Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecules
2.2. Cell Culture
2.3. LED Light Source
2.4. Blue-Violet Light Irradiation
2.5. Measurement of Intracellular ROS
2.6. Cell Viability
2.7. Analysis of Apoptotic Cells
2.8. Western Blot Analyses of Cell Lysates
2.9. Statistics
3. Results
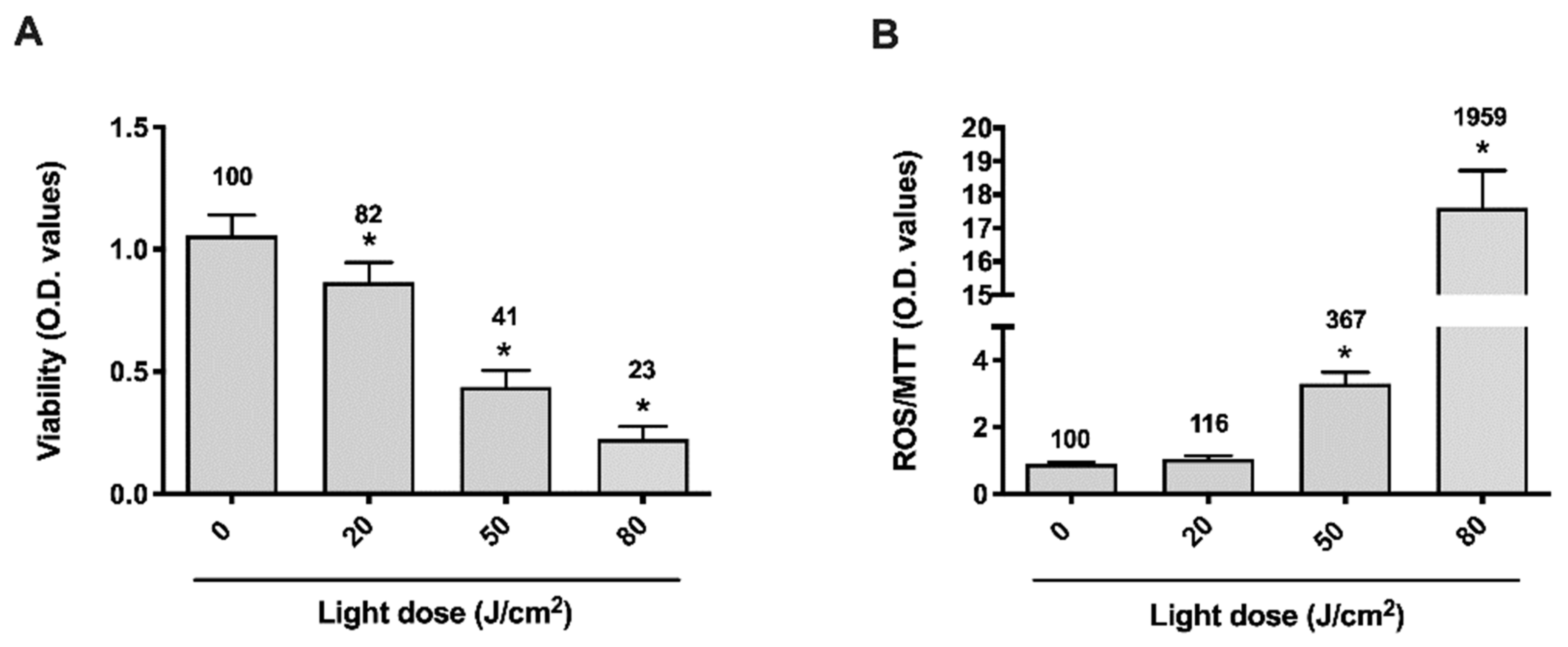
3.1. Blue-Violet LED Dose-Effect
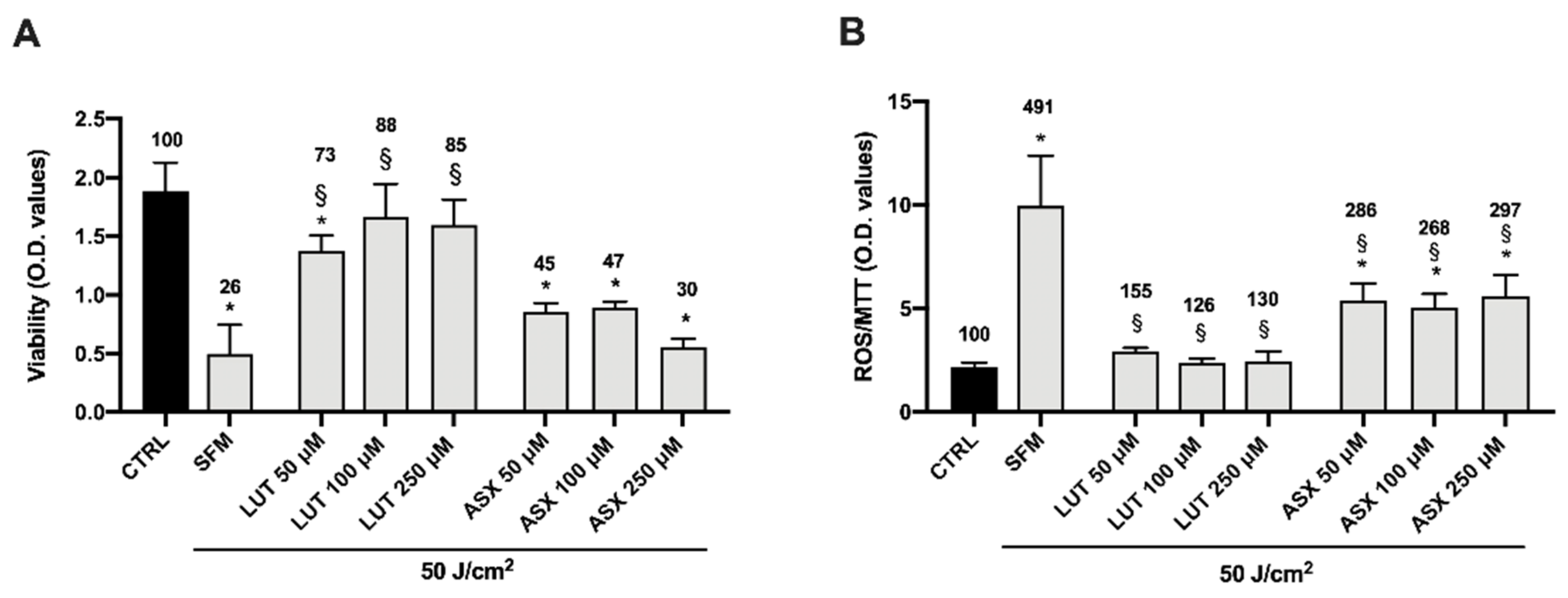
3.2. Dose-Effect Response of Lutein and Astaxanthin
3.3. Cooperative Effect of Lutein and Astaxanthin
3.4. Cell Death Induced by Blue-Violet Light Exposure in HCE-F Cells and Protective Role of Antioxidants
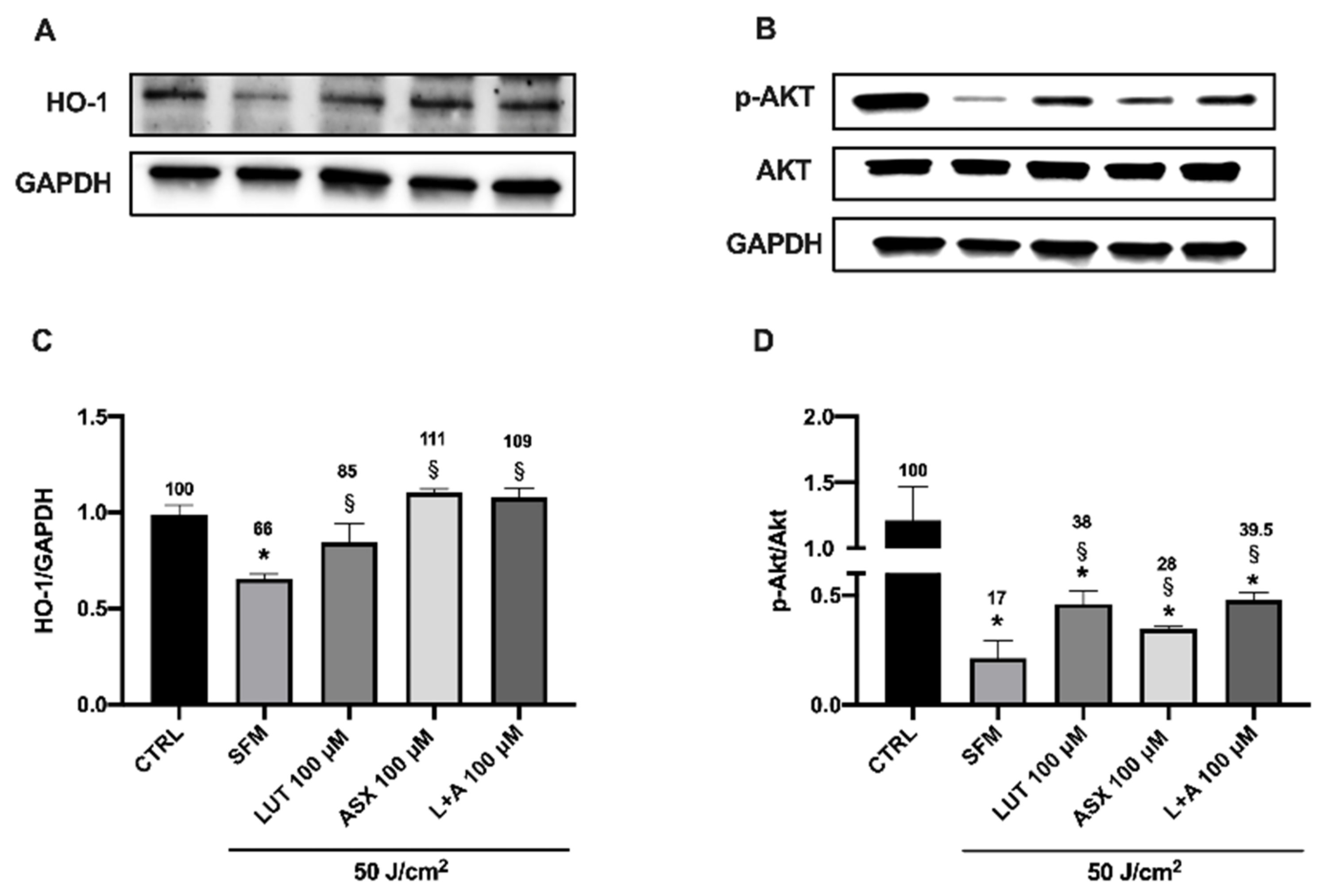
3.5. Western Blot Analysis of Endogenous Antioxidant Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.-C.; Zhou, Y.; Tan, G.; Li, J. Research Progress about the Effect and Prevention of Blue Light on Eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Ferguson, I.; Tsubota, K. Effects of Blue Light on the Circadian System and Eye Physiology. Mol. Vis. 2016, 22, 61–72. [Google Scholar] [PubMed]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-Emitting Diodes (LED) for Domestic Lighting: Any Risks for the Eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, S.E.; Pfaff, N.; Denault, K.A.; Zhang, Z.; (Bert) Hintzen, H.T.; Seshadri, R.; Nakamura, S.; DenBaars, S.P. Robust Thermal Performance of Sr2Si5N8:Eu2+: An Efficient Red Emitting Phosphor for Light Emitting Diode Based White Lighting. Appl. Phys. Lett. 2011, 99, 241106. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.-M.; Wang, G.-S.; Sliney, D.; Yang, C.-H.; Lee, L.-L. White Light-Emitting Diodes (LEDs) at Domestic Lighting Levels and Retinal Injury in a Rat Model. Environ. Health Perspect 2014, 122, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue Light-Induced Inflammatory Marker Expression in the Retinal Pigment Epithelium-Choroid of Mice and the Protective Effect of a Yellow Intraocular Lens Material in Vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.-I.; Seo, Y.-J.; Park, W.-S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue Light Effect on Retinal Pigment Epithelial Cells by Display Devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef]
- Seko, Y.; Pang, J.; Tokoro, T.; Ichinose, S.; Mochizuki, M. Blue Light-Induced Apoptosis in Cultured Retinal Pigment Epithelium Cells of the Rat. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 239, 47–52. [Google Scholar] [CrossRef]
- Roehlecke, C.; Schumann, U.; Ader, M.; Knels, L.; Funk, R.H.W. Influence of Blue Light on Photoreceptors in a Live Retinal Explant System. Mol. Vis. 2011, 17, 876–884. [Google Scholar]
- Nakamura, M.; Yako, T.; Kuse, Y.; Inoue, Y.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Exposure to Excessive Blue LED Light Damages Retinal Pigment Epithelium and Photoreceptors of Pigmented Mice. Exp. Eye Res. 2018, 177, 1–11. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Cui, L.; Li, Y.; Choi, J.S.; Choi, J.-H.; Li, Z.; Kim, G.E.; Choi, W.; Yoon, K.C. Influence of Light Emitting Diode-Derived Blue Light Overexposure on Mouse Ocular Surface. PLoS ONE 2016, 11, e0161041. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in Human Blood and Tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary Identification of the Human Macular Pigment. Vis. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, Zeaxanthin, and Meso-Zeaxanthin: The Basic and Clinical Science Underlying Carotenoid-Based Nutritional Interventions against Ocular Disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.M.; Meagher, K.; Kashani, S.; Beatty, S. What Is Meso -Zeaxanthin, and Where Does It Come From? Eye 2013, 27, 899–905. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications--a Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of Vertebrate Visual Pigments. Vis. Res. 2008, 48, 2022–2041. [Google Scholar] [CrossRef] [Green Version]
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-J.; Taylor, A. Nutritional Antioxidants and Age-Related Cataract and Maculopathy. Exp. Eye Res. 2007, 84, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Krishnadev, N.; Meleth, A.D.; Chew, E.Y. Nutritional Supplements for Age-Related Macular Degeneration. Curr. Opin. Ophthalmol. 2010, 21, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L.; AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Gohto, Y.; Nakazawa, R.; Moriyama, T.; Gellermann, W.; Bernstein, P.S. Effect of an Antioxidant Supplement Containing High Dose Lutein and Zeaxanthin on Macular Pigment and Skin Carotenoid Levels. Sci. Rep. 2020, 10, 10262. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Identification and Quantitation of Carotenoids and Their Metabolites in the Tissues of the Human Eye. Exp. Eye Res. 2001, 72, 215–223. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, H.-C.; Wu, W.-C.; Sahoo, S.L.; Hsu, C.-Y. Novel Lutein Loaded Lipid Nanoparticles on Porcine Corneal Distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus Pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef]
- Cristaldi, M.; Olivieri, M.; Spampinato, G.; Anfuso, C.D.; Scalia, M.; Lupo, G.; Rusciano, D. Isolation and Characterization of a New Human Corneal Epithelial Cell Line: HCE-F. Cornea 2020, 39, 1419–1425. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, P.; Wang, W.; Xu, Y.; Wang, M.; Chen, X.; Dong, X. Long-Term Blue Light Exposure Induces RGC-5 Cell Death in Vitro: Involvement of Mitochondria-Dependent Apoptosis, Oxidative Stress, and MAPK Signaling Pathways. Apoptosis 2014, 19, 922–932. [Google Scholar] [CrossRef]
- Dichlorodihydrofluorescein Diacetate—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/dichlorodihydrofluorescein-diacetate (accessed on 4 November 2021).
- Jakubowski, W.; Bartosz, G. 2,7-Dichlorofluorescin Oxidation and Reactive Oxygen Species: What Does It Measure? Cell Biol. Int. 2000, 24, 757–760. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The Antioxidant and Anti-Inflammatory Effects of Astaxanthin Supplementation on the Expression of MiR-146a and MiR-126 in Patients with Type 2 Diabetes Mellitus: A Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef] [PubMed]
- Radkar, P.; Lakshmanan, P.S.; Mary, J.J.; Chaudhary, S.; Durairaj, S.K. A Novel Multi-Ingredient Supplement Reduces Inflammation of the Eye and Improves Production and Quality of Tears in Humans. Ophthalmol. Ther. 2021, 10, 581–599. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Shinjo, T.; Tanaka, T.; Okuda, H.; Kawaguchi, A.T.; Oh-hashi, K.; Terada, Y.; Isonishi, A.; Morita-Takemura, S.; Tatsumi, K.; Kawaguchi, M.; et al. Propofol Induces Nuclear Localization of Nrf2 under Conditions of Oxidative Stress in Cardiac H9c2 Cells. PLoS ONE 2018, 13, e0196191. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) Signaling in Oxidative Stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Xu, J.; Cai, Y.; Yang, Y.; Liu, Y.; Cao, S. Cytoprotection against Oxidative Stress by Methylnissolin-3-O-β-d-Glucopyranoside from Astragalus Membranaceus Mainly via the Activation of the Nrf2/HO-1 Pathway. Molecules 2021, 26, 3852. [Google Scholar] [CrossRef]
- O’Connor, I.; O’Brien, N. Modulation of UVA Light-Induced Oxidative Stress by β-Carotene, Lutein and Astaxanthin in Cultured Fibroblasts. J. Dermatol. Sci. 1998, 16, 226–230. [Google Scholar] [CrossRef]
- Roberts, J.E. Ocular Phototoxicity. J. Photochem. Photobiol. B 2001, 64, 136–143. [Google Scholar] [CrossRef]
- Jaadane, I.; Rodriguez, G.E.V.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of White Light-Emitting Diode (LED) Exposure on Retinal Pigment Epithelium in Vivo. J. Cell. Mol. Med. 2017, 21, 3453–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Hirasawa, M.; Nagai, N.; Shimmura, S.; Tsubota, K. Disruption of Cell-Cell Junctions and Induction of Pathological Cytokines in the Retinal Pigment Epithelium of Light-Exposed Mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4555–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schick, T.; Ersoy, L.; Lechanteur, Y.T.; Saksens, N.T.; Hoyng, C.B.; Den Hollander, A.I.; Kirchhof, B.; Fauser, S. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina 2016, 36, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.-Y.; Liu, G.-C.; Liu, G.-Y.; Gao, Y.-Y.; Deng, Y.; Wang, W.-Y.; Tong, S.-H.; Wang, L. Is Sunlight Exposure a Risk Factor for Age-Related Macular Degeneration? A Systematic Review and Meta-Analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the Ocular Surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Niwano, Y.; Iwasawa, A.; Tsubota, K.; Ayaki, M.; Negishi, K. Protective Effects of Blue Light-Blocking Shades on Phototoxicity in Human Ocular Surface Cells. BMJ Open Ophthalmol. 2019, 4, e000217. [Google Scholar] [CrossRef] [Green Version]
- Marek, V.; Mélik-Parsadaniantz, S.; Villette, T.; Montoya, F.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A. Blue Light Phototoxicity toward Human Corneal and Conjunctival Epithelial Cells in Basal and Hyperosmolar Conditions. Free Radic. Biol. Med. 2018, 126, 27–40. [Google Scholar] [CrossRef]
- Kaido, M.; Toda, I.; Oobayashi, T.; Kawashima, M.; Katada, Y.; Tsubota, K. Reducing Short-Wavelength Blue Light in Dry Eye Patients with Unstable Tear Film Improves Performance on Tests of Visual Acuity. PLoS ONE 2016, 11, e0152936. [Google Scholar] [CrossRef] [Green Version]
- Jun, I.; Han, S.J.; Shin, H.-S.; Kim, J.; Kim, E.K.; Kim, T.; Yoon, S.C.; Seo, K.Y. Comparison of Ophthalmic Toxicity of Light-Emitting Diode and Organic Light-Emitting Diode Light Sources. Sci. Rep. 2020, 10, 11582. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Álvarez, C.; Osborne, N.N. Enhancement of Corneal Epithelium Cell Survival, Proliferation and Migration by Red Light: Relevance to Corneal Wound Healing. Exp. Eye Res. 2019, 180, 231–241. [Google Scholar] [CrossRef]
- Roberts, J.E.; Dennison, J. The Photobiology of Lutein and Zeaxanthin in the Eye. J. Ophthalmol. 2015, 2015, 687173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and Zeaxanthin Supplementation Reduces Photooxidative Damage and Modulates the Expression of Inflammation-Related Genes in Retinal Pigment Epithelial Cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Li, D.; Zhang, Y.; Zhang, L.; Liao, Z.; Aihemaitijiang, S.; Hou, Y.; Zhan, Z.; Xie, K.; Zhang, Z. Lutein Protected the Retina from Light Induced Retinal Damage by Inhibiting Increasing Oxidative Stress and Inflammation. J. Funct. Foods 2020, 73, 104107. [Google Scholar] [CrossRef]
- Chucair, A.J.; Rotstein, N.P.; SanGiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and Zeaxanthin Protect Photoreceptors from Apoptosis Induced by Oxidative Stress: Relation with Docosahexaenoic Acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5168–5177. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.-Y.; Jin, J.; Lu, G.; Kang, X.-L. Astaxanthin Attenuates the Apoptosis of Retinal Ganglion Cells in Db/Db Mice by Inhibition of Oxidative Stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. Protective Effects of a Dietary Carotenoid, Astaxanthin, against Light-Induced Retinal Damage. J. Pharmacol. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin Protects ARPE-19 Cells from Oxidative Stress via Upregulation of Nrf2-Regulated Phase II Enzymes through Activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar]
- Inoue, Y.; Shimazawa, M.; Nagano, R.; Kuse, Y.; Takahashi, K.; Tsuruma, K.; Hayashi, M.; Ishibashi, T.; Maoka, T.; Hara, H. Astaxanthin Analogs, Adonixanthin and Lycopene, Activate Nrf2 to Prevent Light-Induced Photoreceptor Degeneration. J. Pharmacol. Sci. 2017, 134, 147–157. [Google Scholar] [CrossRef]
- Lin, C.-W.; Yang, C.-M.; Yang, C.-H. Protective Effect of Astaxanthin on Blue Light Light-Emitting Diode-Induced Retinal Cell Damage via Free Radical Scavenging and Activation of PI3K/Akt/Nrf2 Pathway in 661W Cell Model. Mar. Drugs 2020, 18, 387. [Google Scholar] [CrossRef]
- Lee, J.-B.; Kim, S.-H.; Lee, S.-C.; Kim, H.-G.; Ahn, H.-G.; Li, Z.; Yoon, K.C. Blue Light-Induced Oxidative Stress in Human Corneal Epithelial Cells: Protective Effects of Ethanol Extracts of Various Medicinal Plant Mixtures. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4119–4127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennikov, A.; Kitaichi, N.; Fukase, R.; Murata, M.; Noda, K.; Ando, R.; Ohguchi, T.; Kawakita, T.; Ohno, S.; Ishida, S. Amelioration of Ultraviolet-Induced Photokeratitis in Mice Treated with Astaxanthin Eye Drops. Mol. Vis. 2012, 18, 455–464. [Google Scholar] [PubMed]
- Harada, F.; Morikawa, T.; Lennikov, A.; Mukwaya, A.; Schaupper, M.; Uehara, O.; Takai, R.; Yoshida, K.; Sato, J.; Horie, Y.; et al. Protective Effects of Oral Astaxanthin Nanopowder against Ultraviolet-Induced Photokeratitis in Mice. Oxid Med. Cell. Longev. 2017, 2017, 1956104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-J.; Wu, C.; Xu, Z.; Kuse, Y.; Hara, H.; Duh, E.J. Nrf2 Protects Photoreceptor Cells from Photo-Oxidative Stress Induced by Blue Light. Exp. Eye Res. 2017, 154, 151–158. [Google Scholar] [CrossRef]
- Surin, A.M.; Sharipov, R.R.; Krasil’nikova, I.A.; Boyarkin, D.P.; Lisina, O.Y.; Gorbacheva, L.R.; Avetisyan, A.V.; Pinelis, V.G. Disruption of Functional Activity of Mitochondria during MTT Assay of Viability of Cultured Neurons. Biochem. Mosc. 2017, 82, 737–749. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zeng, W.; Wei, J. Edaravone Protects against Hyperosmolarity-Induced Oxidative Stress and Apoptosis in Primary Human Corneal Epithelial Cells. PLoS ONE 2017, 12, e0174437. [Google Scholar] [CrossRef]
- Giurdanella, G.; Longo, A.; Salerno, L.; Romeo, G.; Intagliata, S.; Lupo, G.; Distefano, A.; Platania, C.B.M.; Bucolo, C.; Li Volti, G.; et al. Glucose-Impaired Corneal Re-Epithelialization Is Promoted by a Novel Derivate of Dimethyl Fumarate. Antioxidants 2021, 10, 831. [Google Scholar] [CrossRef]
- Cristaldi, M.; Olivieri, M.; Pezzino, S.; Spampinato, G.; Lupo, G.; Anfuso, C.D.; Rusciano, D. Atropine Differentially Modulates ECM Production by Ocular Fibroblasts, and Its Ocular Surface Toxicity Is Blunted by Colostrum. Biomedicines 2020, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaramillo, M.C.; Zhang, D.D. The Emerging Role of the Nrf2–Keap1 Signaling Pathway in Cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.-J.; Hyun, J.W. Up-Regulation of Nrf2-Mediated Heme Oxygenase-1 Expression by Eckol, a Phlorotannin Compound, through Activation of Erk and PI3K/Akt. Int. J. Biochem. Cell. Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, M.; Zhu, P.; Huang, H.; Zhuang, Q.; Shen, J.; Cai, Y.; Zhao, M.; He, Q. New Insights into the Nrf-2/HO-1 Signaling Axis and Its Application in Pediatric Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, e3214196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential Roles of the PI3 Kinase/Akt Pathway in Regulating Nrf2-Dependent Antioxidant Functions in the RPE. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristaldi, M.; Anfuso, C.D.; Spampinato, G.; Rusciano, D.; Lupo, G. Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage. Appl. Sci. 2022, 12, 1268. https://doi.org/10.3390/app12031268
Cristaldi M, Anfuso CD, Spampinato G, Rusciano D, Lupo G. Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage. Applied Sciences. 2022; 12(3):1268. https://doi.org/10.3390/app12031268
Chicago/Turabian StyleCristaldi, Martina, Carmelina Daniela Anfuso, Giorgia Spampinato, Dario Rusciano, and Gabriella Lupo. 2022. "Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage" Applied Sciences 12, no. 3: 1268. https://doi.org/10.3390/app12031268
APA StyleCristaldi, M., Anfuso, C. D., Spampinato, G., Rusciano, D., & Lupo, G. (2022). Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage. Applied Sciences, 12(3), 1268. https://doi.org/10.3390/app12031268






