Efficient Capture of Sr2+ Ions by a Layered Potassium Neodymium Phosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the K3Nd(PO4)2
2.2.2. Characterizations
2.2.3. Batch Adsorption Experiments
3. Results and Discussion
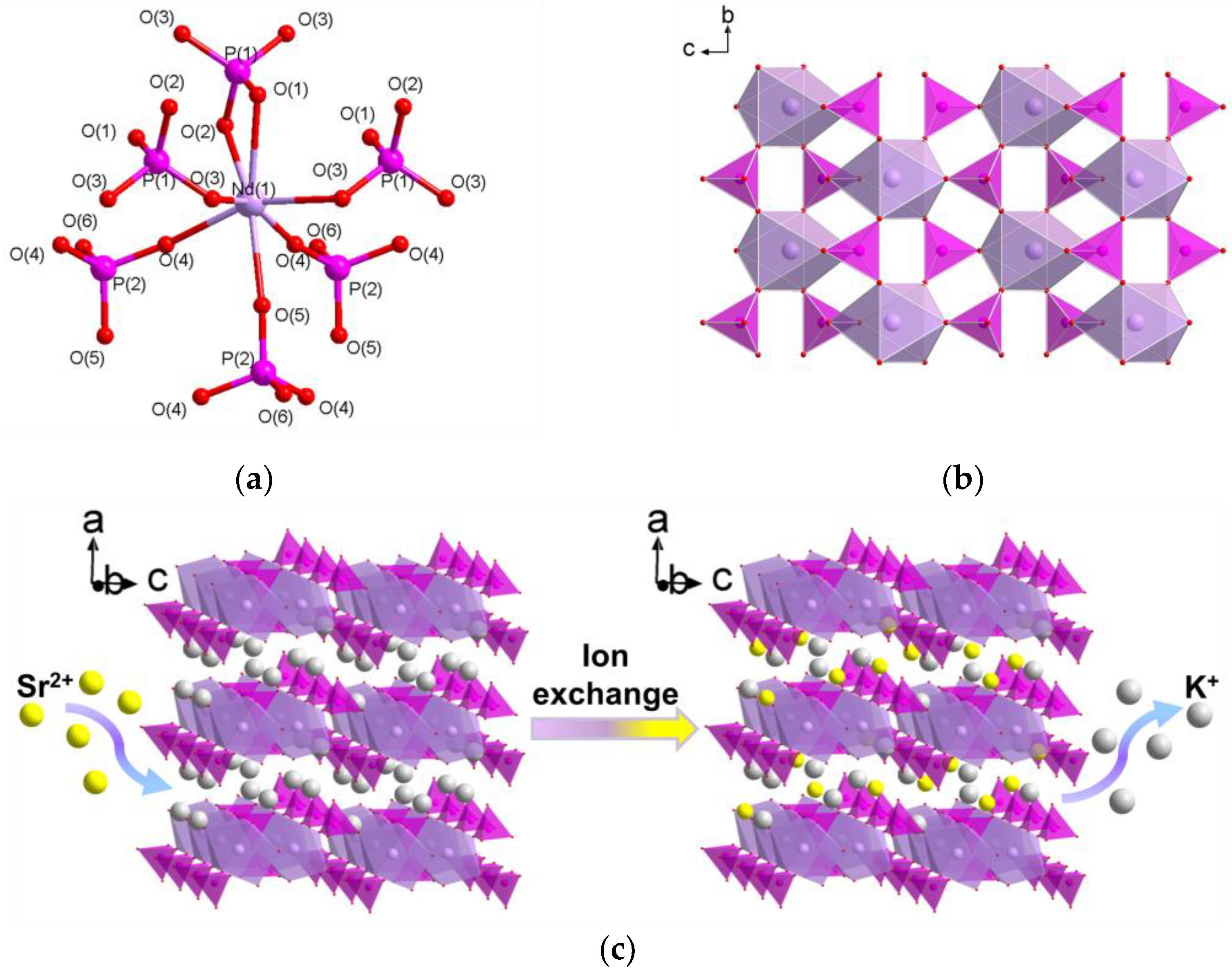
3.1. Crystal Structures
3.2. Removal of Sr2+ from Aqueous Solutions
3.2.1. Adsorption Isotherms
3.2.2. Kinetic Studies of Sr2+ Adsorption
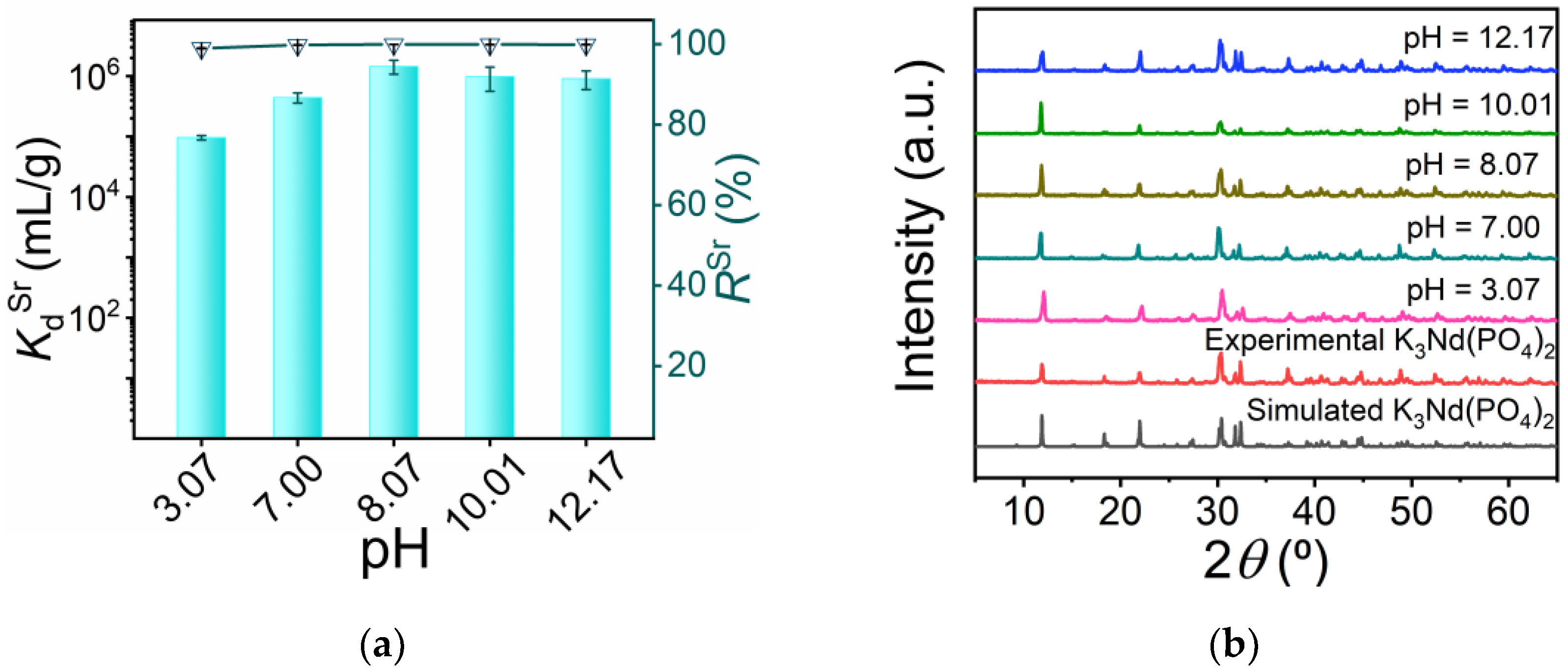
3.2.3. pH-Dependent Experiments
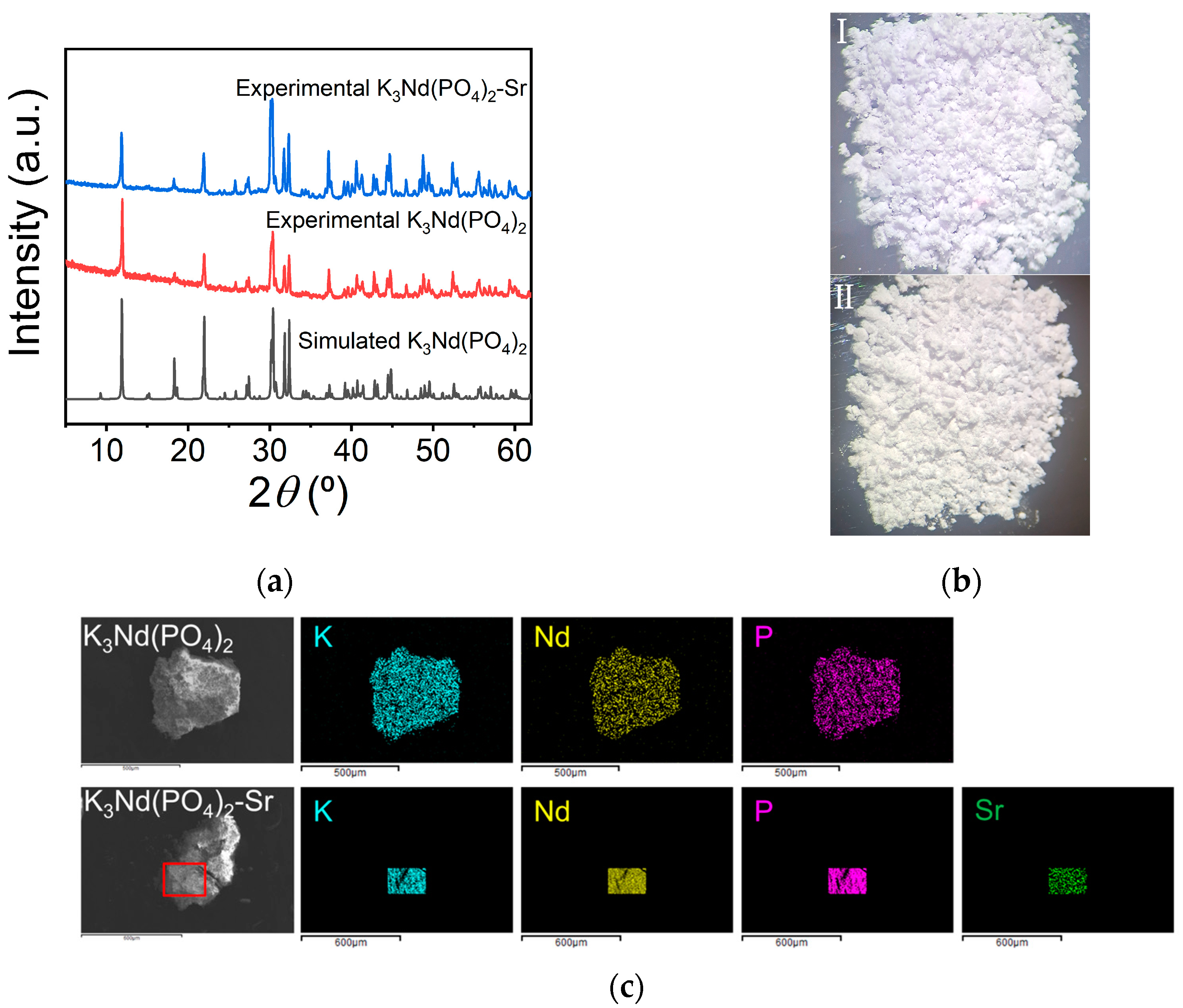
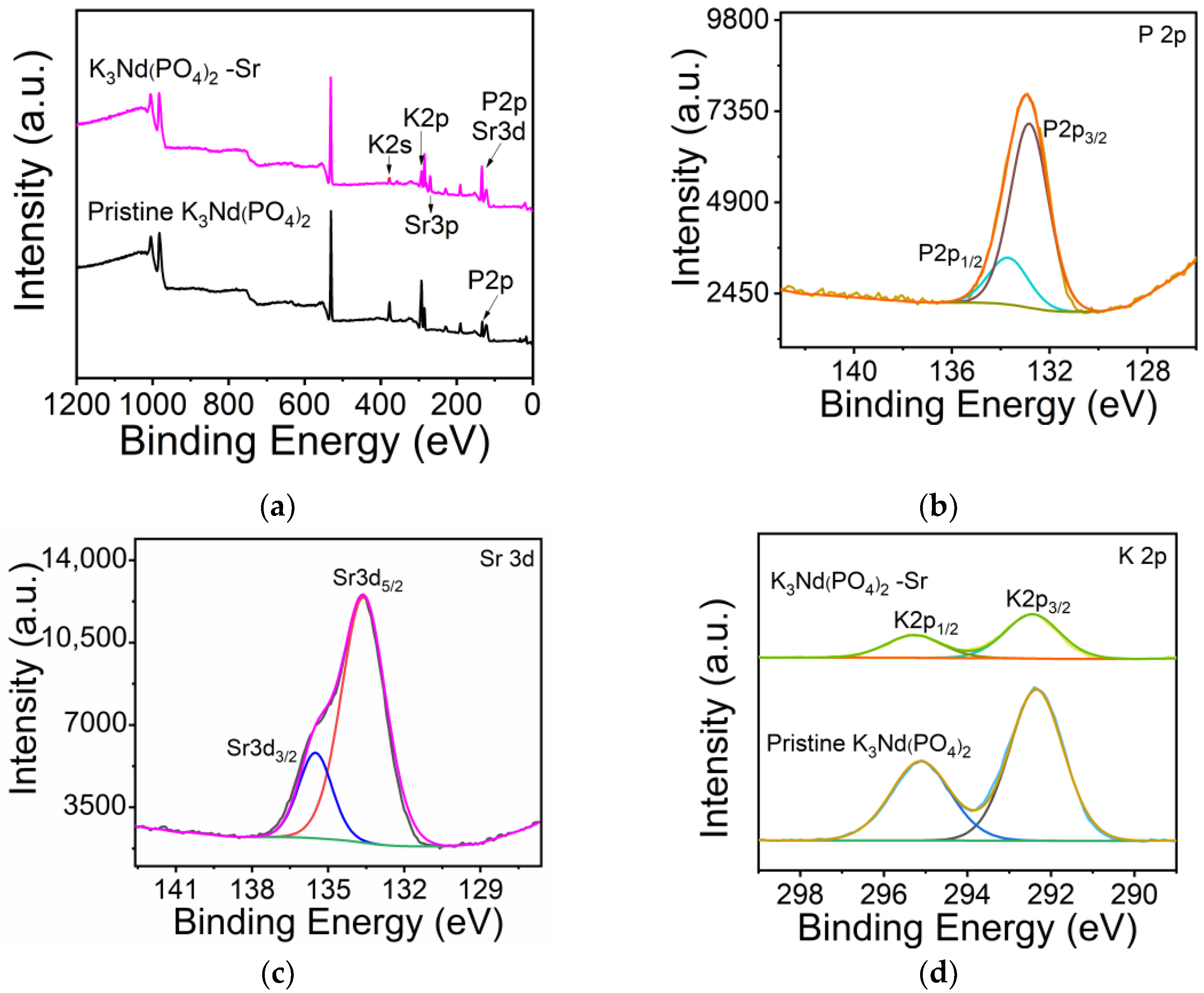
3.3. Adsorption Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard. Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Inan, S. Inorganic ion exchangers for strontium removal from radioactive waste: A review. J. Radioanal. Nucl. Chem. 2022, 331, 1137–1154. [Google Scholar] [CrossRef]
- Lv, T.T.; Ma, W.; Zhang, D.; Zhang, T.; Tang, J.H.; Zeng, X.; Feng, M.L.; Huang, X.Y. Rapid and highly selective Sr2+ uptake by 3D microporous rare earth oxalates with the facile synthesis, high water stability and radiation resistance. Chem. Eng. J. 2022, 435, 134906. [Google Scholar] [CrossRef]
- Zhang, J.R.; Chen, L.H.; Dai, X.; Zhu, L.; Xiao, C.L.; Xu, L.; Zhang, Z.Y.; Alekseev, E.V.; Wang, Y.X.; Zhang, C.; et al. Distinctive two-step intercalation of Sr2+ into a coordination polymer with record high Sr-90 uptake capabilities. Chem 2019, 5, 977–994. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef]
- Casacuberta, N.; Masque, P.; Garcia-Orellana, J.; Garcia-Tenorio, R.; Buesseler, K.O. Sr-90 and Sr-89 in seawater off Japan as a consequence of the Fukushima Dai-ichi nuclear accident. Biogeosciences 2013, 10, 3649–3659. [Google Scholar] [CrossRef]
- Martell, E.A. Atmospheric aspects of strontium-90 fallout. Science 1959, 129, 1197–1206. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, G.S.; Tazoe, H.; Ma, L.L.; Yamada, M.; Xu, D.D. A review of measurement methodologies and their applications to environmental Sr-90. J. Environ. Radioact. 2018, 192, 321–333. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Integrated forward osmosis-adsorption process for strontium-containing water treatment: Pre-concentration and solidification. J. Hazard. Mater. 2021, 414, 125518. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Du, D.; Sun, B.; Yi, X. Monitoring long-term ecological impacts from release of Fukushima radiation water into ocean. Geogr. Sustain. 2021, 2, 95–98. [Google Scholar] [CrossRef]
- Xin, X.; Liu, M.H.; Wang, X.L.; Zhang, T.; Gao, L.Y.; Chen, K. Evolutionary analysis of Japan’s nuclear wastewater discharge events considering the impact of participants’ emotions. Ocean Coast. Manag. 2022, 225, 106231. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Tochaikul, G.; Phattanasub, A.; Khemkham, P.; Saengthamthawee, K.; Danthanavat, N.; Moonkum, N. Radioactive waste treatment technology: A review. Kerntechnik 2022, 87, 208–225. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Metal sulfide ion exchangers: Superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016, 7, 4804–4824. [Google Scholar] [CrossRef]
- Sun, H.Y.; Liu, Y.; Lin, J.; Yue, Z.H.; Li, W.A.; Jin, J.C.; Sun, Q.; Ai, Y.J.; Feng, M.L.; Huang, X.Y. Highly selective recovery of lanthanides by using a layered vanadate with acid and radiation resistance. Angew. Chem. Int. Ed. 2020, 59, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Smiciklas, I.; Dimovic, S.; Plecas, I. Removal of Cs1+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl. Clay Sci. 2007, 35, 139–144. [Google Scholar] [CrossRef]
- Seliman, A.F.; Lasheen, Y.F.; Youssief, M.A.E.; Abo-Aly, M.M.; Shehata, F.A. Removal of some radionuclides from contaminated solution using natural clay: Bentonite. J. Radioanal. Nucl. Chem. 2014, 300, 969–979. [Google Scholar] [CrossRef]
- Chitra, S.; Shanmugamani, A.G.; Sudha, R.; Kalavathi, S.; Paul, B. Selective removal of cesium and strontium by crystalline silicotitanates. J. Radioanal. Nucl. Chem. 2017, 312, 507–515. [Google Scholar] [CrossRef]
- Aguila, B.; Banerjee, D.; Nie, Z.M.; Shin, Y.; Ma, S.Q.; Thallapally, P.K. Selective removal of cesium and strontium using porous frameworks from high level nuclear waste. Chem. Commun. 2016, 52, 5940–5942. [Google Scholar] [CrossRef]
- Manos, M.J.; Ding, N.; Kanatzidis, M.G. Layered metal sulfides: Exceptionally selective agents for radioactive strontium removal. Proc. Natl. Acad. Sci. USA 2008, 105, 3696–3699. [Google Scholar] [CrossRef]
- Qi, X.H.; Du, K.Z.; Feng, M.L.; Li, J.R.; Du, C.F.; Zhang, B.; Huang, X.Y. A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property. J. Mater. Chem. A 2015, 3, 5665–5673. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U. Recent progress in field of synthetic inorganic exchangers having a layered or fibrous structure. J. Chromatogr. A 1974, 102, 5–29. [Google Scholar] [CrossRef]
- Deng, Y.L.; Huang, L.; Dong, X.H.; Wang, L.; Ok, K.M.; Zeng, H.M.; Lin, Z.; Zou, G.H. K2Sb(P2O7)F: Cairo pentagonal layer with bifunctional genes reveal optical performance. Angew. Chem. Int. Ed. 2020, 59, 21151–21156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.C.; Huang, L.C.; Zhu, J.H.; Li, P.P.; Chu, P.K.; Wang, J.H.; Yu, X.F. Phosphorus-based materials for high-performance alkaline metal ion batteries: Progress and prospect. Small 2022, 18, 2201808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yuan, Z.Y. Insights into transition metal phosphate materials for efficient electrocatalysis. ChemCatChem 2020, 12, 3797–3810. [Google Scholar] [CrossRef]
- Munkaila, S.; Dahal, R.; Kokayi, M.; Jackson, T.; Bastakoti, B.P. Hollow structured transition metal phosphates and their applications. Chem. Rec. 2022, 22, e202200084. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, U.H.; Hwang, Y.K.; Chang, J.S.; Jang, N.H. Catalytic and sorption applications of porous nickel phosphate materials. Catal. Today 2019, 324, 154–166. [Google Scholar] [CrossRef]
- Colodrero, R.M.P.; Olivera-Pastor, P.; Cabeza, A.; Bazaga-Garcia, M. Properties and applications of metal phosphates and pyrophosphates as proton conductors. Materials 2022, 15, 1292. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Role of ion exchange in solid-state chemistry. Chem. Rev. 1988, 88, 125–148. [Google Scholar] [CrossRef]
- Lu, X.F.; Chen, Z.H.; Shi, X.R.; Jing, Q.; Lee, M.H. Two pyrophosphates with large birefringences and second-harmonic responses as ultraviolet nonlinear optical materials. Angew. Chem. Int. Ed. 2020, 59, 17648–17656. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Kuwano, J. Ion-exchange properties of nasicon-type phosphates with the frameworks [Ti2(PO4)3] and [Ti1.7Al0.3(PO4)3]. J. Mater. Chem. 1994, 4, 9–12. [Google Scholar] [CrossRef]
- Bevara, S.; Giri, P.; Patwe, S.J.; Achary, S.N.; Mishra, R.K.; Kumar, A.; Sinha, A.K.; Kaushik, C.P.; Tyagi, A.K. Separation of 90Sr from nuclear waste by crystalline complex phosphates of Ce(IV) and Zr(IV). J. Environ. Chem. Eng. 2018, 6, 2248–2261. [Google Scholar] [CrossRef]
- Roelofs, M.G.; Morris, P.A.; Bierlein, J.D. Ion exchange of Rb, Ba, and Sr in KTiOPO4. J. Appl. Phys. 1991, 70, 720–728. [Google Scholar] [CrossRef]
- Daneshvar, K.; Giess, E.A.; Bacon, A.M.; Dawes, D.G.; Gea, L.A.; Boatner, L.A. Ion exchange in potassium titanyl phosphate. Appl. Phys. Lett. 1997, 71, 756–758. [Google Scholar] [CrossRef]
- Zouad, S.; Jeanjean, J.; Loosneskovic, C.; Fedoroff, M.; Piffard, Y. Sorption of strontium and lanthanum on polyantimonic acid and two phosphatoantimonic acids. J. Radioanal. Nucl. Chem. Art. 1994, 182, 193–204. [Google Scholar] [CrossRef]
- Jeanjean, J.; Fedoroff, M.; Zouad, S.; Loosneskovic, C.; Piffard, Y. A comparative study of the sorption of strontium on polyantimonic acid (H2Sb2O6, x H2O) and phosphatoantimonic acid (H3Sb3O6(PO4)2, x H2O). Eur. J. Solid State Inorg. Chem. 1994, 31, 1037–1048. [Google Scholar]
- Clearfield, A. Inorganic ion exchangers with layered structures. Annu. Rev. Mater. Sci. 1984, 14, 205–229. [Google Scholar] [CrossRef]
- Bashir, A.; Ahad, S.; Malik, L.A.; Qureashi, A.; Manzoor, T.; Dar, G.N.; Pandith, A.H. Revisiting the old and golden inorganic material, zirconium phosphate: Synthesis, intercalation, surface functionalization, and metal ion uptake. Ind. Eng. Chem. Res. 2020, 59, 22353–22397. [Google Scholar] [CrossRef]
- Bevara, S.; Achary, S.N.; Patwe, S.J.; Sinha, A.K.; Mishra, R.K.; Kumar, A.; Kaushik, C.P.; Tyagi, A.K. Crystal structure and cation exchanging properties of a novel open framework phosphate of Ce (IV). In Proceedings of the DAE Solid State Physics Symposium, Noida, India, 21–25 December 2015. [Google Scholar]
- Romanchuk, A.Y.; Shekunova, T.O.; Larina, A.I.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K.; Kalmykov, S.N. Sorption of radionuclides onto cerium(IV) hydrogen phosphate Ce(PO4)(HPO4)0.5 (H2O)0.5. Radiochemistry 2019, 61, 719–723. [Google Scholar] [CrossRef]
- Gao, Y.J.; Feng, M.L.; Zhang, B.; Wu, Z.F.; Song, Y.; Huang, X.Y. An easily synthesized microporous framework material for the selective capture of radioactive Cs+ and Sr2+ ions. J. Mater. Chem. A 2018, 6, 3967–3976. [Google Scholar] [CrossRef]
- Li, J.L.; Jin, J.C.; Zou, Y.M.; Sun, H.Y.; Zeng, X.; Huang, X.Y.; Feng, M.L.; Kanatzidis, M.G. Efficient removal of Cs+ and Sr2+ ions by granulous (Me2NH2)4/3(Me3NH)2/3Sn3S7·1.25H2O/polyacrylonitrile composite. ACS Appl. Mater. Interfaces 2021, 13, 13434–13442. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.L.; Sarma, D.; Gao, Y.J.; Qi, X.H.; Li, W.A.; Huang, X.Y.; Kanatzidis, M.G. Efficient removal of [UO2]2+, Cs+, and Sr2+ ions by radiation-resistant gallium thioantimonates. J. Am. Chem. Soc. 2018, 140, 11133–11140. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.Y.P.; Chinn, S.R. Crystal structure and fluorescence lifetime of potassium neodymium orthophosphate, K3Nd(PO4)2, a new laser material. Mater. Res. Bull. 1976, 11, 421–428. [Google Scholar] [CrossRef]
- Mizer, D.; Macalik, L.; Tomaszewski, P.E.; Lisiecki, R.; Godlewska, P.; Matraszek, A.; Szczygiel, I.; Zawadzki, M.; Hanuza, J. Structural and optical properties of nano-sized K3Nd(PO4)2:Yb3+ orthophosphate. J. Nanosci. Nanotechnol. 2009, 9, 5164–5169. [Google Scholar] [CrossRef]
- Farmer, J.M.; Boatner, L.A.; Chakoumakos, B.C.; Rawn, C.J.; Richardson, J. Structural and crystal chemical properties of alkali rare-earth double phosphates. J. Alloys Compd. 2016, 655, 253–265. [Google Scholar] [CrossRef]
- Okada, K.; Ossaka, J. Structures of potassium-sodium sulfate and tripotassium sodium disulfate. Acta Crystallogr. Sect. B Struct. Sci 1980, 36, 919–921. [Google Scholar] [CrossRef]
- Zubkova, N.V.; Pekov, I.V.; Ksenofontov, D.A.; Yapaskurt, V.O.; Pushcharovsky, D.Y.; Sidorov, E.G. Arcanite from fumarole exhalations of the Tolbachik Volcano (Kamchatka, Russia) and its crystal structure. Dokl. Earth Sci. 2018, 4791, 339–341. [Google Scholar] [CrossRef]
- Sarma, D.; Malliakas, C.D.; Subrahmanyam, K.S.; Islama, S.M.; Kanatzidis, M.G. K2xSn4-xS8-x (x = 0.65-1): A new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions. Chem. Sci. 2016, 7, 1121–1132. [Google Scholar] [CrossRef]
- Griffith, C.S.; Luca, V.; Cochrane, J.; Hanna, J.V. Lanthanide/actinide ion-exchange and structural investigations of the layered phosphatoantimonic acid, H3Sb3P2O14·ZH2O. Microporous Mesoporous Mater. 2008, 111, 387–403. [Google Scholar] [CrossRef]
- Pekarek, V.; Benesova, M. A study on uranyl phosphates—I: Sorption properties of uranyl hydrogen phosphate. J. Inorg. Nucl. Chem. 1964, 26, 1743–1751. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics, 3rd ed.; Imperial College Press: London, UK, 1998; pp. 1–892. [Google Scholar]
- Dabrowski, A.; Oscik, J.; Rudzinski, W.; Jaroniec, M. Effects of surface heterogeneity in adsorption from binary liquid mixtures: II. Adsorption from nonideal bulk solutions. J. Colloid Interface Sci. 1979, 69, 287–300. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.M.; Liu, J.D. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, Z.B.; Liu, Y.H.; Cao, X.H.; Liu, Y.T.; Li, Q. Adsorption of U(VI) from aqueous solution by the carboxyl-mesoporous carbon. Chem. Eng. J. 2012, 198, 246–253. [Google Scholar] [CrossRef]
- Lin, M.L.; Zhao, Z.W.; Cui, F.Y.; Xia, S.J. Modeling of equilibrium and kinetics of chlorobenzene (CB) adsorption onto powdered activated carbon (PAC) for drinking water treatment. Desalin. Water Treat. 2012, 44, 245–254. [Google Scholar] [CrossRef]
- Park, Y.; Lee, Y.C.; Shin, W.S.; Choi, S.J. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate-polyacrylonitrile (AMP-PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Protect. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.H.; Wang, Y.L.; Li, X.H.; Wang, Y.Y. Investigation of phosphate removal mechanisms by a lanthanum hydroxide adsorbent using p-XRD, FTIR and XPS. Appl. Surf. Sci. 2021, 557, 149838. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef]
- Pekarek, V.; Vesely, V. A study on uranyl phosphates—II sorption properties of some 1- to 4-valent cations on uranyl hydrogen phosphate heated to various temperatures. J. Inorg. Nucl. Chem. 1965, 27, 1151–1158. [Google Scholar] [CrossRef]
- Li, Z.Q.; Vivas, E.L.; Yang, C.Y.; Suh, Y.J.; Cho, K.K. Layered potassium calcium phosphate with multiple exchangeable cations for Sr(II) and Co(II) removal from water. Sep. Purif. Technol. 2022, 299, 121789. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Shashkova, I.L.; Kitikova, N.V.; Maslova, M.V.; Mudruk, N.V. New heterogeneous synthesis of mixed Ti-Ca-Mg phosphates as efficient sorbents of Cs-137, Sr-90 and Co-60 radionuclides. J. Taiwan Inst. Chem. Eng. 2019, 104, 151–159. [Google Scholar] [CrossRef]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Vasylyeva, H.; Naushad, M.; Mykytyn, I. Adsorption of Sr(II) cations onto phosphated mesoporous titanium dioxide: Mechanism, isotherm and kinetics studies. J. Environ. Chem. Eng. 2019, 7, 103430. [Google Scholar] [CrossRef]
- Mullica, D.F.; Wilson, G.A.; Sappenfield, E.L. Application of an optical beam condenser for UV-Vis spectral studies on monoclinic LnPO4 single-crystals. Appl. Spectrosc. 1994, 48, 345–349. [Google Scholar] [CrossRef]

| Model | R2 | qm (mg/g) | b (L/mg) | n | k |
|---|---|---|---|---|---|
| Langmiur | 0.9938 | 42.60 ± 1.43 | 0.02255 ± 0.00 | - | - |
| Freundlich | 0.8912 | - | - | 4.8673 ± 0.06 | 11.01 ± 0.17 |
| Langmuir–Freundlich | 0.9929 | 40.91 ± 2.62 | 0.02845 ± 0.01 | 0.8932 ± 0.16 | - |
| Adsorbent | qmSr (mg/g) | teSr (min) | Removal Mechanism | pH 1 | Solution Composition 2 | Distribution Coefficient (mL/g) | Ref |
|---|---|---|---|---|---|---|---|
| 2D 3-K3Nd(PO4)2 | 42.60 | 1440 | IE 4 | 8.03 | Sr | 1.46 × 106 | This work |
| 2D-K2Zr(PO4)2 | 52.83 | 90 | IE | 7.00 | Sr | ~30,000 | [32] |
| 2D-UO2HPO4·4H2O | 98.57 | UM 5 | OTH 6 | 2.00 | Sr | 6.90 × 103 | [61] |
| 2D-K3HCa(PO4)2 | 384.00 | 120 | IE | 7.00 | Sr | 2.30 × 104 | [62] |
| 2D-H3Sb3P2O14 | 314.73 | <15 | IE | 2.1–2.5 | Sr | >106 | [36] |
| 2D-Ce(PO4)(HPO4)0.5(H2O)0.5 | UM | UM | AD 7 | >9.00 | Np, Am, U, Th, Sr | UM | [40] |
| 3D-K2Ce(PO4)2 | 45.65 | 60 | IE | 14.00 | Sr | ~8000 | [32] |
| 3D-H5Sb5O12(PO4)2·7.3H2O | 123.19 | NC 8 | IE | <1.00 | Sr | >105 | [35] |
| Ca0.7Mg0.3HPO4·2H2O | UM | UM | NC | 5.00 | Cs, Sr, Co | 0.14 × 103 | [63] |
| Ca2.65Mg3(NH4)1.3(PO4)4(CO3)0.3·6H2O | UM | UM | NC | 5.00 | Cs, Sr, Co | 7.91 × 103 | [63] |
| phosphated montmorillonite | 12.5 | UM | IE/SC 9 | 5.00 | Sr, Cs | UM | [64] |
| 2P-TiO2 | 94.1 | 60 | AD | 8.00 | Sr | UM | [65] |
| 8P-TiO2 | 128.9 | 60 | AD | 9.00 | Sr | UM | [65] |
| 4P-TiO2 | 172.5 | 60 | AD | 9.00 | Sr | UM | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Sun, H.; Guo, Y.; Cheng, C.; Zhuang, T.; Liu, J.; Feng, M.; Huang, X. Efficient Capture of Sr2+ Ions by a Layered Potassium Neodymium Phosphate. Appl. Sci. 2023, 13, 497. https://doi.org/10.3390/app13010497
Yao Y, Sun H, Guo Y, Cheng C, Zhuang T, Liu J, Feng M, Huang X. Efficient Capture of Sr2+ Ions by a Layered Potassium Neodymium Phosphate. Applied Sciences. 2023; 13(1):497. https://doi.org/10.3390/app13010497
Chicago/Turabian StyleYao, Yuexin, Haiyan Sun, Yanling Guo, Cheng Cheng, Tinghui Zhuang, Jiating Liu, Meiling Feng, and Xiaoying Huang. 2023. "Efficient Capture of Sr2+ Ions by a Layered Potassium Neodymium Phosphate" Applied Sciences 13, no. 1: 497. https://doi.org/10.3390/app13010497
APA StyleYao, Y., Sun, H., Guo, Y., Cheng, C., Zhuang, T., Liu, J., Feng, M., & Huang, X. (2023). Efficient Capture of Sr2+ Ions by a Layered Potassium Neodymium Phosphate. Applied Sciences, 13(1), 497. https://doi.org/10.3390/app13010497







