Predicting the Onset of Freezing of Gait Using EEG Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Equipment
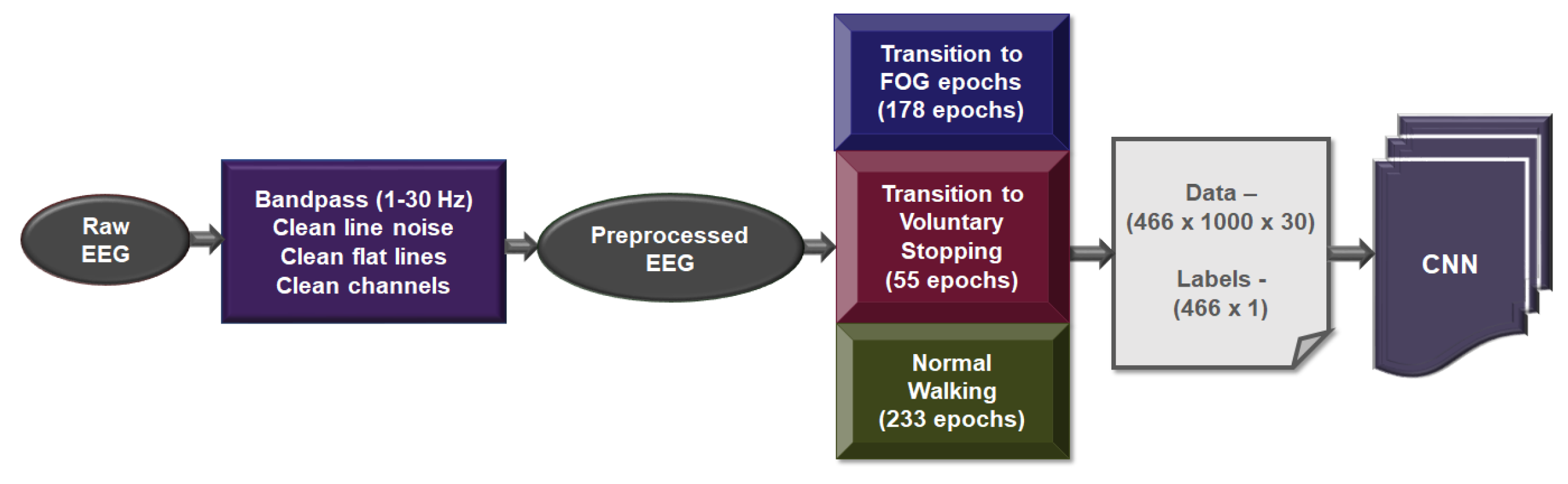
2.4. EEG Processing
3. Results and Discussion
Classification Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieuwboer, A.; Giladi, N. Characterizing freezing of gait in Parkinson’s disease: Models of an episodic phenomenon. Mov. Disord. 2013, 28, 1509–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giladi, N.; Kao, R.; Fahn, S. Freezing phenomenon in patients with parkinsonian syndromes. Mov. Disord. Off. J. Mov. Disord. Soc. 1997, 12, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Moore, O.; Peretz, C.; Giladi, N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 2192–2195. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Mazzà, C.; Lord, S.; Rochester, L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov. Disord. 2016, 31, 1293–1313. [Google Scholar] [CrossRef]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. J. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [CrossRef]
- Mazilu, S.; Hardegger, M.; Zhu, Z.; Roggen, D.; Tröster, G.; Plotnik, M.; Hausdorff, J.M. Online detection of freezing of gait with smartphones and machine learning techniques. In Proceedings of the 2012 6th International Conference on Pervasive Computing Technologies for Healthcare (PervasiveHealth) and Workshops, San Diego, CA, USA, 21–24 May 2012; pp. 123–130. [Google Scholar]
- Zhao, Y.; Tonn, K.; Niazmand, K.; Fietzek, U.M.; D’Angelo, L.T.; Ceballos-Baumann, A.; Lueth, T.C. Online FOG identification in Parkinson’s disease with a time-frequency combined algorithm. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 192–195. [Google Scholar]
- Tripoliti, E.E.; Tzallas, A.T.; Tsipouras, M.G.; Rigas, G.; Bougia, P.; Leontiou, M.; Konitsiotis, S.; Chondrogiorgi, M.; Tsouli, S.; Fotiadis, D.I. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput. Methods Programs Biomed. 2013, 110, 12–26. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Samà, A.; Pérez-López, C.; Català, A.; Arostegui, J.M.M.; Cabestany, J.; Bayés, A.; Alcaine, S.; Mestre, B.; Prats, A.; et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS ONE 2017, 12, e0171764. [Google Scholar] [CrossRef]
- Samà, A.; Rodríguez-Martín, D.; Pérez-López, C.; Català, A.; Alcaine, S.; Mestre, B.; Prats, A.; Crespo, M.C.; Bayés, A. Determining the optimal features in freezing of gait detection through a single waist accelerometer in home environments. Pattern Recognit. Lett. 2018, 105, 135–143. [Google Scholar] [CrossRef]
- Handojoseno, A.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.; Nguyen, H.T. The detection of Freezing of Gait in Parkinson’s disease patients using EEG signals based on Wavelet decomposition. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 69–72. [Google Scholar]
- Coste, C.A.; Sijobert, B.; Pissard-Gibollet, R.; Pasquier, M.; Espiau, B.; Geny, C. Detection of freezing of gait in Parkinson disease: Preliminary results. Sensors 2014, 14, 6819–6827. [Google Scholar] [CrossRef]
- Mazilu, S.; Calatroni, A.; Gazit, E.; Mirelman, A.; Hausdorff, J.M.; Tröster, G. Prediction of freezing of gait in Parkinson’s from physiological wearables: An exploratory study. IEEE J. Biomed. Health Inform. 2015, 19, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Handojoseno, A.M.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.G.; Nguyen, H.T. Analysis and Prediction of the Freezing of Gait Using EEG Brain Dynamics. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Handojoseno, A.M.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.G.; Nguyen, H.T. Using EEG spatial correlation, cross frequency energy, and wavelet coefficients for the prediction of Freezing of Gait in Parkinson’s Disease patients. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4263–4266. [Google Scholar]
- Cao, Z.; John, A.R.; Chen, H.T.; Martens, K.E.; Georgiades, M.; Gilat, M.; Nguyen, H.T.; Lewis, S.J.; Lin, C.T. Identification of EEG dynamics during freezing of gait and voluntary stopping in patients with Parkinson’s disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up and Go” Test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar] [CrossRef]
- Zampieri, C.; Salarian, A.; Carlson-Kuhta, P.; Aminian, K.; Nutt, J.G.; Horak, F.B. The instrumented timed up and go test: Potential outcome measure for disease modifying therapies in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Lawhern, V.J.; Solon, A.J.; Waytowich, N.R.; Gordon, S.M.; Hung, C.P.; Lance, B.J. EEGNet: A compact convolutional neural network for EEG-based brain–computer interfaces. J. Neural Eng. 2017, 15, 056013. [Google Scholar] [CrossRef] [Green Version]
- Schirrmeister, R.T.; Springenberg, J.T.; Fiederer, L.D.J.; Glasstetter, M.; Eggensperger, K.; Tangermann, M.; Hutter, F.; Burgard, W.; Ball, T. Deep learning with convolutional neural networks for EEG decoding and visualization. Hum. Brain Mapp. 2017, 38, 5391–5420. [Google Scholar] [CrossRef] [Green Version]
- Brugman, H.; Russel, A.; Nijmegen, X. Annotating Multi-mediaMulti-modal Resources with ELAN. In Proceedings of the 4th International Conference on Language Resources and Language Evaluation (LREC), Lisbon, Portugal, 26–28 May 2004; pp. 2065–2068. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.E.; Iansek, R.; Kirkwood, B. A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Mov. Disord. 2009, 24, 64–71. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Park. Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]

| Subject No. | No. of Normal Walking Epochs | No. of Transition to FOG Epochs | No. of Transition Voluntary Stopping Epochs |
|---|---|---|---|
| 1 | 11 | 8 | 3 |
| 2 | 12 | 8 | 4 |
| 3 | 1 | 1 | 0 |
| 4 | 33 | 33 | 0 |
| 5 | 5 | 2 | 3 |
| 6 | 8 | 5 | 3 |
| 7 | 7 | 1 | 6 |
| 8 | 15 | 11 | 4 |
| 9 | 8 | 8 | 0 |
| 10 | 23 | 23 | 0 |
| 11 | 5 | 0 | 5 |
| 12 | 30 | 24 | 6 |
| 13 | 3 | 0 | 3 |
| 14 | 15 | 15 | 0 |
| 15 | 33 | 26 | 7 |
| 16 | 7 | 1 | 6 |
| 17 | 17 | 12 | 5 |
| Model | Accuracy | F1-Score | Coh-Kappa | Sensitivity | Specificity |
|---|---|---|---|---|---|
| EEGNet | 88.09 ± 4.25% | 80.09 ± 4.62% | 68.30 ± 2.50% | 94.42 ± 4.65% | 96.21 ± 3.52% |
| Shallow ConvNet | 89.9 ± 2.31% | 89.21 ± 3.94% | 70.11 ± 3.91% | 96.49 ± 2.97% | 94.36 ± 3.60% |
| Deep ConvNet | 92.28 ± 2.70% | 93.02 ± 2.03% | 72.94 ± 2.27% | 96.89 ± 2.04% | 96.91 ± 2.09% |
| Model | Accuracy | F1-Score | Coh-Kappa | Sensitivity | Specificity |
|---|---|---|---|---|---|
| EEGNet | 87.28 ± 5.89% | 87.61 ± 5.53% | 69.19 ± 4.37% | 84.89 ± 5.72% | 84.16 ± 4.71% |
| Shallow ConvNet | 87.92 ± 4.3% | 82.16 ± 3.02% | 71.14 ± 4.84% | 86.23 ± 3.71% | 85.55 ± 4.62% |
| Deep ConvNet | 87.83 ± 5.35% | 84.81 ± 5.86% | 70.6 ± 5% | 86.37 ± 3.31% | 84.72 ± 2.49% |
| Model | Accuracy | F1-Score | Coh-Kappa | Sensitivity | Specificity |
|---|---|---|---|---|---|
| EEGNet | 71.92 ± 5.64% | 69.49 ± 5.38% | 52.57 ± 4.63% | 87.8 ± 5.90% | 84.02 ± 4.06% |
| Shallow ConvNet | 73.68 ± 3.87% | 73.53 ± 3.76% | 57.14 ± 4.53% | 89.28 ± 4.59% | 86.2 ± 3.37% |
| Deep ConvNet | 75.43 ± 1.48% | 72.52 ± 1.44% | 58.11 ± 1.64% | 92.85 ± 1.70% | 75.86 ± 1.75% |
| Model | Accuracy | F1-Score | Coh-Kappa | Sensitivity | Specificity |
|---|---|---|---|---|---|
| EEGNet | 70.85 ± 3.25% | 70.79 ± 3.86% | 52.54 ± 5.89% | 83.83 ± 5.65% | 82.80 ± 4.13% |
| Shallow ConvNet | 73.45 ± 3.69% | 72.84 ± 3.61% | 54.43 ± 4.92% | 88.91 ± 5.08% | 86.34 ± 5.62% |
| Deep ConvNet | 74.65 ± 4.19% | 71.54 ± 4.7% | 57.52 ± 3.42% | 91.18 ± 5.04% | 74.46 ± 4.79% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, A.R.; Cao, Z.; Chen, H.-T.; Martens, K.E.; Georgiades, M.; Gilat, M.; Nguyen, H.T.; Lewis, S.J.G.; Lin, C.-T. Predicting the Onset of Freezing of Gait Using EEG Dynamics. Appl. Sci. 2023, 13, 302. https://doi.org/10.3390/app13010302
John AR, Cao Z, Chen H-T, Martens KE, Georgiades M, Gilat M, Nguyen HT, Lewis SJG, Lin C-T. Predicting the Onset of Freezing of Gait Using EEG Dynamics. Applied Sciences. 2023; 13(1):302. https://doi.org/10.3390/app13010302
Chicago/Turabian StyleJohn, Alka Rachel, Zehong Cao, Hsiang-Ting Chen, Kaylena Ehgoetz Martens, Matthew Georgiades, Moran Gilat, Hung T. Nguyen, Simon J. G. Lewis, and Chin-Teng Lin. 2023. "Predicting the Onset of Freezing of Gait Using EEG Dynamics" Applied Sciences 13, no. 1: 302. https://doi.org/10.3390/app13010302
APA StyleJohn, A. R., Cao, Z., Chen, H.-T., Martens, K. E., Georgiades, M., Gilat, M., Nguyen, H. T., Lewis, S. J. G., & Lin, C.-T. (2023). Predicting the Onset of Freezing of Gait Using EEG Dynamics. Applied Sciences, 13(1), 302. https://doi.org/10.3390/app13010302







