; Chen, C.Y.; Huang, S.L.; Lin, Y.S. Effects of Infusion and Storage on Antioxidant Activity and Total Phenolic Content of Black Tea. Appl. Sci. 2020, 10, 2685. [Google Scholar] [CrossRef] [Green Version]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [Green Version]
- Kandyliari, A.; Elmaliklis, I.N.; Kontopoulou, O.; Tsafkopoulou, M.; Komninos, G.; Ntzatha, C.; Petsas, A.; Karantonis, H.C.; Koutelidakis, A. An epidemiological study report on the antioxidant and phenolic content of selected mediterranean functional foods, their consumption association with the Body Mass Index, and consumers purchasing behavior in a sample of healthy Greek adults. Appl. Sci. 2021, 11, 7818. [Google Scholar] [CrossRef]
- Nordin, N.H.; Molan, A.L.; Chua, W.H.; Kruger, M.C. Total phenolic contents and antioxidant activities of selenium-rich black tea versus regular black tea. Am. J. Life Sci. Res. 2017, 5, 40–50. [Google Scholar]
- Klepacka, J.; Tońska, E.; Rafałowski, R.; Czarnowska-Kujawska, M.; Opara, B. Tea as a source of biologically active compounds in the human diet. Molecules 2021, 26, 1487. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fu, Y.Q.; Chen, J.X.; Wang, F.; Feng, Z.H.; Yin, J.F.; Zeng, L.; Xu, Y.Q. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Influence of seasonal and yearly variation on phenolic profiles, caffeine, and antioxidant activities of green tea (Camellia sinensis (L.) Kuntze) from Azores. Appl. Sci. 2021, 11, 7439. [Google Scholar] [CrossRef]
- Nardini, M. Phenolic compounds in food: Characterization and health benefits. Molecules 2022, 27, 783. [Google Scholar] [CrossRef]
- Nobari, H.; Saedmocheshi, S.; Chung, L.H.; Suzuki, K.; Maynar-Mariño, M.; Pérez-Gómez, J. An overview on how exercise with green tea consumption can prevent the production of reactive oxygen species and improve sports performance. Int. J. Environ. Res. Public Health 2022, 19, 218. [Google Scholar] [CrossRef]
- Yonekura, Y.; Terauchi, M.; Hirose, A.; Odai, T.; Kato, K.; Miyasaka, N. Daily coffee and green tea consumption is inversely associated with body mass index, body fat percentage, and cardio-ankle vascular index in middle-aged Japanese women: A cross-sectional study. Nutrients 2020, 12, 1370. [Google Scholar] [CrossRef]
- Arceusz, A.; Wesołowski, M. Essential metals and phenolic acids in commercial herbs and spices. Multivariate analysis of correlations among them. Open Chem. 2015, 13, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, I.D.; Dhungana, S.K.; Kim, D.G. Comparison of quality characteristic and antioxidant potential of cultivated pu-erh and gushu pu-erh tea extracts at two temperatures. J. Pure Appl. Microbiol. 2018, 12, 1155–1161. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Oxalate, magnesium and calcium content in selected kinds of tea: Impact on human health. Eur. Food Res. Technol. 2016, 242, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Analytical assessment of bio- and toxic elements distribution in pu-erh and fruit teas in view of chemometric approach. Biol. Trace Elem. Res. 2016, 174, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Charrier, M.J.S.; Savage, G.P.; Vanhanen, L. Oxalate content and calcium binding capacity of tea and herbal teas. Asia Pac. J. Clin. Nutr. 2002, 11, 298–301. [Google Scholar] [CrossRef]
- Garbowska, B.; Wieczorek, J.K.; Polak-Śliwińska, M.; Wieczorek, Z.J. The content of minerals, bioactive compounds and anti-nutritional factors in tea infusions. J. Elem. 2017, 23, 369–380. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E. Effect of brewing method various tea types on content of soluble oxalates. Żywność Nauka Technol. Jakość 2012, 1, 187–195. (In Polish). Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-9ba88d48-90e3-4984-aca9-7b00aa5c0082?q=bwmeta1.element.agro-75c458ae-44d0-43ed-9b11-b35bc96076ca;6&qt=CHILDREN-STATELESS (accessed on 25 February 2022). (In Polish).
- Barghouthy, Y.; Corrales, M.; Doizi, S.; Somani, B.K.; Traxer, O. Tea and coffee consumption and pathophysiology related to kidney stone formation: A systematic review. World J. Urol. 2021, 39, 2417–2426. [Google Scholar] [CrossRef]
- Honow, R.; Gu, K.L.R.; Hesse, A.; Siener, R. Oxalate content of green tea of different origin, quality, preparation and time of harvest. Urol. Res. 2010, 38, 377–381. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Protective effects of epigallocatechin-3-gallate from green tea in various kidney diseases. Adv. Nutr. 2019, 10, 112–121. [Google Scholar] [CrossRef]
- Gaeini, Z.; Bahadoran, Z.; Mirmiran, P.; Azizi, F. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: Findings from a population with low coffee and high tea consumption. Nutr. Metab. 2019, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Yagin, N.L.; Mahdavi, R.; Nikniaz, Z. Oxalate content of different drinkable dilutions of tea infusions after different brewing times. Health Promot. Perspect. 2012, 2, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Chowaniak, M.; Niemiec, M.; Zhu, Z.; Rashidov, N.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; Kuboń, M.; Fayzullo, S.A.; Mahmadyorzoda, U.M.; et al. Quality assessment of wild and cultivated green tea from different regions of China. Molecules 2021, 26, 3620. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Taher, F.; Llantada, A.; Do, Q.; Sapp, T.; Sommerhalter, M. Effect of Water Hardness on Catechin and Caffeine Content in Green Tea Infusions. Molecules 2021, 26, 3485. [Google Scholar] [CrossRef] [PubMed]
- Karak, T.; Kutu, F.R.; Nath, J.R.; Sonar, I.; Paul, R.K.; Boruah, R.K.; Sanyal, S.; Sabhapondit, S.; Dutta, A.K. Micronutrients (B, Co, Cu, Fe, Mn, Mo and Zn) content in made tea (Camellia sinensis L.) and tea infusion with health prospect: A critical review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2996–3034. [Google Scholar] [CrossRef] [PubMed]
- Kilic, C.; Can, Z.; Yilmaz, A.; Yildiz, S.; Turna, H. Antioxidant properties of same herbal teas (green tea, senna, corn silk, rosemary) brewed at different temperatures. Int. J. Second. Metab. 2017, 4, 142–148. [Google Scholar] [CrossRef]
- Konieczynski, P.; Viapiana, A.; Wesolowski, M. Comparison of infusions from black and green teas (Camellia sinensis L. Kuntze) and Erva-mate (Ilex paraguariensis A. St.-Hil.) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- Rode, J.; Bazin, D.; Dessombz, A.; Benzerara, Y.; Letavernier, E.; Tabibzadeh, N.; Hoznek, A.; Tligui, M.; Traxer, O.; Daudon, M.; et al. Daily green tea infusions in hypercalciuric renal stone patients: No evidence for increased stone risk factors or oxalate-dependent stones. Nutrients 2019, 11, 256. [Google Scholar] [CrossRef] [Green Version]
- Safdar, N.; Sarfaraz, A.; Kazmi, Z.; Yasmin, A. Ten different brewing methods of green tea: Comparative antioxidant study. J. Appl. Biol. 2016, 4, 033–040. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.S.; Araujo, M.E.M.; Rodriguez, L.G.; Julio, A.; Mendes, B.G.; dos Santos, R.M.B.; Simoes, J.A.M. Influence of preparation procedures on the phenolic content, antioxidant and antidiabetic activities of green and black teas. Braz. J. Pharm. Sci. 2019, 55, 7695. [Google Scholar] [CrossRef]
- Rocha, D.P.; Pinto, G.F.; Silva, S.M.; Squissato, A.L.; Silva, S.G. A multi-pumping flow system for spectrophotometric determination of oxalate in tea. Microchem. J. 2020, 157, 104938. [Google Scholar] [CrossRef]
- Savage, G.; Klunklin, W. Oxalates are Found in Many Different European and Asian Foods-Effects of Cooking and Processing. J. Food Res. 2018, 7, 76–81. [Google Scholar] [CrossRef]
- Savage, G.; Charrier, M.; Vanhanen, L. Bioavailability of soluble oxalate from tea and the effect of consuming milk with the tea. Eur. J. Clin. Nutr. 2003, 57, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Siener, R.; Seidler, A.; Voss, S.; Hesse, A. Oxalate content of beverages. J. Food Comps. Anal. 2017, 63, 184–188. [Google Scholar] [CrossRef]
- Zaguła, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmiański, J. Comparison of the effectiveness of water-based extraction of substances from dry tea leaves with the use of magnetic field assisted extraction techniques. Molecules 2017, 22, 1656. [Google Scholar] [CrossRef] [Green Version]
- Mehra, A.; Baker, C.L. Leaching and bioavailability of aluminium, copper and manganese from tea (Camellia sinensis). Food Chem. 2007, 100, 1456–1463. [Google Scholar] [CrossRef]
- Erdemir, U.S. Contribute of tea (Camellia sinensis L.) to recommended daily intake of Mg, Mn, and Fe: An in vitro bioaccessibility assessment. J. Food Compos. Anal. 2018, 69, 71–77. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P. Plant Phenolics; Hafner Publishing Company: New York, NY, USA, 1972. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, P.; Miner, B. Pye Unicam Atomic Absorption Data Book; Pye Unicam Ltd.: Cambridge, UK, 1984. [Google Scholar]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; di Serio, M.; Amoresano, A. Quantification of polyphenols and metals in Chinese tea infusions by Mass Spectrometry. Foods 2020, 9, 835. [Google Scholar] [CrossRef]
- Ma, B.; Wang, J.; Xu, C.; Wang, Z.; Yin, D.; Zhou, B.; Ma, C. Interrelation analysis between phenolic compounds and in vitro antioxidant activities in Pu-erh tea. LWT 2022, 158, 113117. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, Y.; Lin, Z.; Liang, Y. Processing and chemical constituents of Pu-erh tea: A review. Food Res. Int. 2013, 53, 608–618. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ohland, C.; Jobin, C.; Sang, S. Degradation of black tea theaflavin through C-ring cleavage by gut microbiota. Food Sci. Hum. 2022, 11, 598–605. [Google Scholar] [CrossRef]
- Pongrac, P.; Tolra, R.; Hajiboland, R.; Vogel-Mikus, K.; Kelemen, M.; Vavpetic, P.; Pelicon, P.; Bercelo, J.; Regvar, M.; Poschenrieder, C. Contrasting allocation of magnesium, calcium and manganese in leaves of tea (Camellia sinensis (L.) Kuntze) plants may explain their different extraction efficiency into tea. Food Chem. Toxicol. 2020, 135, 110974. [Google Scholar] [CrossRef]
- Siener, R.; Hesse, A. Effect of black tea consumption on urinary risk factors for kidney stone formation. Nutrients 2021, 13, 4434. [Google Scholar] [CrossRef]
- Jaeger, P.; Robertson, W.G. Role of dietary intake and intestinal absorption of oxalate in calcium stone formation. Nephron Physiol. 2004, 98, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New insights on the role of manganese in Alzheimer’s disease and Parkinson’s disease. Int. J. Environ. Res. 2019, 16, 3546. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M. Nutrition Standards for the Polish Population; Food and Nutrition Institute: Warszawa, Poland, 2017; ISBN 978-83-86060-89-4. [Google Scholar]
- Podwika, W.; Kleszcz, K.; Krośniak, M.; Zagrodzki, P. Copper, Manganese, Zinc, and Cadmium in Tea Leaves of Different Types and Origin. Biol. Trace Elem. Res. 2018, 183, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ. Monit. Assess. 2016, 188, 183. [Google Scholar] [CrossRef] [Green Version]
| Tea Type | Extraction Time [min] | Total Phenolic Compounds [mg/100 mL] | Oxalates [mg/100 mL] | Mineral Elements [µg/100 mL] | |||
|---|---|---|---|---|---|---|---|
| Ca | Na | Cu | Mn | ||||
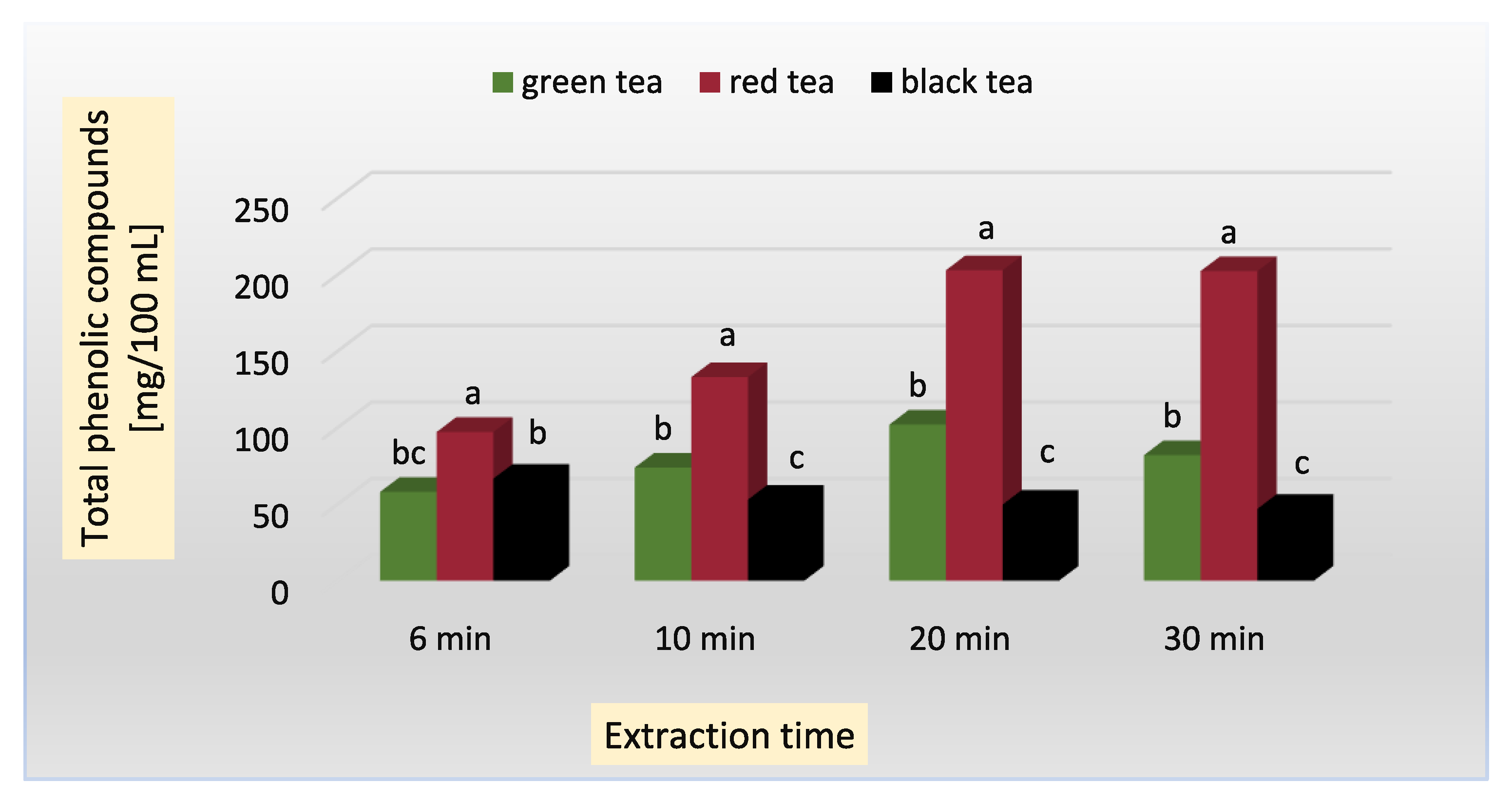
| green | 6 | 58.0 ± 0.81 fg | 18.00 ± 0.00 fg | 132.1 ± 1.89 g | 44.83 ± 0.04 c | 3.90 ± 0.03 c | 370.8 ± 0.03 c |
| 10 | 73.9 ± 1.57 de | 24.24 ± 2.30 cd | 144.1 ± 2.14 f | 42.88 ± 0.11 c | 4.10 ± 0.04 c | 470.1 ± 2.16 b | |
| 20 | 102.0 ± 0.98 c | 26.96 ± 1.77 b | 164.2 ± 2.06 e | 42.10 ± 0.16 c | 6.12 ± 0.11 b | 560.2 ± 2.46 a | |
| 30 | 82.0 ± 2.30 d | 30.88 ± 1.35 a | 179.3 ± 1.99 d | 42.15 ± 0.10 c | 5.86 ± 0.22 b | 539.1 ± 3.19 a | |
| red (pu-erh) | 6 | 97.2 ± 2.26 c | 15.06 ± 0.74 g | 250.3 ±2.45 c | 260.12 ± 1.06 a | 2.40 ± 0.03 f | 140.4 ± 2.22 f |
| 10 | 133.0 ± 3.17 b | 21.80 ± 0.24 de | 255.1 ± 2.87 bc | 261.37 ± 2.04 a | 3.09 ± 0.02 c | 190.2 ± 4.11 e | |
| 20 | 202.9 ± 3.00 a | 29.05 ± 0.14 ab | 262.2 ± 2.88 b | 261.00 ± 1.11 a | 2.30 ± 0.04 f | 230.1 ± 0.09 d | |
| 30 | 202.3 ± 5.74 a | 28.71 ± 0.64 ab | 282.2 ± 1.66 a | 262.13 ± 0.22 a | 2.60 ± 0.07 e | 236.3 ± 1.42 d | |
| black | 6 | 66.6 ± 1.42 ef | 17.19 ± 0.78 fg | 49.3 ± 0.22 k | 61.12 ± 1.70 b | 8.42 ± 0.55 a | 172.4 ± 3.14 e |
| 10 | 52.9 ± 4.41 gh | 17.54 ± 0.74 fg | 55.7 ± 0.67 j | 63.22 ± 2.34 b | 4.14 ± 0.21 c | 223.1 ± 2.03 d | |
| 20 | 49.7 ± 1.18 gh | 18.50 ± 0.90 f | 65.1 ± 0.76 i | 64.11 ± 0.66 b | 4.30 ± 0.20 c | 240.1± 2.89 d | |
| 30 | 46.9 ± 0.98 h | 19.93 ± 1.11 e | 78.1 ± 0.11 h | 64.28 ± 0.53 b | 5.40 ± 0.04 b | 257.0 ± 3.11 d | |
| Tea Type | Extraction Time [min] | Total Phenolic Compounds [%] * | Oxalates [%] * | Mineral Elements [%] * | |||
|---|---|---|---|---|---|---|---|
| Ca | Na | Cu | Mn | ||||
| green | 6 | * | * | * | * | * | * |
| 10 | ↑ 27 | ↑ 34 | ↑ 9 | ↓ 5 | ↑ 5 | ↑ 27 | |
| 20 | ↑ 75 | ↑ 49 | ↑ 24 | ↓ 6 | ↑ 57 | ↑ 51 | |
| 30 | ↑ 41 | ↑ 71 | ↑ 35 | ↓ 6 | ↑ 50 | ↑ 45 | |
| red | 6 | * | * | * | * | * | * |
| 10 | ↑ 36 | ↑ 45 | ↑ 2 | - ** | ↑ 29 | ↑ 35 | |
| 20 | ↑ 108 | ↑ 93 | ↑ 5 | - ** | ↓ 4 | ↑ 64 | |
| 30 | ↑ 108 | ↑ 91 | ↑ 13 | - ** | ↑ 8 | ↑ 68 | |
| black | 6 | * | * | * | * | * | * |
| 10 | ↓ 21 | ↑ 2 | ↑ 13 | ↑ 3 | ↓ 50 | ↑ 29 | |
| 20 | ↓ 25 | ↑ 7 | ↑ 32 | ↑ 5 | ↓ 49 | ↑ 39 | |
| 30 | ↓ 30 | ↑ 15 | ↑ 58 | ↑ 5 | ↓ 36 | ↑ 49 | |
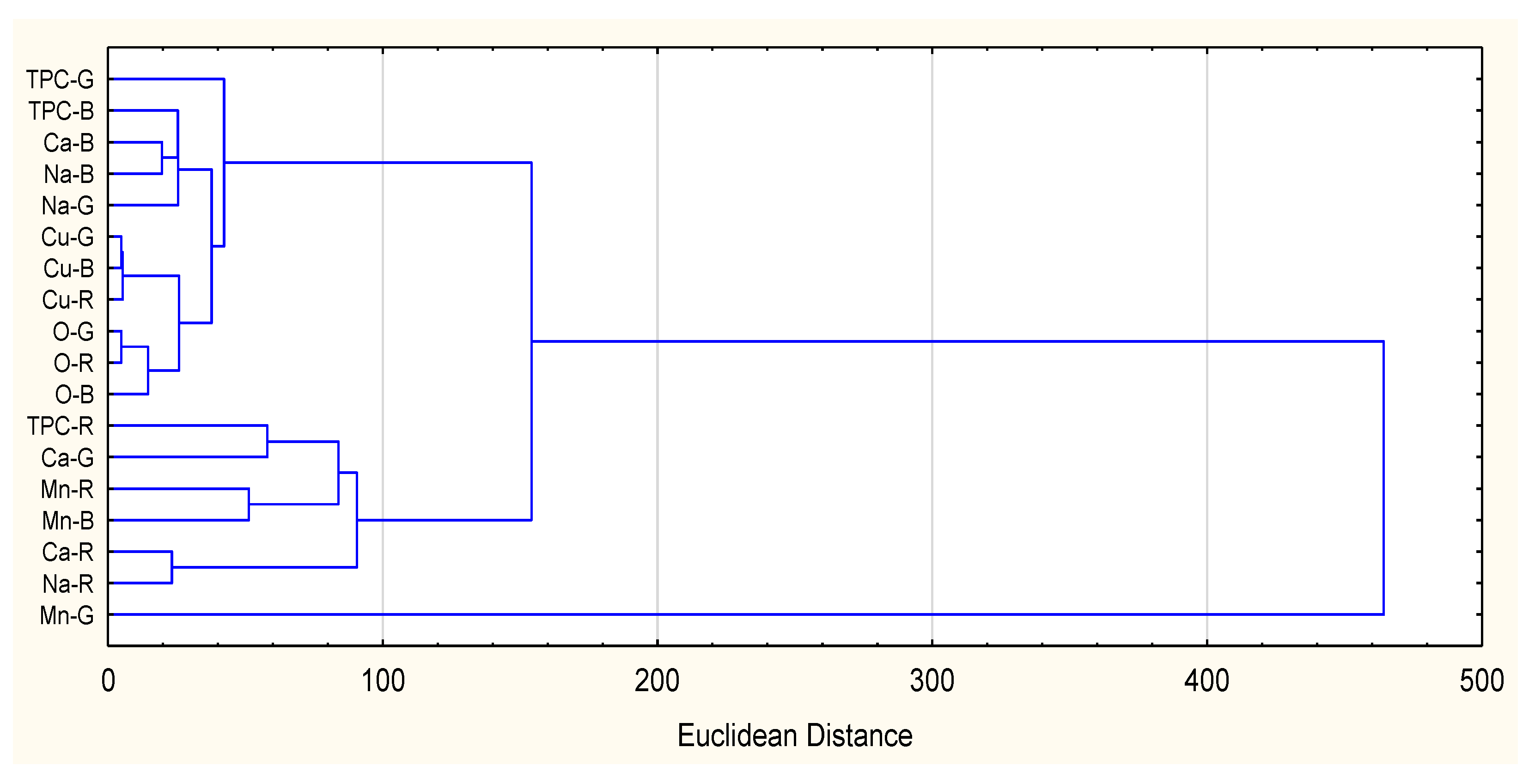
| TPC | Oxalates | Ca | Na | Cu | |
| Oxalates | 0.6209 * | ||||
| Ca | 0.8516 * | 0.5126 | |||
| Na | 0.8045 * | 0.1357 | 0.8322 * | ||
| Cu | −0.5482 | −0.0901 | −0.6807 * | −0.7211 * | |
| Mn | −0.1678 | 0.5703 | −0.0532 | −0.5869 * | 0.3125 |
| Type of Tea | |||
|---|---|---|---|
| Average Content of Minerals Determined in Tea Groups, RDA *, DDC *, and AI * | Green | Red | Black |
| Ca (mg/L) | 1.79 | 2.82 | 0.78 |
| Selected brewing time (min) * | 30 | 30 | 30 |
| RDA (mg/person) | 1000–1200 | 1000–1200 | 1000–1200 |
| DDC (%) | 0.15–0.18 | 0.24–0.28 | 0.07–0.08 |
| Na (mg/L) | 0.44–0.42 | 2.60–2.62 | 0.61–0.64 |
| Selected brewing time * | 6–30 | 6–30 | 6–30 |
| AI (mg/person) | 1300–1500 | 1300–1500 | 1300–1500 |
| DDC (%) | 0.03 | 0.17–0.20 | 0.04–0.05 |
| Cu (mg/L) | 0.06 | 0.03 | 0.08 |
| Selected brewing time * | 20–30 | 10 | 6 |
| RDA (mg/person) | 0.90 | 0.90 | 0.90 |
| DDC (%) | 6.66 | 3.33 | 8.88 |
| Mn (mg/L) | 5.60–5.39 | 2.30–2.36 | 2.23–2.57 |
| Selected brewing time * | 20–30 | 20–30 | 10–30 |
| AI (mg/person) | 1.8–2.3 | 1.8–2.3 | 1.8–2.3 |
| DDC (%) | 243–311 | 102–131 | 111–142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klepacka, J. Tea Infusions as a Source of Phenolic Compounds in the Human Diet. Appl. Sci. 2022, 12, 4227. https://doi.org/10.3390/app12094227
Klepacka J. Tea Infusions as a Source of Phenolic Compounds in the Human Diet. Applied Sciences. 2022; 12(9):4227. https://doi.org/10.3390/app12094227
Chicago/Turabian StyleKlepacka, Joanna. 2022. "Tea Infusions as a Source of Phenolic Compounds in the Human Diet" Applied Sciences 12, no. 9: 4227. https://doi.org/10.3390/app12094227