Design and Implementation of Anatomically Inspired Mesenteric and Intestinal Vascular Patterns for Personalized 3D Bioprinting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Images
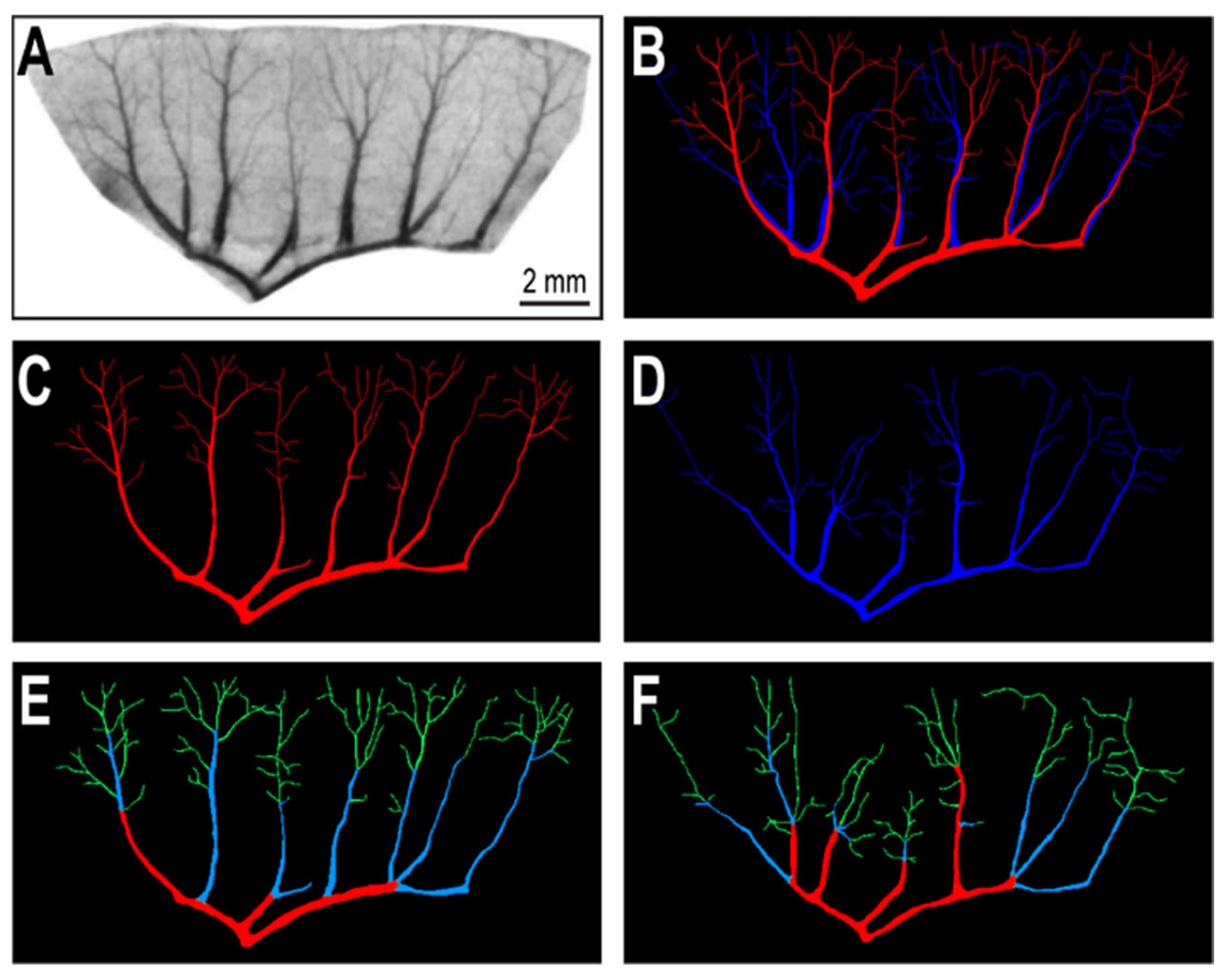
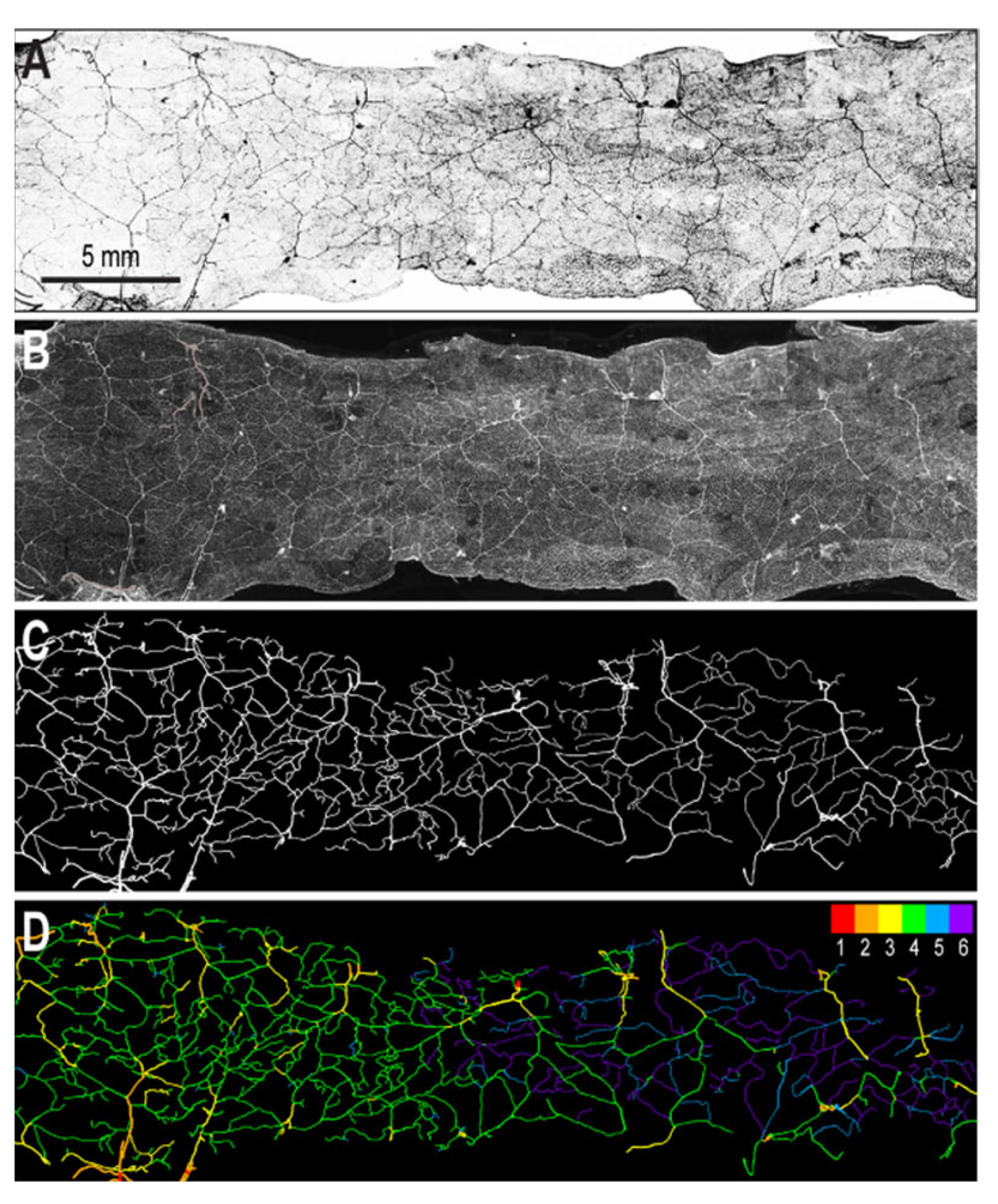
2.2. Segmentation of Vascular Patterns and VESGEN 2D Image Processing
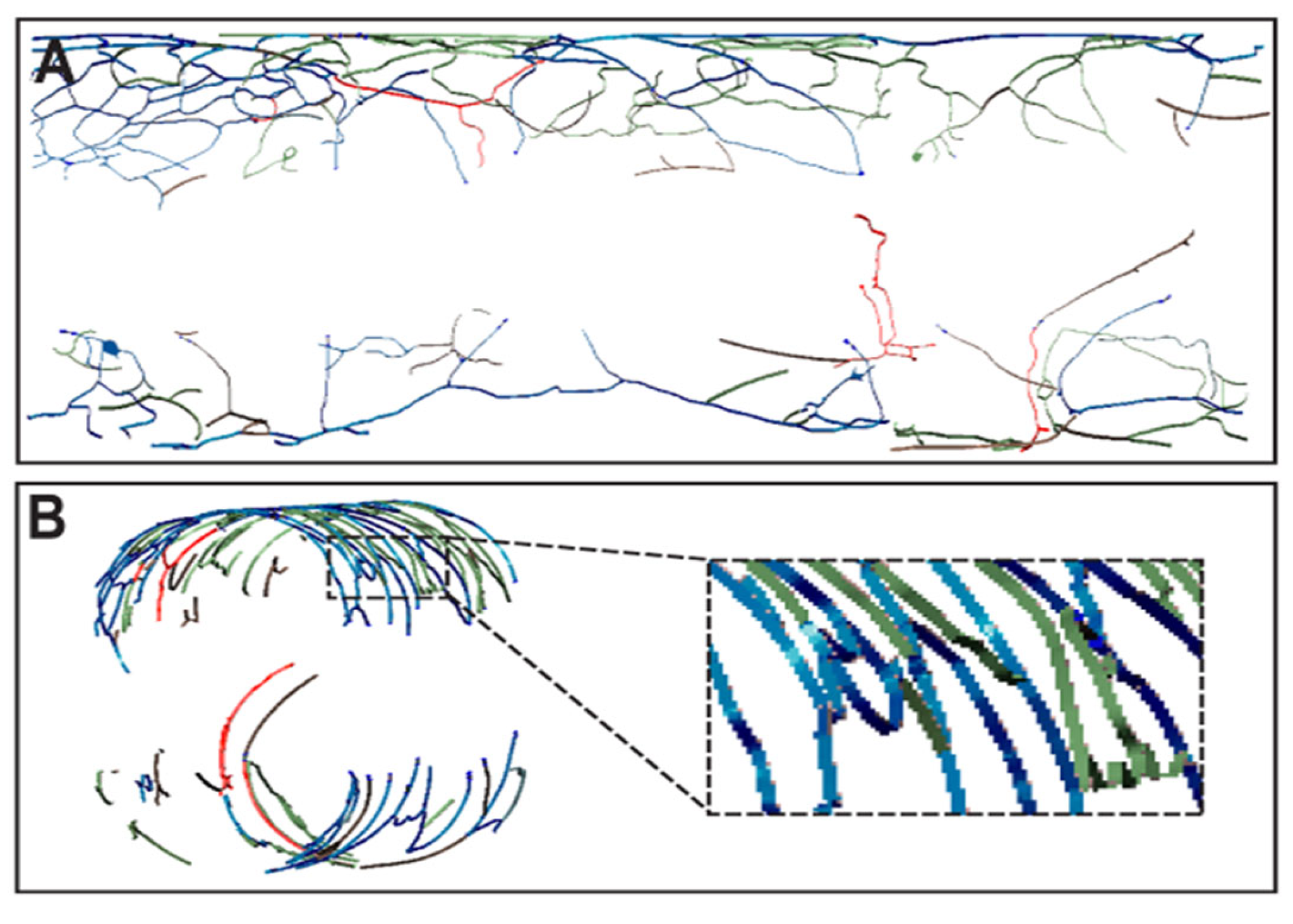
2.3. Preparation of Images for Printing
2.4. Printing of Vascular Patterns
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelby, R.D.; Raab, R.; Besner, G.E.; McElroy, S.J. Hope on the horizon: Promising novel therapies for necrotizing enterocolitis. Pediatr. Res. 2020, 88, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hewes, S.A.; Wilson, R.L.; Estes, M.K.; Shroyer, N.F.; Blutt, S.E.; Grande-Allen, K.J. In vitro models of the small intestine: Engineering challenges and engineering solutions. Tissue Eng. Part B Rev. 2020, 26, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Shi, W.; Black, A.R.; Kuss, M.A.; Pang, X.; He, Y.; Liu, B.; Duan, B. Repair and regeneration of small intestine: A review of current engineering approaches. Biomaterials 2020, 240, 119832. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Nguyen, T.V.; Garcia-Mojica, S.; Shah, V.; Le, A.V.; Peier, A.; Visconti, R.; Parker, E.M.; Presnell, S.C.; Nguyen, D.G.; et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience 2018, 2, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 2020, 20, 22–29. [Google Scholar] [CrossRef]
- Maina, R.M.; Barahona, M.J.; Geibel, P.; Lysyy, T.; Finotti, M.; Isaji, T.; Wengerter, B.; Mentone, S.; Dardik, A.; Geibel, J.P. Hydrogel-based 3D bioprints repair rat small intestine injuries and integrate into native intestinal tissue. J. Tissue Eng. Regen. Med. 2020, 15, 129–138. [Google Scholar] [CrossRef]
- Wengerter, B.C.; Emre, G.; Park, J.Y.; Geibel, J. Three-dimensional Printing in the Intestine. Clin. Gastroenterol. Hepatol. 2016, 14, 1081–1085. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. Intestinal Villi Model with Blood Capillaries Fabricated Using Collagen-Based Bioink and Dual-Cell-Printing Process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G.H. An intestinal model with a finger-like villus structure fabricated using a bioprinting process and collagen/SIS-based cell-laden bioink. Theranostics 2020, 10, 2495–2508. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Popovic, N.; Vujosevic, S.; Popovic, T. Regional Patterns in Retinal Microvascular Network Geometry in Health and Disease. Sci. Rep. 2019, 9, 16340. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Khalilpour, A.; Cecen, B.; Maharjan, S.; Shin, S.R.; Khademhosseini, A. Multiscale bioprinting of vascularized models. Biomaterials 2018, 198, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, N.I. Three-Dimensional Bioprinting of Anatomically Realistic Tissue Constructs for Disease Modeling and Drug Testing. Tissue Eng. Part C Methods 2021, 27, 225–231. [Google Scholar] [CrossRef]
- Lagatuz, M.; Vyas, R.J.; Predovic, M.; Lim, S.; Jacobs, N.; Martinho, M.; Valizadegan, H.; Kao, D.; Oza, N.; Theriot, C.A.; et al. Vascular Patterning as Integrative Readout of Complex Molecular and Physiological Signaling by VESsel GENeration Analysis. J. Vasc. Res. 2021, 58, 207–230. [Google Scholar] [CrossRef]
- Vickerman, M.B.; Keith, P.A.; McKay, T.L.; Gedeon, D.J.; Watanabe, M.; Montano, M.; Karunamuni, G.; Kaiser, P.K.; Sears, J.E.; Ebrahem, Q.; et al. VESGEN 2D: Automated, User-Interactive Software for Quantification and Mapping of Angiogenic and Lymphangiogenic Trees and Networks. Anat. Rec. 2009, 292, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Cadle, R.; Moldovan, L.; Parsons-Wingerter, P.; Moldovan, N.I. An Image Analysis-Based Workflow for 3D Bioprinting of Anatomically Realistic Retinal Vascular Patterns. Bioprinting 2021, 23, e00152. [Google Scholar] [CrossRef]
- Merfeld-Clauss, S.; Gollahalli, N.; March, K.L.; Traktuev, D.O. Adipose Tissue Progenitor Cells Directly Interact with Endothelial Cells to Induce Vascular Network Formation. Tissue Eng. Part A 2010, 16, 2953–2966. [Google Scholar] [CrossRef] [Green Version]
- Bogorad, M.I.; DeStefano, J.; Karlsson, J.; Wong, A.D.; Gerecht, S.; Searson, P.C. Review: In vitro microvessel models. Lab Chip 2015, 15, 4242–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons-Wingerter, P.; Reinecker, H.-C. For application to human spaceflight and ISS experiments: VESGEN mapping of microvascular network remodeling during intestinal inflammation. Gravit. Space Biol. Bull. 2012, 26, 2–12. [Google Scholar] [PubMed]
- Parsons-Wingerter, P.; Radhakrishnan, K.; Vickerman, M.B.; Kaiser, P.K. Oscillation of Angiogenesis with Vascular Dropout in Diabetic Retinopathy by VESsel GENeration Analysis (VESGEN). Investig. Opthalmol. Vis. Sci. 2010, 51, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Parsons-Wingerter, P.; Mckay, T.L.; Leontiev, D.; Vickerman, M.B.; Condrich, T.K.; Dicorleto, P.E. Lymphangiogenesis by blind-ended vessel sprouting is concurrent with hemangiogenesis by vascular splitting. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 233–247. [Google Scholar] [CrossRef]
- Scardulla, F.; Pasta, S.; D’Acquisto, L.; Sciacca, S.; Agnese, V.; Vergara, C.; Quarteroni, A.M.; Clemenza, F.; Bellavia, D.; Pilato, M. Shear stress alterations in the celiac trunk of patients with a continuous-flow left ventricular assist device as shown by in-silico and in-vitro flow analyses. J. Heart Lung Transplant. 2017, 36, 906–913. [Google Scholar] [CrossRef]
- Scardulla, F.; Bellavia, D.; D’Acquisto, L.; Raffa, G.M.; Pasta, S. Particle image velocimetry study of the celiac trunk hemodynamic induced by continuous-flow left ventricular assist device. Med. Eng. Phys. 2017, 47, 47–54. [Google Scholar] [CrossRef]
- Unthank, J.L.; Bohlen, H.G. Intestinal microvascular growth during maturation in diabetic juvenile rats. Circ. Res. 1988, 63, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef] [Green Version]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; O’Leary, D.P. Defining the mesentery as an organ and what this means for understanding its roles in digestive disorders. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 703–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anghelina, M.; Krishnan, P.; Moldovan, L.; Moldovan, N.I. Monocytes/Macrophages Cooperate with Progenitor Cells during Neovascularization and Tissue Repair: Conversion of Cell Columns into Fibrovascular Bundles. Am. J. Pathol. 2006, 168, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.; Macklin, B.L.; Lin, Y.-Y.; Zhou, L.; Lai, M.J.; Lee, G.; Gerecht, S.; Duh, E.J. iPSC-derived endothelial cell response to hypoxia via SDF1a/CXCR4 axis facilitates incorporation to revascularize ischemic retina. JCI Insight 2020, 5, e131828. [Google Scholar] [CrossRef] [Green Version]
- Tronolone, J.J.; Jain, A. Engineering New Microvascular Networks On-Chip: Ingredients, Assembly, and Best Practices. Adv. Funct. Mater. 2021, 31, 2007199. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; Fitzgerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.; Sheehy, S.P.; Park, T.-E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef]
- Hakam, M.S.; Imani, R.; Abolfathi, N.; Fakhrzadeh, H.; Sharifi, A.M. Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: An in-vitro study. Bio-Med. Mater. Eng. 2016, 27, 669–682. [Google Scholar] [CrossRef]
- Lee, W.; Bae, C.Y.; Kwon, S.; Son, J.; Kim, J.; Jeong, Y.; Yoo, S.-S.; Park, J.-K. Cellular Hydrogel Biopaper for Patterned 3D Cell Culture and Modular Tissue Reconstruction. Adv. Health Mater. 2012, 1, 635–639. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadle, R.; Rogozea, D.; Moldovan, L.; Moldovan, N.I. Design and Implementation of Anatomically Inspired Mesenteric and Intestinal Vascular Patterns for Personalized 3D Bioprinting. Appl. Sci. 2022, 12, 4430. https://doi.org/10.3390/app12094430
Cadle R, Rogozea D, Moldovan L, Moldovan NI. Design and Implementation of Anatomically Inspired Mesenteric and Intestinal Vascular Patterns for Personalized 3D Bioprinting. Applied Sciences. 2022; 12(9):4430. https://doi.org/10.3390/app12094430
Chicago/Turabian StyleCadle, Rachel, Dan Rogozea, Leni Moldovan, and Nicanor I. Moldovan. 2022. "Design and Implementation of Anatomically Inspired Mesenteric and Intestinal Vascular Patterns for Personalized 3D Bioprinting" Applied Sciences 12, no. 9: 4430. https://doi.org/10.3390/app12094430




