Preparation of Ca- and Na-Modified Activated Clay as a Promising Heterogeneous Catalyst for Biodiesel Production via Transesterification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Catalysts
2.3. Characterizations
2.4. Catalytic Activity Test and Optimization of Transesterification Conditions
2.5. Kinetics Experiment
2.6. Catalyst Recycling
3. Results
3.1. Base Strength and Basicity
3.2. CO2-TPD Analysis
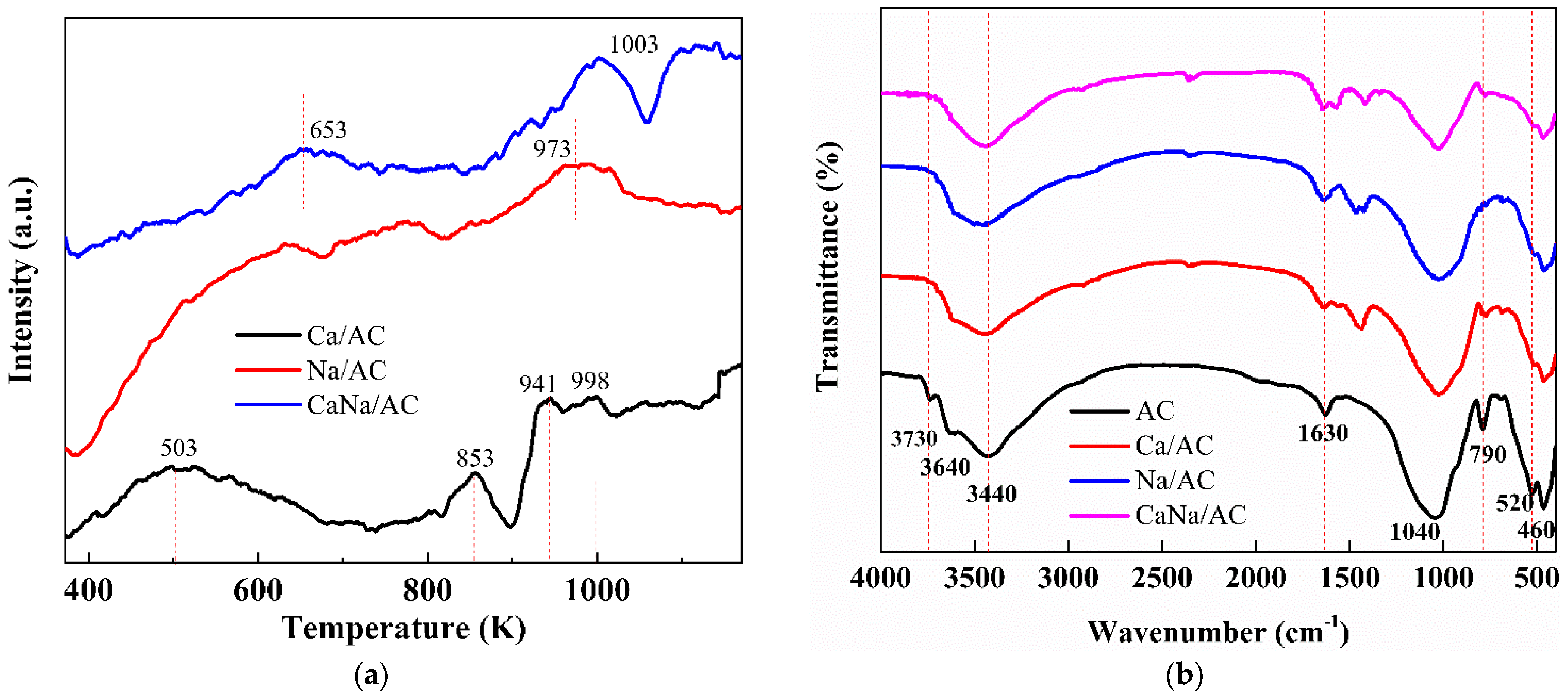
3.3. FT-IR Analysis
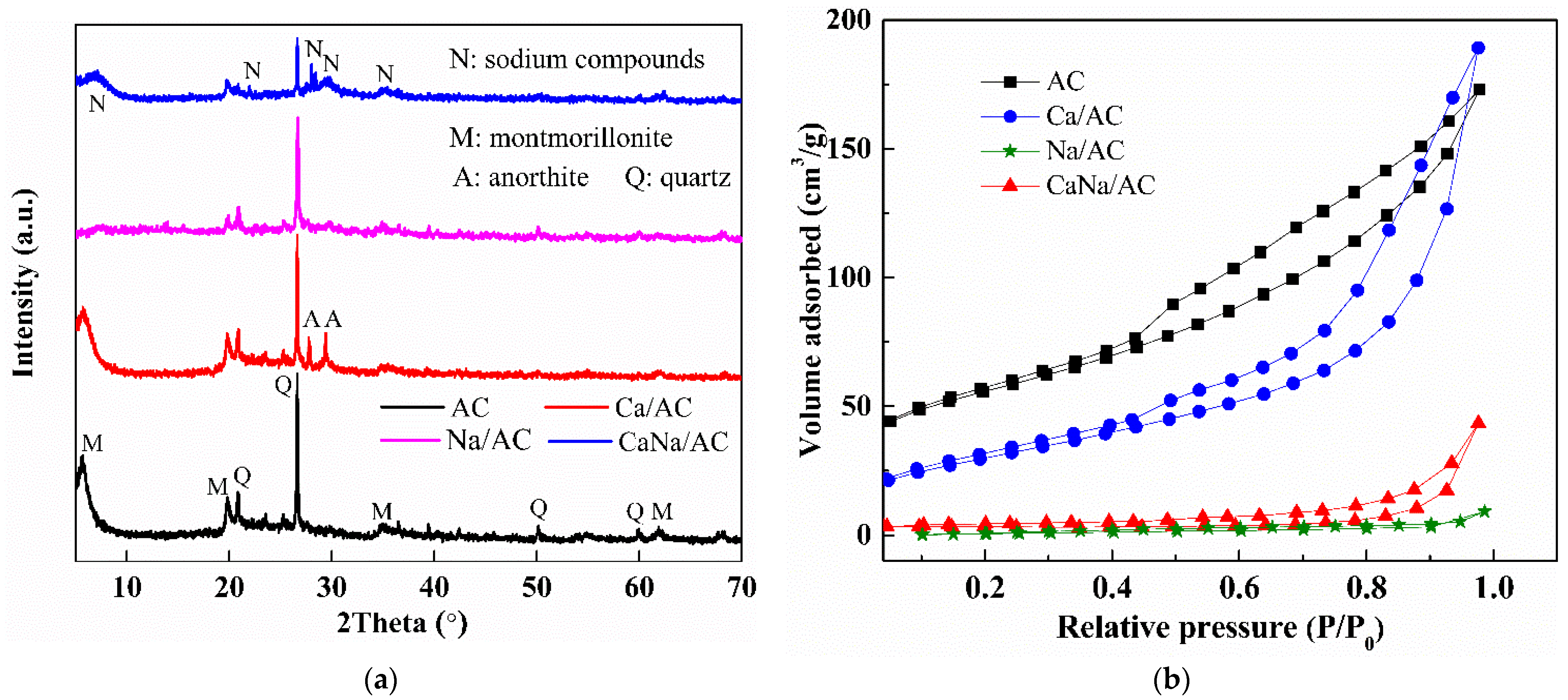
3.4. XRD Analysis
3.5. BET Analysis
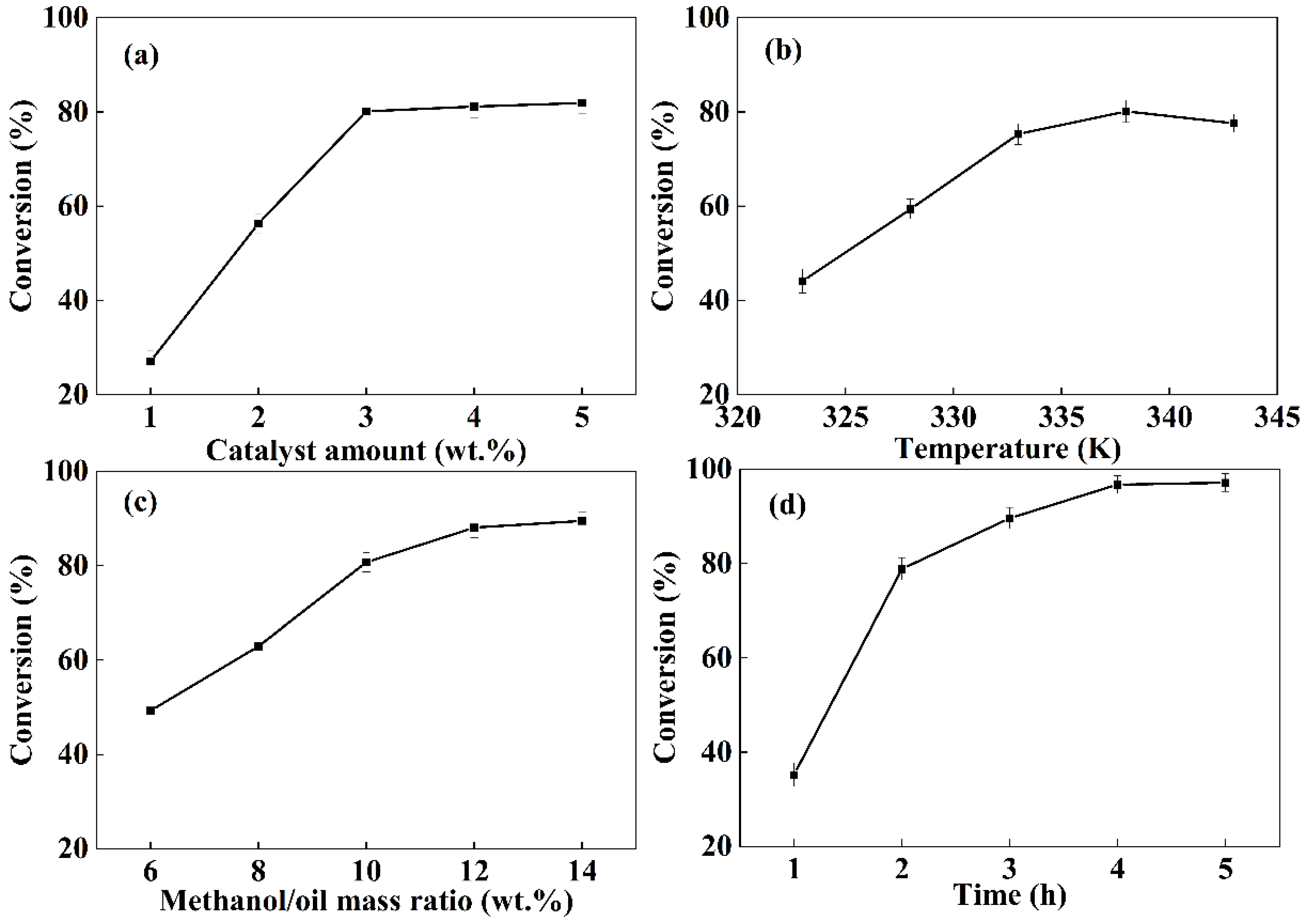
3.6. Influence of Reaction Conditions on the Transesterification
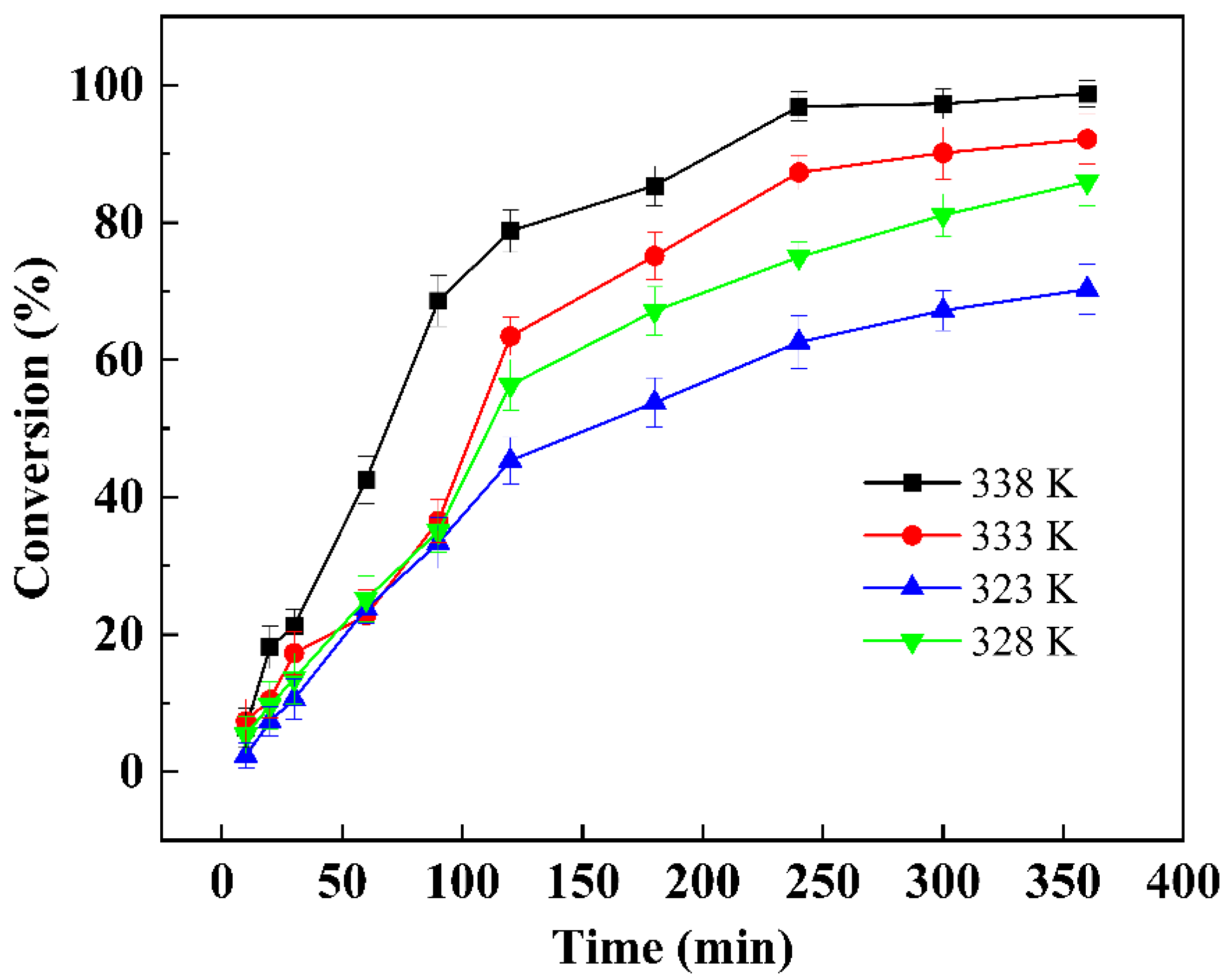
3.7. Kinetic Analysis
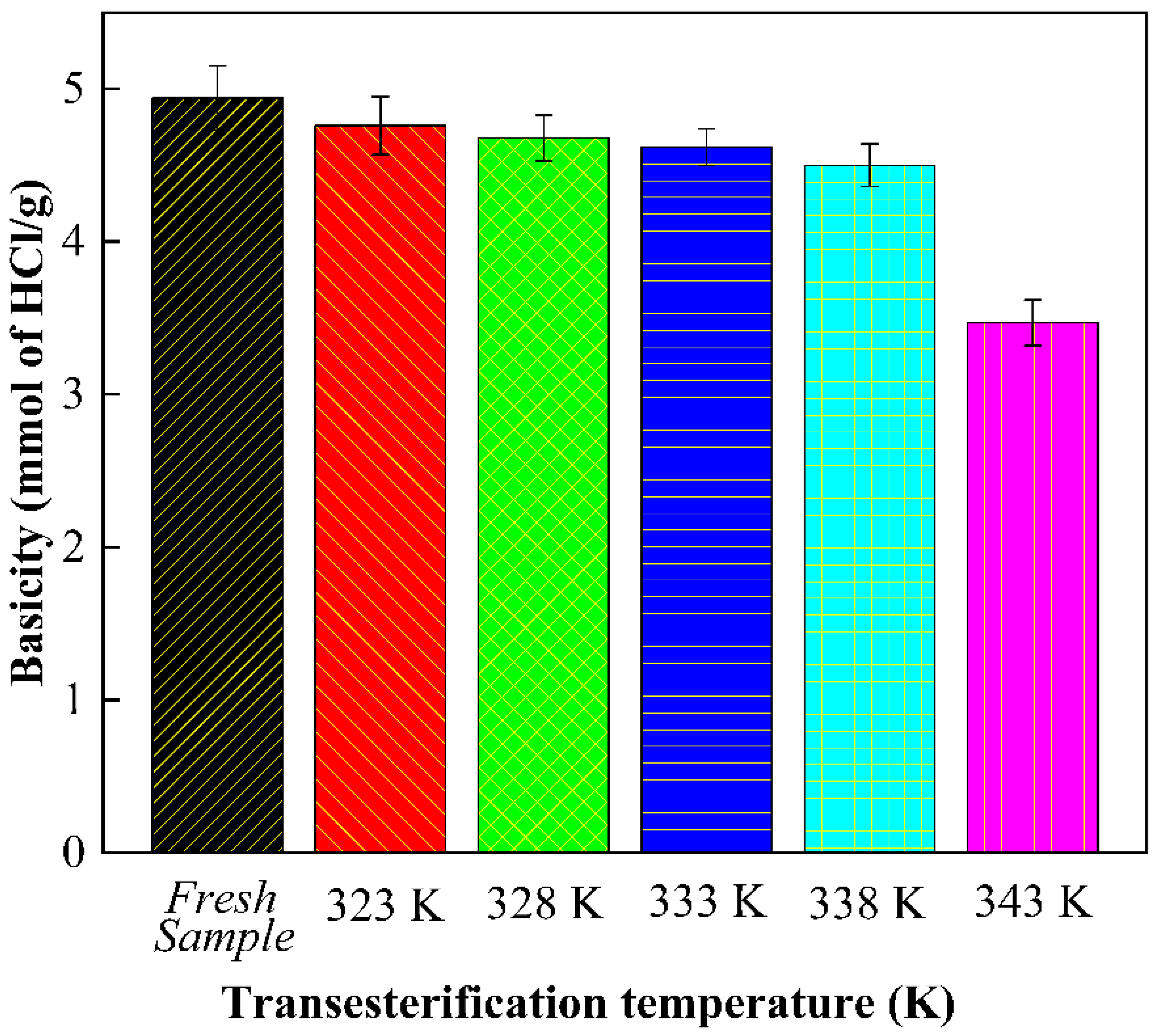
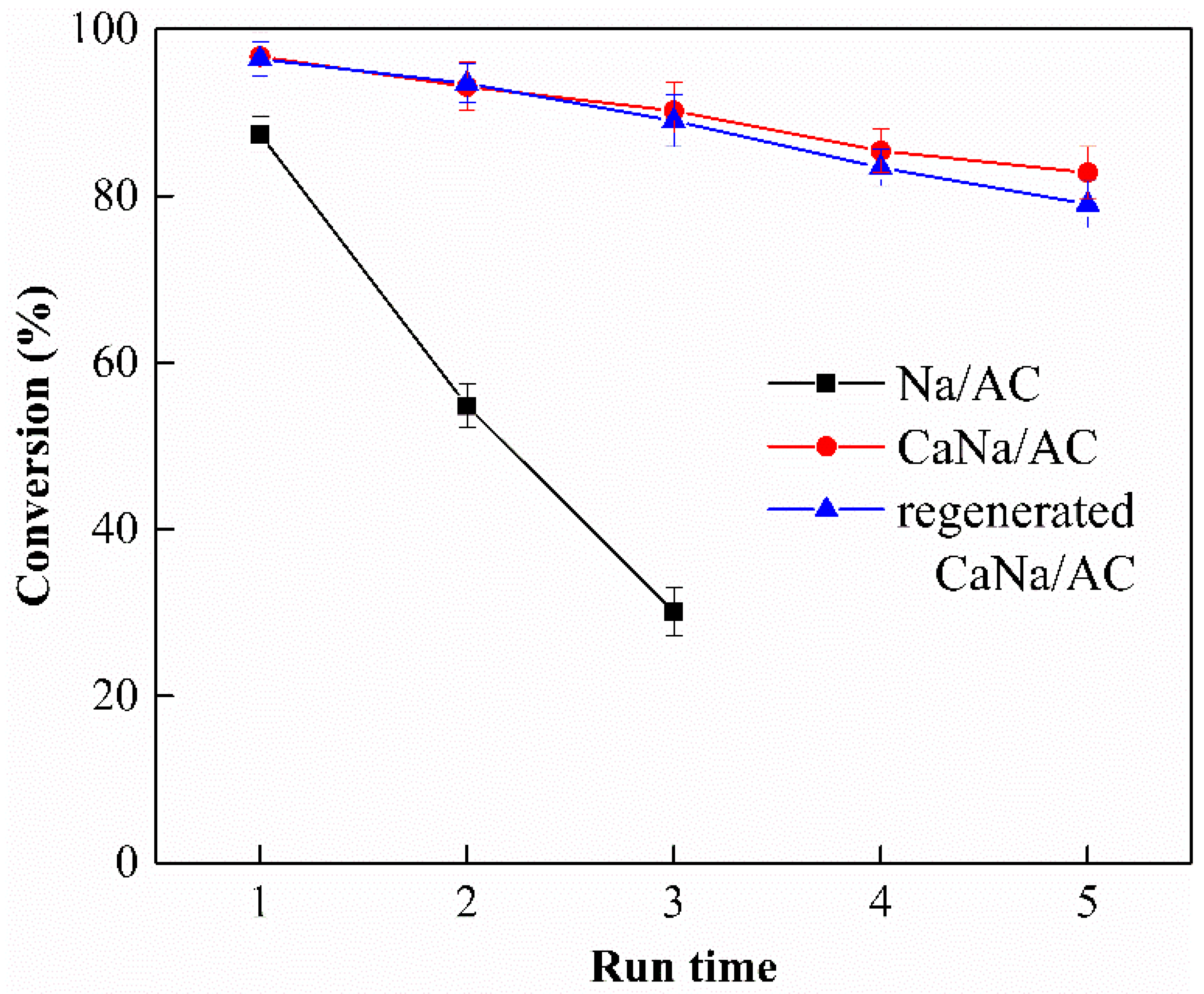
3.8. Reusability of Catalyst
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bento, H.B.S.; Reis, C.E.R.; Cunha, P.G.; Carvalho, A.K.F.; De Castro, H.F. One-pot fungal biomass-to-biodiesel process: Influence of the molar ratio and the concentration of acid heterogenous catalyst on reaction yield and costs. Fuels 2021, 300, 120968. [Google Scholar] [CrossRef]
- Dhawan, M.S.; Barton, S.C.; Yadav, G.D. Interesterification of triglycerides with methyl acetate for the co-production biodiesel and triacetin using hydrotalcite as a heterogenous base catalyst. Catal. Today 2021, 375, 101–111. [Google Scholar] [CrossRef]
- Moraes, P.S.; Engelmann, J.I.; Igansi, A.V.; Sant Anna Cadaval, T.R., Jr.; Antonio De Almeida Pinto, L. Nile tilapia industrialization waste: Evaluation of the yield, quality and cost of the biodiesel production process. J. Clean. Prod. 2021, 287, 125041. [Google Scholar] [CrossRef]
- Lourenço, V.A.; Nadaleti, W.C.; Vieira, B.M.; Li, H. Investigation of ethyl biodiesel via transesterification of rice bran oil: Bioenergy from residual biomass in Pelotas, Rio Grande do Sul-Brazil. Renew. Sust. Energ. Rev. 2021, 144, 111016. [Google Scholar] [CrossRef]
- Aronica, L.A.; Albano, G. Supported Metal Catalysts for the Synthesis of N-Heterocycles. Catalysts 2022, 12, 68. [Google Scholar] [CrossRef]
- Fechete, I.; Wang, Y.; Védrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.J.A.; Ramli, A.; Jumbri, K.; Uemura, Y. Tailoring the surface area and the acid-base properties of ZrO2 for biodiesel production from Nannochloropsis sp. Sci. Rep. 2019, 9, 16223. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jeon, H.; Park, J.T.; Kim, J.H. Synthesis of hierarchical flower-shaped hollow MgO microspheres via ethylene-glycol-mediated process as a base heterogeneous catalyst for transesterification for biodiesel production. Biomass Bioenergy 2020, 142, 105788. [Google Scholar] [CrossRef]
- Saman, S.; Balouch, A.; Talpur, F.N.; Memon, A.A.; Mousavi, B.M.; Verpoort, F. Green synthesis of MgO nanocatalyst by usingZiziphus mauritiana leaves and seeds for biodiesel production. Appl. Organomet. Chem. 2021, 35, e6199. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Soliman, M.M.A.; Pinto, T.V.; Silva, S.M.; Costa, P.; Alegria, E.C.B.A.; Freire, C. Highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass Bioenergy 2021, 145, 105936. [Google Scholar] [CrossRef]
- Đặng, T.; Nguyễn, X.; Chou, C.; Chen, B. Preparation of cancrinite-type zeolite from diatomaceous earth as transesterification catalysts for biodiesel production. Renew. Energy 2021, 174, 347–358. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Ibrahim, S.M.; Yakout, S.M.; El-Zaidy, M.E.; Abdeltawab, A.A. Synthesis of Na+ trapped bentonite/zeolite-P composite as a novel catalyst for effective production of biodiesel from palm oil; Effect of ultrasonic irradiation and mechanism. Energ. Convers. Manag. 2019, 196, 739–750. [Google Scholar] [CrossRef]
- Đặng, T.; Chen, B.; Lee, D. Optimization of biodiesel production from transesterification of triolein using zeolite LTA catalysts synthesized from kaolin clay. J. Taiwan Inst. Chem. E 2017, 79, 14–22. [Google Scholar] [CrossRef]
- Da Costa, J.M.; de Andrade Lima, L.R.P. Transesterification of cotton oil with ethanol for biodiesel using a KF/bentonite solid catalyst. Fuels 2021, 293, 120446. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Ingole, P.G.; Lee, S. Use of activated bentonite-alginate composite beads for efficient removal of toxic Cu2+ and Pb2+ ions from aquatic environment. Int. J. Biol. Macromol. 2020, 164, 3145–3154. [Google Scholar] [CrossRef]
- Gozali Balkanloo, P.; Mahmoudian, M.; Hosseinzadeh, M.T. A comparative study between MMT-Fe3O4/PES, MMT-HBE/PES, and MMT-acid activated/PES mixed matrix membranes. Chem. Eng. J. 2020, 396, 125188. [Google Scholar] [CrossRef]
- Funes, I.G.A.; Peralta, M.E.; Pettinari, G.R.; Carlos, L.; Parolo, M.E. Facile modification of montmorillonite by intercalation and grafting: The study of the binding mechanisms of a quaternary alkylammonium surfactant. Appl. Clay Sci. 2020, 195, 105738. [Google Scholar] [CrossRef]
- Huang, G.; Song, Y.; Liu, C.; Yang, J.; Lu, J.; Liu, Z.; Liu, Z. Acid activated montmorillonite for gas-phase catalytic dehydration of monoethanolamine. Appl. Clay Sci. 2019, 168, 116–124. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation—Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Bhatti, U.H.; Kazmi, W.W.; Muhammad, H.A.; Min, G.H.; Nam, S.C.; Baek, I.H. Practical and inexpensive acid-activated montmorillonite catalysts for energy-efficient CO2 capture. Green Chem. 2020, 22, 6328–6333. [Google Scholar] [CrossRef]
- Ayodele, O.B.; Abdullah, A.Z. Exploring kaolinite as dry methane reforming catalyst support: Influences of chemical activation, organic ligand functionalization and calcination temperature. Appl. Catal. A Gen. 2019, 576, 20–31. [Google Scholar] [CrossRef]
- Saka, C.; Eygi, M.S.; Balbay, A. Cobalt loaded organic acid modified kaolin clay for the enhanced catalytic activity of hydrogen release via hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2021, 46, 3876–3886. [Google Scholar] [CrossRef]
- Flessner, U.; Jones, D.J.; Rozière, J.; Zajac, J.; Storaro, L.; Lenarda, M.; Pavan, M.; Jiménez-López, A.; Rodríguez-Castellón, E.; Trombetta, M.; et al. A study of the surface acidity of acid-treated montmorillonite clay catalysts. J. Mol. Catal. A Chem. 2001, 168, 247–256. [Google Scholar] [CrossRef]
- Wei, T.; Pan, Y.; Lu, G.; Tong, Z.; Xiao, H. Activated Clay Prepared by Waste Acid Recycling: Technology and Mechanism. Environ. Eng. Sci. 2010, 27, 531–535. [Google Scholar] [CrossRef]
- Jeenpadiphat, S.; Tungasmita, D.N. Esterification of oleic acid and high acid content palm oil over an acid-activated bentonite catalyst. Appl. Clay Sci. 2014, 87, 272–277. [Google Scholar] [CrossRef]
- Çakırca, E.E.; Akın, A.N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production. Sustain. Chem. Pharm. 2021, 20, 100378. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D. Preparation and Reaction Kinetics Studies of Na-based Mixed Metal Oxide for Transesterification. Energ. Fuel. 2009, 23, 5160–5164. [Google Scholar] [CrossRef]
- Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energy 2009, 34, 1145–1150. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Temrak, A.; Nuchdang, S.; Kingkam, W.; Puripunyavanich, V.; Thanapimmetha, A.; Saisriyoot, M.; Srinophakun, P. Catalytic behavior of La2O3-promoted SO42−/ZrO2 in the simultaneous esterification and transesterification of palm oil. Energ. Rep. 2021, 7, 5374–5385. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Krstić, J.B.; Zdujić, M.V.; Veselinović, L.M.; Veljović, D.N.; Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Transesterification of used cooking sunflower oil catalyzed by hazelnut shell ash. Renew. Energy 2022, 183, 103–113. [Google Scholar] [CrossRef]
- Fang, X.; Xia, L.; Li, S.; Hong, Z.; Yang, M.; Xu, X.; Xu, J.; Wang, X. Superior 3DOM Y2Zr2O7 supports for Ni to fabricate highly active and selective catalysts for CO2 methanation. Fuels 2021, 293, 120460. [Google Scholar] [CrossRef]
- Yaseen, M.; Ullah, M.; Subhan, S.; Ahmad, W.; Subhan, F.; Shakir, M. Recovery and characterization of useful benzene derivatives from spent engine oil through solvent extraction. Chem. Eng. Res. Des. 2021, 175, 51–60. [Google Scholar] [CrossRef]
- Yaseen, M.; Ammara, O.; Ahmad, W.; Shakir, M.; Subhan, S.; Subhan, F.; Khan, K.; Iqbal, M.S. Preparation of titanium carbide reinforced polymer based composite nanofibers for enhanced humidity sensing. Sens. Actuator A Phys. 2021, 332, 113201. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 273–278. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Xu, Y.; Liu, Q.; Qian, G. CaFeAl mixed oxide derived heterogeneous catalysts for transesterification of soybean oil to biodiesel. Bioresour. Technol. 2015, 190, 438–441. [Google Scholar] [CrossRef]
- Mashhadinezhad, M.; Shirini, F.; Mamaghani, M. Nanoporous Na+-montmorillonite perchloric acid as an efficient heterogeneous catalyst for synthesis of merocyanine dyes based on isoxazolone and barbituric acid. Microporous Mesoporous Mater. 2018, 262, 269–282. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Pugazhendhi, A.; Kim, J.; Lee, S.; Nooruddin, T. Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuels 2021, 300, 121018. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, S.; Ullah, S.; Subhan, F.; Ahmad, W.; Shakir, M.; Tong, Z. Oxidative desulfurization of model and real petroleum distillates using Cu or Ni impregnated banana peels derived activated carbon–NaClO catalyst–oxidant system. Chem. Eng. Res. Des. 2022, 179, 107–118. [Google Scholar] [CrossRef]
- Subhan, S.; Yaseen, M.; Ahmad, B.; Tong, Z.; Subhan, F.; Ahmad, W.; Sahibzada, M. Fabrication of MnO2 NPs incorporated UiO-66 for the green and efficient oxidative desulfurization and denitrogenation of fuel oils. J. Environ. Chem. Eng. 2021, 9, 105179. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]
- Gajek, M.; Rapacz-Kmita, A.; Stodolak-Zych, E.; Zarzecka-Napierała, M.; Wilk, M.; Magdziarz, A.; Dudek, M. Microstructure and mechanical properties of diopside and anorthite glazes with high abrasion resistance. Ceram. Int. 2022, 48, 6792–6798. [Google Scholar] [CrossRef]
- Tabrizi, S.H.; Tanhaei, B.; Ayati, A.; Ranjbari, S. Substantial improvement in the adsorption behavior of montmorillonite toward Tartrazine through hexadecylamine impregnation. Environ. Res. 2022, 204, 111965. [Google Scholar] [CrossRef]
- Yang, G.; Deng, Y.; Ding, H.; Lin, Z.; Shao, Y.; Wang, Y. A facile approach to synthesize MCM-41 mesoporous materials from iron ore tailing: Influence of the synthesis conditions on the structural properties. Appl. Clay Sci. 2015, 111, 61–66. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rahman, A.U.; Rashid, H.U.; Sahibzada, M.; Subhan, S.; Tong, Z. Hydrodesulfurization of dibenzothiophene using Pd-promoted Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts coupled with ionic liquids at ambient operating conditions. RSC Adv. 2019, 9, 10371–10385. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energ. Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Vishal, D.; Dubey, S.; Goyal, R.; Dwivedi, G.; Baredar, P.; Chhabra, M. Optimization of alkali-catalyzed transesterification of rubber oil for biodiesel production & its impact on engine performance. Renew. Energy 2020, 158, 167–180. [Google Scholar] [CrossRef]
- Esonye, C.; Onukwuli, O.D.; Ofoefule, A.U. Chemical kinetics of a two-step transesterification of dyacrodes edulis seed oil using acid-alkali catalyst. Chem. Eng. Res. Des. 2019, 145, 245–257. [Google Scholar] [CrossRef]
- Takase, M.; Zhang, M.; Feng, W.; Chen, Y.; Zhao, T.; Cobbina, S.J.; Yang, L.; Wu, X. Application of zirconia modified with KOH as heterogeneous solid base catalyst to new non-edible oil for biodiesel. Energ. Convers. Manag. 2014, 80, 117–125. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Lin, K.A.; Park, Y.; Kwon, E.E. Biodiesel synthesis from bio-heavy oil through thermally induced transesterification. J. Clean. Prod. 2021, 294, 126347. [Google Scholar] [CrossRef]
- Subhan, S.; Ur Rahman, A.; Yaseen, M.; Ur Rashid, H.; Ishaq, M.; Sahibzada, M.; Tong, Z. Ultra-fast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant. Fuels 2019, 237, 793–805. [Google Scholar] [CrossRef]
- Jayakumar, M.; Karmegam, N.; Gundupalli, M.P.; Bizuneh Gebeyehu, K.; Tessema Asfaw, B.; Chang, S.W.; Ravindran, B.; Kumar Awasthi, M. Heterogeneous base catalysts: Synthesis and application for biodiesel production—A review. Bioresour. Technol. 2021, 331, 125054. [Google Scholar] [CrossRef]
- Ishak, N.; Estephane, J.; Dahdah, E.; Chalouhi, L.M.; Nassreddine, S.; El Khoury, B.; Aouad, S. Outstanding activity of a biodiesel coated K2O/fumed silica catalyst in the transesterification reaction. J. Environ. Chem. Eng. 2021, 9, 104665. [Google Scholar] [CrossRef]
- Hoque, M.E.; Singh, A.; Chuan, Y.L. Biodiesel from low cost feedstocks: The effects of process parameters on the biodiesel yield. Biomass Bioenergy 2011, 35, 1582–1587. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Udiantoro; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Shahla, S.; Ngoh, G.C.; Yusoff, R. The evaluation of various kinetic models for base-catalyzed ethanolysis of palm oil. Bioresour. Technol. 2012, 104, 1–5. [Google Scholar] [CrossRef]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl–LDH derived heterogeneous catalysts for the ethanol transesterification of canola oil to biodiesel. Appl. Catal. B 2009, 88, 42–49. [Google Scholar] [CrossRef]
- Ziȩba, A.; Pacuła, A.; Drelinkiewicz, A. Transesterification of triglycerides with methanol catalyzed by heterogeneous zinc hydroxy nitrate catalyst. Evaluation of variables affecting the activity and stability of catalyst. Energy Fuel. 2010, 24, 634–645. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S. Model Study on Transesterification of Soybean Oil to Biodiesel with Methanol Using Solid Base Catalyst. J. Phys. Chem. A 2010, 114, 3750–3755. [Google Scholar] [CrossRef]
- Yaseen, M.; Ullah, S.; Ahmad, W.; Subhan, S.; Subhan, F. Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuels 2021, 284, 119102. [Google Scholar] [CrossRef]
- Muhammad, Y.; Rashid, H.U.; Subhan, S.; Rahman, A.U.; Sahibzada, M.; Tong, Z. Boosting the hydrodesulfurization of dibenzothiophene efficiency of Mn decorated (Co/Ni)-Mo/Al2O3 catalysts at mild temperature and pressure by coupling with phosphonium based ionic liquids. Chem. Eng. J. 2019, 375, 121957. [Google Scholar] [CrossRef]
- Khan, I.W.; Naeem, A.; Farooq, M.; Ghazi, Z.A.; Saeed, T.; Perveen, F.; Malik, T. Biodiesel production by valorizing waste non-edible wild olive oil using heterogeneous base catalyst: Process optimization and cost estimation. Fuels 2022, 320, 123828. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gebeyehu, K.B.; Selvakumar, K.V.; Parvathy, S.; Kim, W.; Karmegam, N. Waste Ox bone based heterogeneous catalyst synthesis, characterization, utilization and reaction kinetics of biodiesel generation from Jatropha curcas oil. Chemosphere 2022, 288, 132534. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Mahdinia, S.; Oryani, B.; Cho, J.; Kwon, E.E.; Bozorgian, A.; Rashidi Nodeh, H.; Darajeh, N.; Mehranzamir, K. Biodiesel production from wild mustard (Sinapis Arvensis) seed oil using a novel heterogeneous catalyst of LaTiO3 nanoparticles. Fuels 2022, 307, 121759. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Macina, A.; Naccache, R. Graphitic carbon nitrides: Efficient heterogeneous catalysts for biodiesel production. Nano Energy 2020, 78, 105306. [Google Scholar] [CrossRef]
- Mares, E.K.L.; Gonçalves, M.A.; Da Luz, P.T.S.; Da Rocha Filho, G.N.; Zamian, J.R.; Da Conceição, L.R.V. Acai seed ash as a novel basic heterogeneous catalyst for biodiesel synthesis: Optimization of the biodiesel production process. Fuels 2021, 299, 120887. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M.; El-Sherbeeny, A.M.; Ahmed Soliman, A.T.; Abd Elgawad, A.E.E. Effective transformation of waste sunflower oil into biodiesel over novel K+ trapped clay nanotubes (K+/KNTs) as a heterogeneous catalyst; response surface studies. Microporous Mesoporous Mater. 2020, 306, 110465. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Rehan, M.; Saeed, M.; Lam, S.S.; Nizami, A.S.; Waseem, A.; Sultana, S.; Zafar, M. Production of high quality biodiesel from novel non-edible Raphnus raphanistrum L. seed oil using copper modified montmorillonite clay catalyst. Environ. Res. 2021, 193, 110398. [Google Scholar] [CrossRef]
- Bargole, S.S.; Singh, P.K.; George, S.; Saharan, V.K. Valorisation of low fatty acid content waste cooking oil into biodiesel through transesterification using a basic heterogeneous calcium-based catalyst. Biomass Bioenergy 2021, 146, 105984. [Google Scholar] [CrossRef]

| Sample | Basic Strength (H_) | Basicity (mmol of HCl·g−1) | Conversion (%) |
|---|---|---|---|
| AC | H_ < 7.2 | - | - |
| Ca/AC | 7.2 < H_ < 9.8 | 1.4 | 7.3 |
| Na/AC | 12.2 < H_ < 15.0 | 3.3 | 58.6 |
| CaNa/AC | 15.0 < H_ < 18.4 | 5.0 | 81.3 |
| Sample | Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| AC | 138 | 0.27 | 6 |
| Ca/AC | 110 | 0.28 | 11 |
| Na/AC | 13 | 0.02 | 2 |
| CaNa/AC | 15 | 0.07 | 29 |
| Temperature (K) | Slope | Intercept | R2 |
|---|---|---|---|
| 323 | 0.961 | −2.47 | 0.970 |
| 328 | 0.988 | −2.26 | 0.989 |
| 333 | 0.985 | −2.12 | 0.979 |
| 338 | 0.980 | −1.91 | 0.982 |
| Catalyst | Calcination Conditions | Transesterification Conditions | Conversion Rate (%) | References | |||
|---|---|---|---|---|---|---|---|
| Catalyst Amount (wt.%) | Methanol/Oil Molar Ratio | Temperature (K) | Reaction Time (h) | ||||
| Na/SiO2/TiO2 | 773 K/5 h | 9 | 20:1 | 343 | 2 | 97 | [62] |
| KOH/Waste Ox bone | 1173 K/3 h | 5 | 12:1 | 338 | 4 | 97 | [63] |
| LaTiO3 | 973 K/3 h | 5 | 4:1 | 353 | 1 | 90 | [64] |
| Treated carbon nitrides | 943 K/3 h | 5 | 24:1 | 423 | 3 | 96 | [65] |
| Acai seed ash | 1073 K/4 h | 12 | 18:1 | 373 | 1 | 98 | [66] |
| K+ trapped clay nanotubes | 573 K/4 h | 6 | 15:1 | 363 | 4 | 98 | [67] |
| Copper modified montmorillonite clay | 773 K/4 h | 4 | 15:1 | 423 | 5 | 89 | [68] |
| Marble waste powder | 1123 K/2 h | 7 | 16:1 | 338 | 3 | 95 | [69] |
| CaNa/AC | 473 K/2 h | 3 | 12:1 | 338 | 4 | 97 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Muhammad, Y.; Yu, S.; Fu, T.; Liu, K.; Tong, Z.; Hu, X.; Zhang, H. Preparation of Ca- and Na-Modified Activated Clay as a Promising Heterogeneous Catalyst for Biodiesel Production via Transesterification. Appl. Sci. 2022, 12, 4667. https://doi.org/10.3390/app12094667
Wang Y, Muhammad Y, Yu S, Fu T, Liu K, Tong Z, Hu X, Zhang H. Preparation of Ca- and Na-Modified Activated Clay as a Promising Heterogeneous Catalyst for Biodiesel Production via Transesterification. Applied Sciences. 2022; 12(9):4667. https://doi.org/10.3390/app12094667
Chicago/Turabian StyleWang, Yue, Yaseen Muhammad, Sishan Yu, Tian Fu, Kun Liu, Zhangfa Tong, Xueling Hu, and Hanbing Zhang. 2022. "Preparation of Ca- and Na-Modified Activated Clay as a Promising Heterogeneous Catalyst for Biodiesel Production via Transesterification" Applied Sciences 12, no. 9: 4667. https://doi.org/10.3390/app12094667






