Physiological and Biochemical Variations in Celery by Imidacloprid and Fenpyroximate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Analytical Techniques
2.3.1. Regular Index Analysis
2.3.2. Determination of Physiological Parameters
2.3.3. Pesticide Half-Lives and Statistical Analyses
3. Results
3.1. Imidacloprid and Fenpyroximate Residue Concentration
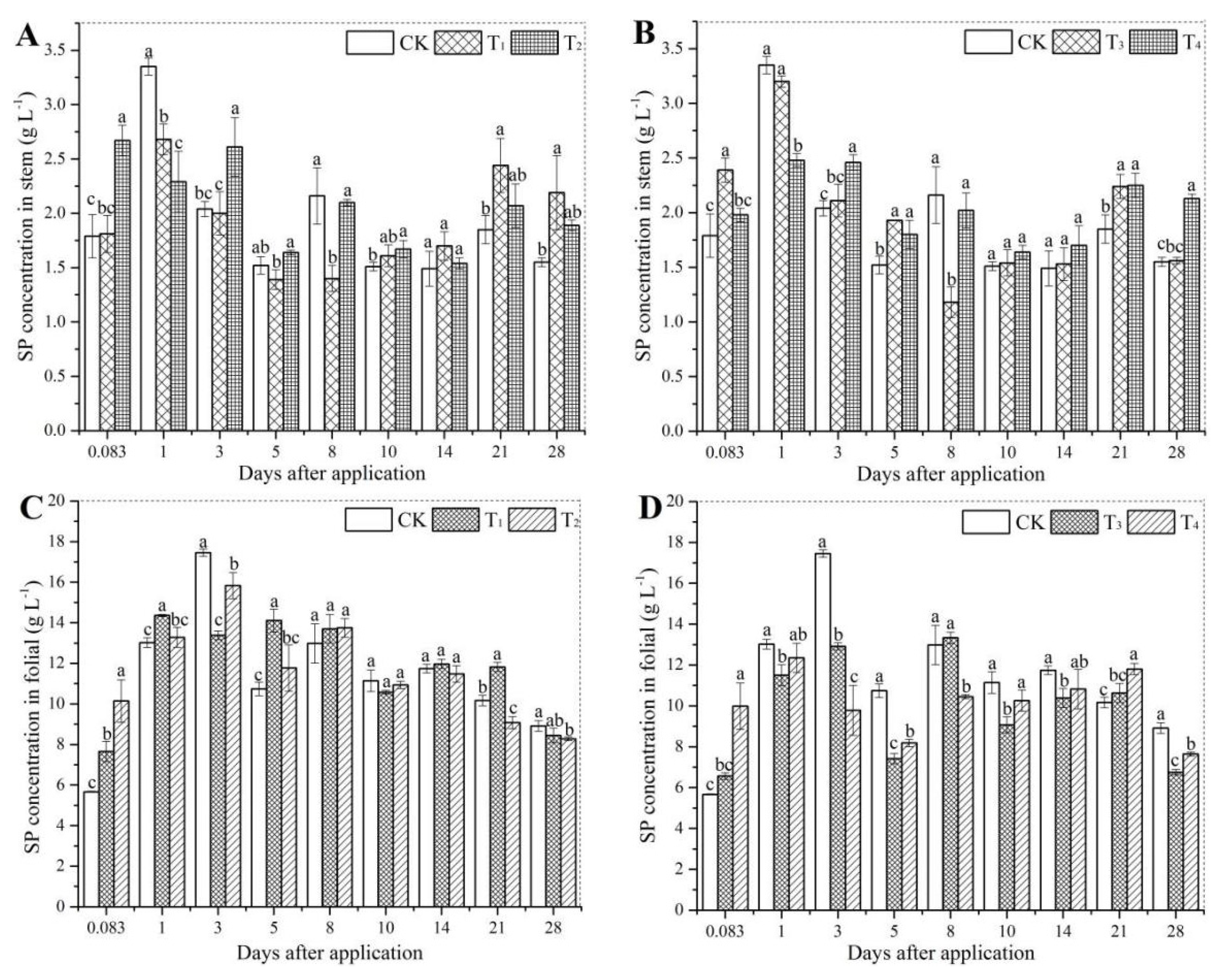
3.2. Soluble Protein Accumulation
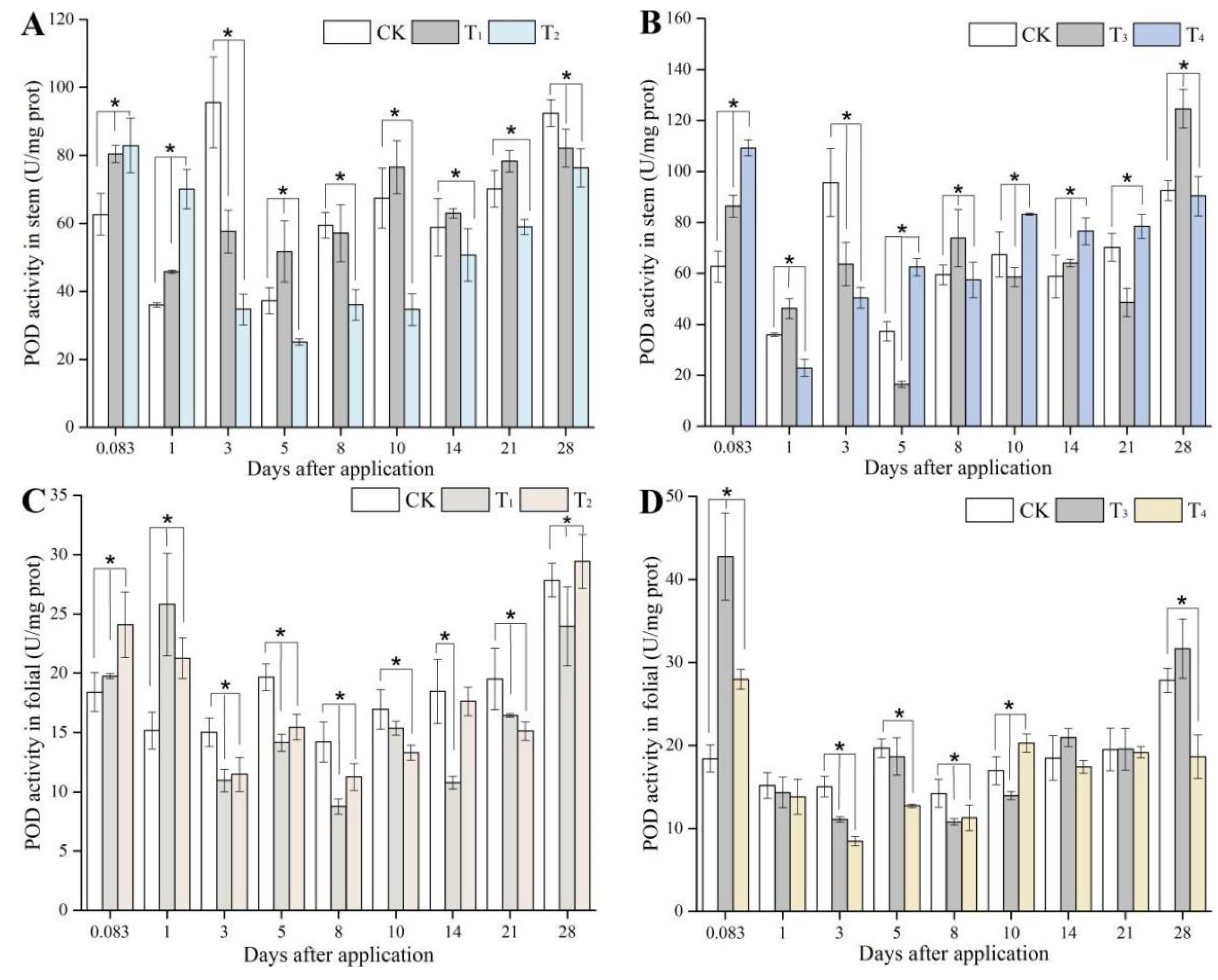
3.3. Peroxidase Activity
3.4. Superoxide Dismutase Activity
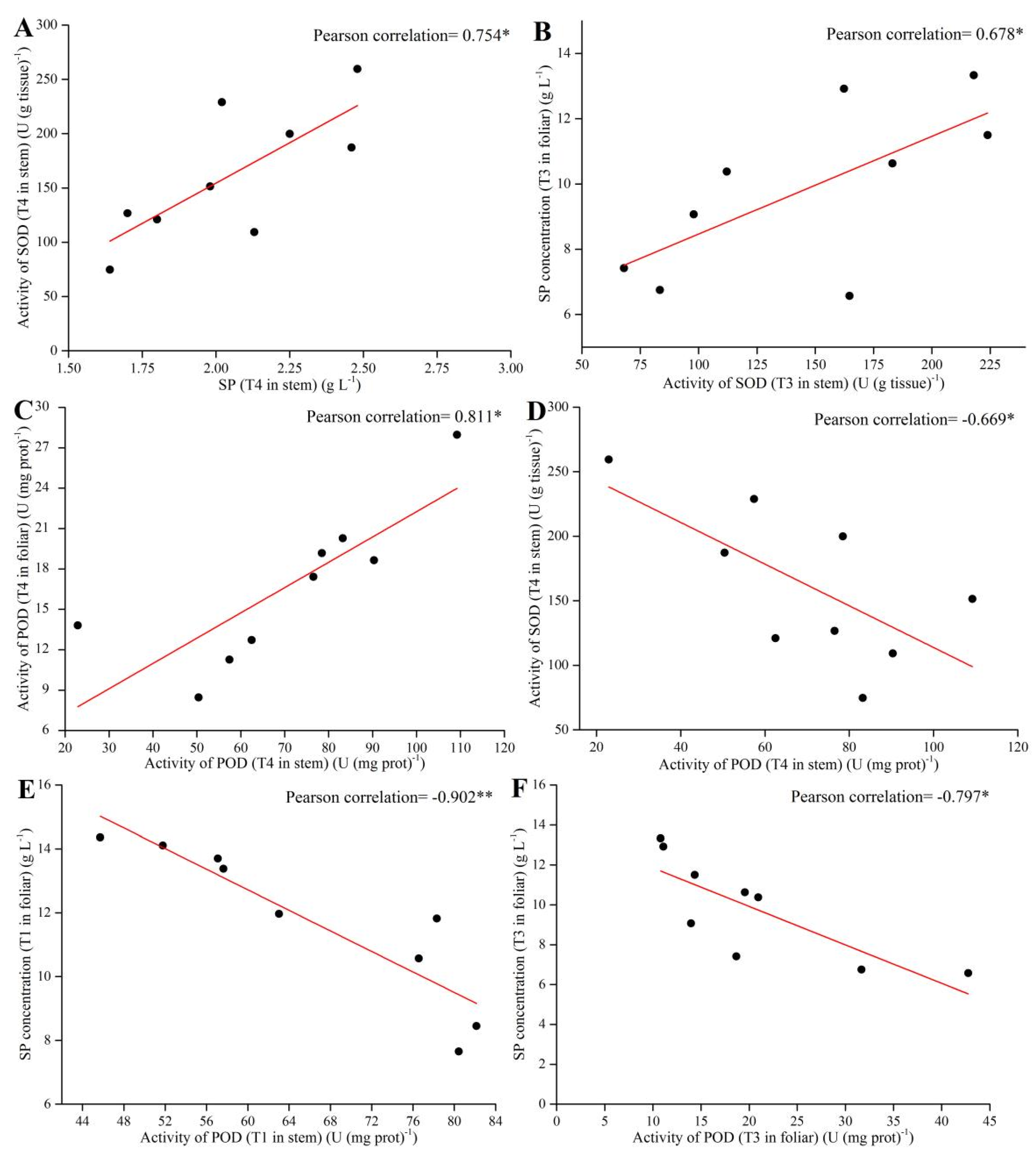
3.5. Pearson Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etesami, H.; Fatemi, H.; Rizwan, M. Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: A review. Ecotoxicol. Environ. Saf. 2021, 225, 112769. [Google Scholar] [CrossRef] [PubMed]
- Homayoonzadeh, M.; Hosseininaveh, V.; Haghighi, S.R.; Talebi, K.; Roessner, U.; Maali-Amiri, R. Evaluation of physiological and biochemical responses of pistachio plants (Pistacia vera L.) exposed to pesticides. Ecotoxicology 2021, 30, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. World Food and Agriculture-Statistical Yearbook 2020. Rome. Available online: https://www.fao.org/documents/card/en/c/cb1329en (accessed on 21 April 2022).
- Li, X.; Giles, D.K.; Niederholzer, F.J.; Andaloro, J.T.; Lang, E.B.; Watson, L.J. Evaluation of an unmanned aerial vehicle as a new method of pesticide application for almond crop protection. Pest Manag. Sci. 2021, 77, 527–537. [Google Scholar] [CrossRef]
- Amirgaliev, N.A.; Askarova, M.; Opp, C.; Medeu, A.; Kulbekova, R.; Medeu, A.R. Water Quality Problems Analysis and Assessment of the Ecological Security Level of the Transboundary Ural-Caspian Basin of the Republic of Kazakhstan. Appl. Sci. 2022, 12, 2059. [Google Scholar] [CrossRef]
- Melendez-Pastor, I.; Hernández, E.I.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Lucas, I.G.; Vidal, M.M.J. Occurrence of Pesticides Associated with an Agricultural Drainage System in a Mediterranean Environment. Appl. Sci. 2021, 11, 10212. [Google Scholar] [CrossRef]
- Berg, H. Pesticide use in rice and rice-fish farms in the Mekong Delta, Vietnam. Crop Prot. 2001, 20, 897–905. [Google Scholar] [CrossRef]
- Guler, G.O.; Cakmak, Y.S.; Dagli, Z.; Aktumsek, A.; Ozparlak, H. Organochlorine pesticide residues in wheat from Konya region, Turkey. Food Chem. Toxicol. 2010, 48, 1218–1221. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Ni, Z.; Qu, M.; Zhong, D.; Ye, C.; Tang, F. Pesticides in persimmons, jujubes and soil from China: Residue levels, risk assessment and relationship between fruits and soils. Sci. Total Environ. 2016, 542, 620–628. [Google Scholar] [CrossRef]
- Golge, O.; Hepsag, F.; Kabak, B. Health risk assessment of selected pesticide residues in green pepper and cucumber. Food Chem. Toxicol. 2018, 121, 51–64. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, S.; Chen, Z.; Du, H.; Zhu, Q.; Dong, Z.; Li, H. Risk assessment of pesticide residues in dietary intake of celery in China. Regul. Toxicol. Pharm. 2015, 73, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Koh, H.M.; Dayal, H.; Ren, Y.; Li, S.F.Y. Optimisation of an aglycone-enhanced celery extract with germinated soy supplementation using response surface methodology. Foods 2021, 10, 2505. [Google Scholar] [CrossRef] [PubMed]
- Arsenov, D.; Župunski, M.; Pajević, S.; Borišev, M.; Nikolić, N.; Mimica-Dukić, N. Health assessment of medicinal herbs, celery and parsley related to cadmium soil pollution-potentially toxic elements (PTEs) accumulation, tolerance capacity and antioxidative response. Environ. Geochem. Health 2021, 43, 2927–2943. [Google Scholar] [CrossRef]
- Sutthi, N.; Panase, A.; Chitmanat, C.; Sookying, S.; Ratworawong, K.; Panase, P. Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aquac. Rep. 2020, 18, 100551. [Google Scholar] [CrossRef]
- GabAllah, M.; Kandeil, A.; Mousa, A.E.B.; Ali, M.A. Antiviral activity of water extracts of some medicinal and nutritive plants from the Apiaceae family. Nov. Res. Microbiol. J. 2020, 4, 725–735. [Google Scholar]
- Kharchenko, V.; Moldovan, A.; Golubkina, N.; Koshevarov, A.; Caruso, G. Antioxidant status of celery (Apium graveolens L.). Veg. Crops Russia 2020, 2, 82–86. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Li, Y.; Chen, L.; Wang, Y.; Xu, Z.; Jiang, J.; Tang, T.; Chen, W.; Wang, Q. Determination of imidacloprid and fenpyroximate in celery by QuEChERS—LC-MS/MS. Acta Agric. Zhejiangensis 2018, 30, 261–267. (In Chinese) [Google Scholar]
- Chen, L.; Wu, C.; Xu, M.; Cang, T.; Wang, X.; Zhao, X.; Zhang, C. Assessment of carbendazim residues and safety in celery under different cultivation conditions. Bull. Environ. Contam. Toxicol. 2021, 107, 276–280. [Google Scholar] [CrossRef]
- China Food and Drug Administration. Standard GB 2763-2021. 2021. Available online: http://www.jgs.moa.gov.cn/nybz/202103/t20210318_6364007.htm (accessed on 21 April 2022).
- Kang, L.; Liu, H.; Zhao, D.; Pan, C.; Wang, C. Pesticide residue behavior and risk assessment in celery after Se nanoparticles application. Foods 2021, 10, 1987. [Google Scholar] [CrossRef]
- Jie, M.; Gao, Y.; Kuang, D.; Shi, Y.; Wang, H.; Jing, W. Relationship between imidacloprid residues and control effect on cotton aphids in arid region. Environ. Geochem. Health 2021, 43, 1941–1952. [Google Scholar] [CrossRef]
- Grimalt, S.; Thompson, D.; Chartrand, D.; McFarlane, J.; Helson, B.; Lyons, B.; Meating, J.; Scarr, T. Foliar residue dynamics of azadirachtins following direct stem injection into white and green ash trees for control of emerald ash borer. Pest Manag. Sci. 2011, 67, 1277–1284. [Google Scholar] [CrossRef]
- Morales, S.I.; Martínez, A.M.; Figueroa, J.I.; Campos-García, J.; Gómez-Tagle, A.; Lobit, P.; Smagghe, G.; Pineda, S. Foliar persistence and residual activity of four insecticides of different mode of action on the predator Engytatus varians (Hemiptera: Miridae). Chemosphere 2019, 235, 76–83. [Google Scholar] [CrossRef]
- Pes, M.P.; Melo, A.A.; Stacke, R.S.; Zanella, R.; Perini, C.R.; Silva, F.M.A.; Guedes, J.V.C. Translocation of chlorantraniliprole and cyantraniliprole applied to corn as seed treatment and foliar spraying to control Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2020, 15, e0229151. [Google Scholar] [CrossRef] [Green Version]
- Gierer, F.; Vaughan, S.; Slater, M.; Thompson, H.M.; Elmore, J.S.; Girling, R.D. A review of the factors that influence pesticide residues in pollen and nectar: Future research requirements for optimising the estimation of pollinator exposure. Environ. Pollut. 2019, 249, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Lentola, A.; David, A.; Abdul-Sada, A.; Tapparo, A.; Goulson, D.; Hill, E.M. Ornamental plants on sale to the public are a significant source of pesticide residues with implications for the health of pollinating insects. Environ. Pollut. 2017, 228, 297–304. [Google Scholar] [CrossRef]
- Han, Y.; Mo, R.; Yuan, X.; Zhong, D.; Tang, F.; Ye, C.; Liu, Y. Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere 2017, 180, 42–47. [Google Scholar] [CrossRef]
- Seifrtova, M.; Halesova, T.; Sulcova, K.; Riddellova, K.; Erban, T. Distributions of imidacloprid, imidacloprid-olefin and imidacloprid-urea in green plant tissues and roots of rapeseed (Brassica napus) from artificially contaminated potting soil. Pest Manag. Sci. 2017, 73, 1010–1016. [Google Scholar] [CrossRef]
- Brar, P.K.; Kang, B.K.; Rasool, R.; Chhuneja, P.K. Dissipation kinetics of imidacloprid in cotton flower, nectariferous tissue, pollen and Apis mellifera products using QuEChERS method. Pest Manag. Sci. 2021, 78, 662–670. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Devakumar, B.; Li, B.; Huang, X. Novel far-red-emitting SrGdAlO4: Mn 4+ phosphors with excellent responsiveness to phytochrome P FR for plant growth lighting. RSC Adv. 2018, 8, 39307–39313. [Google Scholar] [CrossRef] [Green Version]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 transcription factor enhances heat and drought stress tolerance in wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Vitória, A.P.; Cunha, M.D.; Azevedo, R.A. Ultrastructural changes of radish leaf exposed to cadmium. Environ. Exp. Bot. 2006, 58, 47–52. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Nie, M.; Cheng, D.; Chen, J.; Wang, S.; Lv, J.; Niu, Y. Effects of Foliar Application of Nano-Se on Photosynthetic Characteristics and Se Accumulation in Paeonia Ostii. 2021. Available online: https://assets.researchsquare.com/files/rs-951366/v1/f435bc11-01ef-47a2-897d-60ef471c5691.pdf?c=1637245799 (accessed on 21 April 2022).
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631, 1100–1108. [Google Scholar] [CrossRef]
- Davies, L.C.; Carias, C.C.; Novais, J.M.; Martins-Dias, S. Phytoremediation of textile effluents containing azo dye by using Phragmites australis in a vertical flow intermittent feeding constructed wetland. Ecol. Eng. 2005, 25, 594–605. [Google Scholar] [CrossRef]
- Shi, S.; Feng, J.; Liang, Y.; Sun, H.; Yang, X.; Su, Z.; Luo, L.; Wang, J.; Zhang, W. Lycium barbarum polysaccharide-Iron (III) chelate as peroxidase mimics for total antioxidant capacity assay of fruit and vegetable food. Foods 2021, 10, 2800. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Zou, J.; Xu, J.; Wang, M.; Cai, H.; Yuan, B.; Ma, J. ABTS as an electron shuttle to accelerate the degradation of diclofenac with horseradish peroxidase-catalyzed hydrogen peroxide oxidation. Sci. Total Environ. 2021, 798, 149276. [Google Scholar] [CrossRef]
- Khan, A.L.; Numan, M.; Bilal, S.; Asaf, S.; Lee, I.J. Mangrove’s rhizospheric engineering with bacterial inoculation improve degradation of diesel contamination. J. Hazard. Mater. 2022, 423, 127046. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Melo, A.A.R.; Luan, D.K.M.; Sanchez, D.E.J.; Reis, A.R.D. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. R 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. The effect of drought stress on the superoxide dismutase and chlorophyll content in durum wheat genotypes. Adv. Life Sci. 2021, 8, 119–123. [Google Scholar]
- Shih, M.C.; Chang, C.M.; Kang, S.M.; Tsai, M.L. Effect of different parts (leaf, stem and stalk) and seasons (summer and winter) on the chemical compositions and antioxidant activity of Moringa oleifera. Int. J. Mol. Sci. 2011, 12, 6077–6088. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.L.; He, Z.Q.; Chen, N.F.; Tang, Z.Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.Q.; Noor, Y.; Hu, W.Z.; Hannan, F.; Zhou, W.J. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef]
- Li, H.B.; Zheng, Y.T.; Sun, D.D.; Wang, J.J.; Du, Y.Z. Combined effects of temperature and avermectins on life history and stress response of the western flower thrips, Frankliniella occidentalis. Pestic. Biochem. Phys. 2014, 108, 42–48. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, M.; Liu, Z.G.; Wang, M.; Sun, Y.; Zheng, W.K.; Lu, H. Copper-based-zinc-boron foliar fertilizer improved yield, quality, physiological characteristics, and microelement concentration of celery (Apium graveolens L.). Environ. Pollut. Bioavailab. 2019, 31, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar]


| Pesticides | Tissues | Dosage (g a.i.hm−2) | Residual Kinetics | Correlation Coefficient (R) | Half-Life (T1/2, Days) |
|---|---|---|---|---|---|
| IMI | stem | 30 | Ct = 0.1864 e−0.1898t | 0.8154 | 3.65 |
| 300 | Ct = 2.3015 e−0.2400t | 0.9182 | 2.89 | ||
| leaf | 30 | Ct = 0.7349 e−0.1259t | 0.8478 | 5.51 | |
| 300 | Ct = 9.8642 e−0.1707t | 0.8782 | 4.06 | ||
| FEN | stem | 37.5 | Ct = 0.9038 e−0.1671t | 0.9914 | 4.15 |
| 375 | Ct = 7.0234 e−0.1748t | 0.9773 | 3.97 | ||
| leaf | 37.5 | Ct = 1.4326 e−0.1441t | 0.9466 | 4.81 | |
| 375 | Ct = 11.0467 e−0.1411t | 0.9547 | 4.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Luo, Y.; Jiang, J.; Li, Y.; Wang, X.; He, H.; Fang, N.; Zhao, X.; Liu, Y.; Wang, Q. Physiological and Biochemical Variations in Celery by Imidacloprid and Fenpyroximate. Appl. Sci. 2022, 12, 4306. https://doi.org/10.3390/app12094306
Zhang C, Luo Y, Jiang J, Li Y, Wang X, He H, Fang N, Zhao X, Liu Y, Wang Q. Physiological and Biochemical Variations in Celery by Imidacloprid and Fenpyroximate. Applied Sciences. 2022; 12(9):4306. https://doi.org/10.3390/app12094306
Chicago/Turabian StyleZhang, Changpeng, Yuqin Luo, Jinhua Jiang, Yanjie Li, Xiangyun Wang, Hongmei He, Nan Fang, Xueping Zhao, Ying Liu, and Qiang Wang. 2022. "Physiological and Biochemical Variations in Celery by Imidacloprid and Fenpyroximate" Applied Sciences 12, no. 9: 4306. https://doi.org/10.3390/app12094306
APA StyleZhang, C., Luo, Y., Jiang, J., Li, Y., Wang, X., He, H., Fang, N., Zhao, X., Liu, Y., & Wang, Q. (2022). Physiological and Biochemical Variations in Celery by Imidacloprid and Fenpyroximate. Applied Sciences, 12(9), 4306. https://doi.org/10.3390/app12094306






