Effects of Bilberry Extract on Hepatic Cholesterol Metabolism in HepG2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Total Antioxidant Capacity
2.3. HepG2 Cell Culture and Cytotoxicity
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Effects of BE
3.2. Cytotoxicity of BE-Treated HepG2 Cells
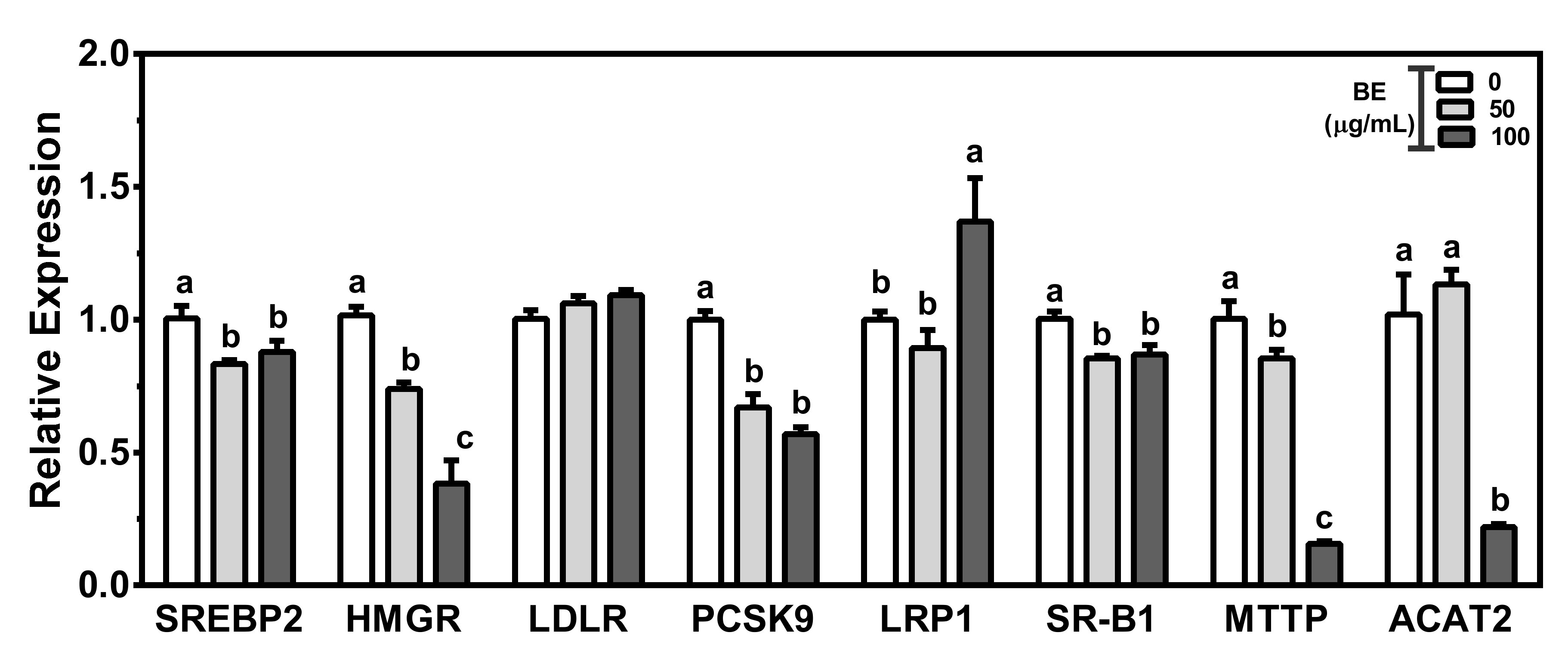
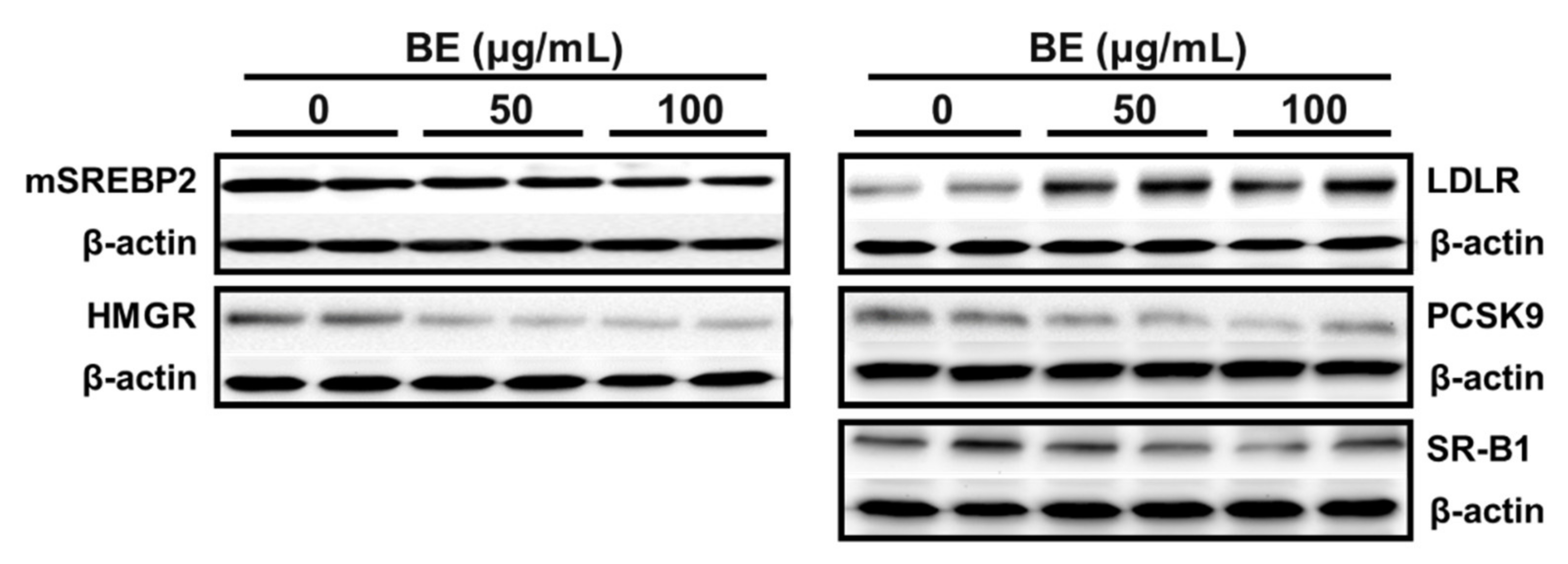
3.3. BE Altered the Expression of Genes for Biosynthesis and Flux of Cholesterol
3.4. Regulation of Genes for Biliary Cholesterol Efflux by BE
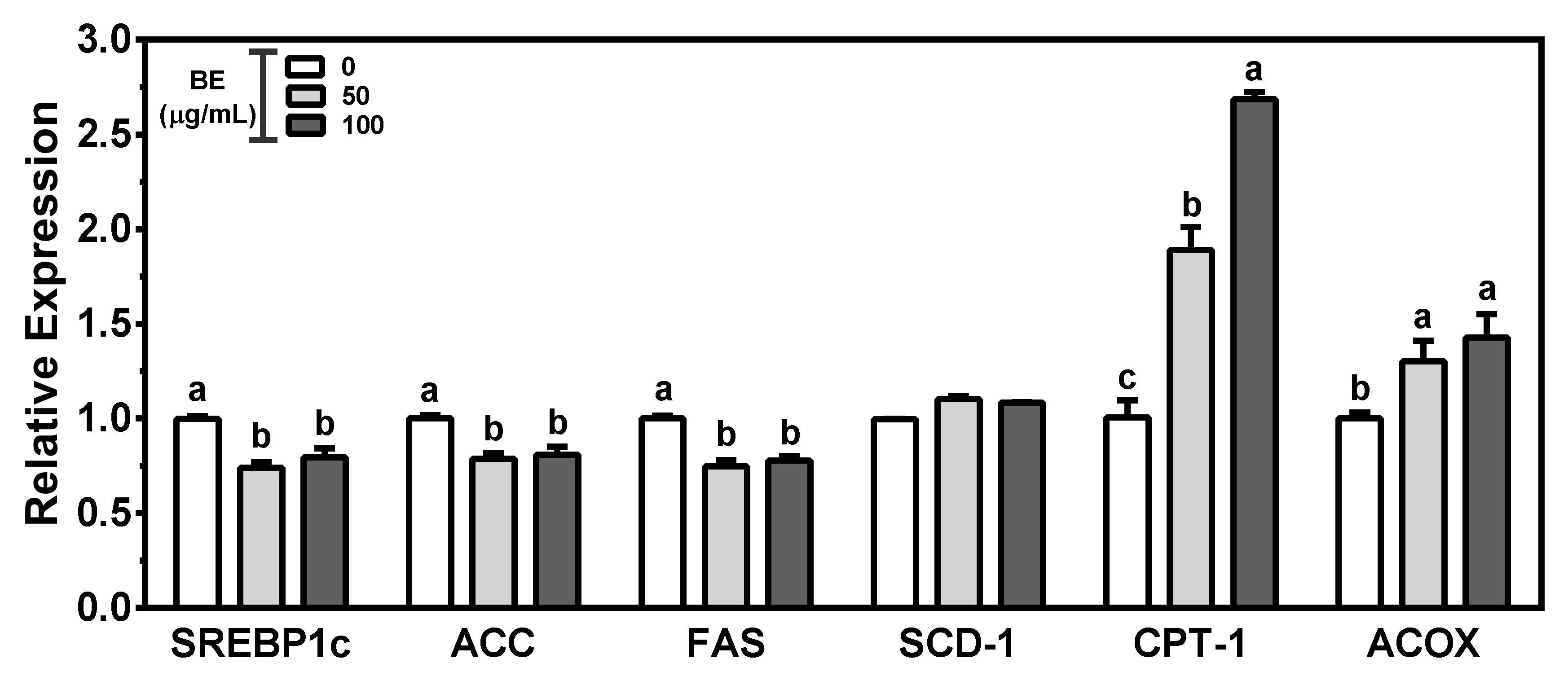
3.5. BE on Genes for Fatty Acid Metabolism
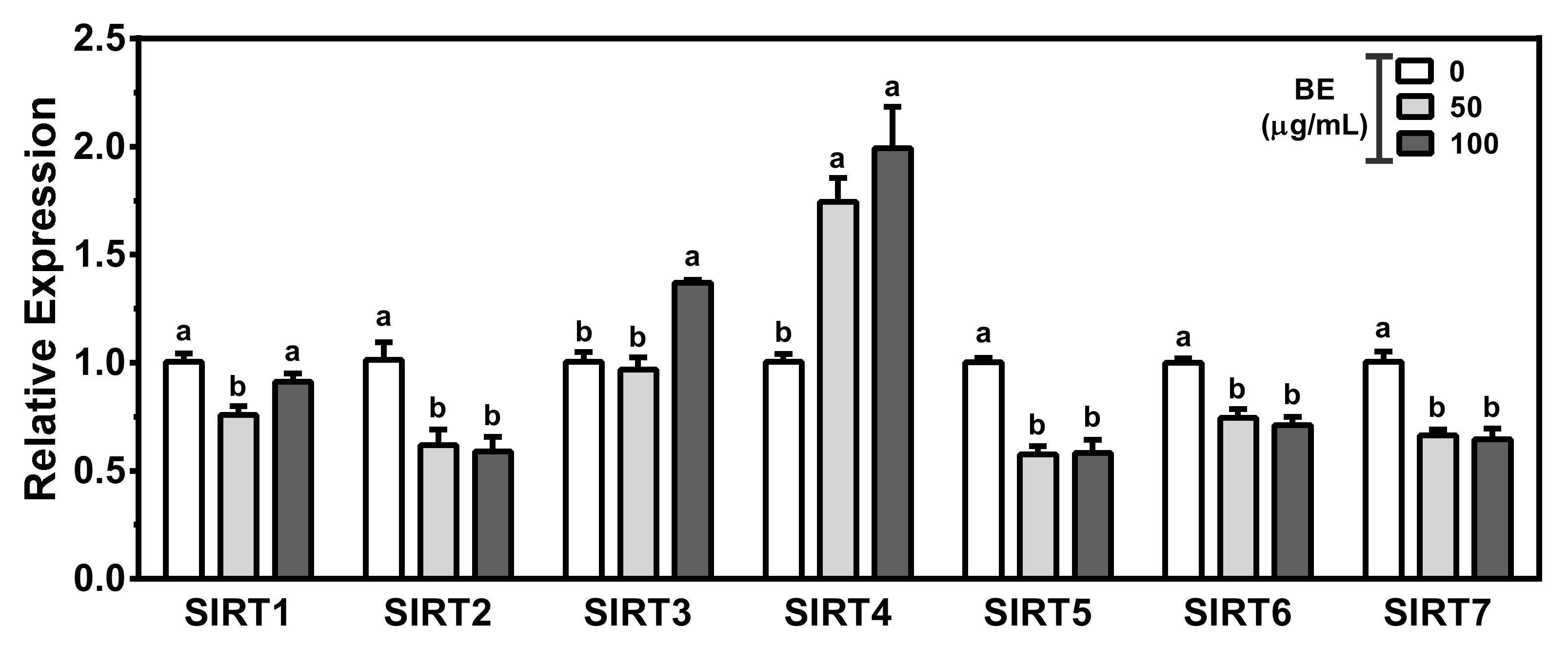
3.6. Alteration of Sirtuins by BE Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Ma, X.; Ye, J.; Zhang, S.; Chen, Z.; Jiang, S. Effects of Dietary Supplementation with Bilberry Extract on Growth Performance, Immune Function, Antioxidant Capacity, and Meat Quality of Yellow-Feathered Chickens. Animals 2021, 11, 1989. [Google Scholar] [CrossRef]
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2007, 8, 11–28. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Lange, Y.; Steck, T.L. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog. Lipid. Res. 2008, 47, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Groen, A.K.; Bloks, V.W.; Verkade, H.; Kuipers, F. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Aspects Med. 2014, 37, 77–88. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- D’Arcy, B.M.; Swingle, M.R.; Schambeau, L.; Pannell, L.; Prakash, A.; Honkanen, R.E. Development of a Synthetic 3-ketosteroid Delta(1)-dehydrogenase for the Generation of a Novel Catabolic Pathway Enabling Cholesterol Degradation in Human Cells. Sci. Rep. 2019, 9, 5969. [Google Scholar] [CrossRef] [Green Version]
- Temel, R.E.; Brown, J.M. A new framework for reverse cholesterol transport: Non-biliary contributions to reverse cholesterol transport. World J. Gastroenterol. 2010, 16, 5946–5952. [Google Scholar] [CrossRef]
- Grefhorst, A.; Verkade, H.J.; Groen, A.K. The TICE Pathway: Mechanisms and Lipid-Lowering Therapies. Methodist Debakey Cardiovasc. J. 2019, 15, 70–76. [Google Scholar] [CrossRef]
- Temel, R.E.; Brown, J.M. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Simonson, W. Update on statin drugs for lipid disorders. Geriatr. Nurs. 2018, 39, 350–351. [Google Scholar] [CrossRef]
- Kashani, A.; Sallam, T.; Bheemreddy, S.; Mann, D.L.; Wang, Y.; Foody, J.M. Review of side-effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am. J. Cardiol. 2008, 101, 1606–1613. [Google Scholar] [CrossRef]
- Viljoen, A.; Wierzbicki, A.S. Colesevelam: An improved bile acid sequestrant for treating hypercholesterolemia and improving diabetes. Expert Rev. Endocrinol. Metab. 2010, 5, 825–834. [Google Scholar] [CrossRef]
- Van Bruggen, F.H.; Nijhuis, G.B.J.; Zuidema, S.U.; Luijendijk, H. Serious adverse events and deaths in PCSK9 inhibitor trials reported on ClinicalTrials.gov: A systematic review. Expert Rev. Clin. Pharmacol. 2020, 13, 787–796. [Google Scholar] [CrossRef]
- Lemus, H.N.; Mendivil, C.O. Adenosine triphosphate citrate lyase: Emerging target in the treatment of dyslipidemia. J. Clin. Lipidol. 2015, 9, 384–389. [Google Scholar] [CrossRef]
- Sprecher, D.L. Raising high-density lipoprotein cholesterol with niacin and fibrates: A comparative review. Am. J. Cardiol. 2000, 86, 46L–50L. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Quinones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ulbricht, C.; Basch, E.; Basch, S.; Bent, S.; Boon, H.; Burke, D.; Costa, D.; Falkson, C.; Giese, N.; Goble, M.; et al. An evidence-based systematic review of bilberry (Vaccinium myrtillus) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2009, 6, 162–200. [Google Scholar] [CrossRef]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [Green Version]
- Grohmann, T.; Litts, C.; Horgan, G.; Zhang, X.; Hoggard, N.; Russell, W.; de Roos, B. Efficacy of Bilberry and Grape Seed Extract Supplement Interventions to Improve Glucose and Cholesterol Metabolism and Blood Pressure in Different Populations—A Systematic Review of the Literature. Nutrients 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Spalinger, M.R.; Muller, I.; Lang, S.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins prevent IFN-gamma-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion 2014, 90, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Lv, X.D.; Wang, G.E.; Li, Y.F.; Kurihara, H.; He, R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014, 65, 594–601. [Google Scholar] [CrossRef]
- Kropat, C.; Mueller, D.; Boettler, U.; Zimmermann, K.; Heiss, E.H.; Dirsch, V.M.; Rogoll, D.; Melcher, R.; Richling, E.; Marko, D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol. Nutr. Food Res. 2013, 57, 545–550. [Google Scholar] [CrossRef]
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.X. Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2020, 12, 3252. [Google Scholar] [CrossRef]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013, 10, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Stefanut, M.N.; Cata, A.; Pop, R.; Tanasie, C.; Boc, D.; Ienascu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Asgary, S.; RafieianKopaei, M.; Sahebkar, A.; Shamsi, F.; Goli-malekabadi, N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Kim, M.; Kim, B. The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion. Foods 2021, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lee, S.; Choi, Y.; Kim, B. The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism. Appl. Sci. 2021, 11, 10018. [Google Scholar] [CrossRef]
- Mommersteeg, P.M.; Denollet, J.; Spertus, J.A.; Pedersen, S.S. Health status as a risk factor in cardiovascular disease: A systematic review of current evidence. Am. Heart J. 2009, 157, 208–218. [Google Scholar] [CrossRef]
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [Green Version]
- Rienks, J.; Barbaresko, J.; Nothlings, U. Review Association of Polyphenol Biomarkers with Cardiovascular Disease and Mortality Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovas. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Quinones, M.; Miguel, M.; Aleixandre, A. The polyphenols, naturally occurring compounds with beneficial effects on cardiovascular disease. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Lan, F.; He, R.R.; Kurihara, H. Protective effects of bilberry (Vaccinium myrtillus L.) extract against endotoxin-induced uveitis in mice. J. Agric. Food Chem. 2010, 58, 4731–4736. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yao, X.S.; Tsi, D.; Yau, C.C.; Chia, C.S.; Nagai, H.; Kurihara, H. Protective effects of bilberry (Vaccinium myrtillus L.) extract on KBrO3-induced kidney damage in mice. J. Agric. Food Chem. 2008, 56, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Portincasa, P.; Tso, P. Transintestinal cholesterol excretion: A secondary, nonbiliary pathway contributing to reverse cholesterol transport. Hepatology 2017, 66, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Bayazid, A.B.; Chun, E.M.; Al Mijan, M.; Park, S.H.; Moon, S.-K.; Lim, B.O. Anthocyanins profiling of bilberry (Vaccinium myrtillus L.) extract that elucidates antioxidant and anti-inflammatory effects. Food Agric. Immunol. 2021, 32, 713–726. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Sterol regulatory element binding proteins (SREBPs): Controllers of lipid synthesis and cellular uptake. Nutr. Rev. 1998, 56, S1–S3. [Google Scholar] [CrossRef]
- Madison, B.B. Srebp2: A master regulator of sterol and fatty acid synthesis. J. Lipid. Res. 2016, 57, 333–335. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. Defining the Cholesterol Lowering Mechanism of Bergamot (Citrus bergamia) Extract in HepG2 and Caco-2 Cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef]
- Wong, T.Y.; Lin, S.M.; Leung, L.K. The Flavone Luteolin Suppresses SREBP-2 Expression and Post-Translational Activation in Hepatic Cells. PLoS ONE 2015, 10, e0135637. [Google Scholar] [CrossRef]
- Liu, L.K.; Chou, F.P.; Chen, Y.C.; Chyau, C.C.; Ho, H.H.; Wang, C.J. Effects of mulberry (Morus alba L.) extracts on lipid homeostasis in vitro and in vivo. J. Agric. Food Chem. 2009, 57, 7605–7611. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Sun, H.; Tang, M.; Zhao, J.; Zhang, Z.; Sun, X.; He, S. Red raspberry extract (Rubus idaeus L. shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019, 133, 110796. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Cheng, J.J.; Yu, M.H.; Chung, D.J.; Huang, C.N.; Wang, C.J. Solanum nigrum polyphenols reduce body weight and body fat by affecting adipocyte and lipid metabolism. Food Funct. 2020, 11, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Van de Sluis, B.; Wijers, M.; Herz, J. News on the molecular regulation and function of hepatic low-density lipoprotein receptor and LDLR-related protein 1. Curr. Opin. Lipidol. 2017, 28, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Moon, Y.A.; Horton, J.D. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 2004, 279, 50630–50638. [Google Scholar] [CrossRef] [Green Version]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Song, K.H.; Kim, Y.H.; Im, A.R.; Kim, Y.H. Black Raspberry Extract Enhances LDL Uptake in HepG2 Cells by Suppressing PCSK9 Expression to Upregulate LDLR Expression. J. Med. Food 2018, 21, 560–567. [Google Scholar] [CrossRef]
- Lee, D.-H.; Choi, S.-S.; Kim, B.-B.; Kim, S.-Y.; Kang, B.-S.; Lee, S.-J.; Park, H.-J. Effect of alcohol-free red wine concentrates on cholesterol homeostasis: An in vitro and in vivo study. Process. Biochem. 2013, 48, 1964–1971. [Google Scholar] [CrossRef]
- Yu, Y.; Si, Y.; Song, G.; Luo, T.; Wang, J.; Qin, S. Ethanolic extract of propolis promotes reverse cholesterol transport and the expression of ATP-binding cassette transporter A1 and G1 in mice. Lipids 2011, 46, 805–811. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhao, G.J. Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARgamma/LXRalpha pathway. Food Funct. 2018, 9, 624–635. [Google Scholar] [CrossRef]
- Shelness, G.S.; Sellers, J.A. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001, 12, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Siddiqi, S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parini, P.; Davis, M.; Lada, A.T.; Erickson, S.K.; Wright, T.L.; Gustafsson, U.; Sahlin, S.; Einarsson, C.; Eriksson, M.; Angelin, B.; et al. ACAT2 is localized to Hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation 2004, 110, 2017–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieda, A.; Wada, M.; Moriyasu, Y.; Okuno, Y.; Zaima, N.; Moriyama, T. Ellagic Acid Suppresses ApoB Secretion and Enhances ApoA-1 Secretion from Human Hepatoma Cells, HepG2. Molecules 2021, 26, 3885. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Ma, C.; Xing, S.; An, Y.; Feng, J.; Dang, H.; Huang, W.; Qiao, L.; Cheng, J.; Xie, L. White tea and its active polyphenols lower cholesterol through reduction of very-low-density lipoprotein production and induction of LDLR expression. Biomed. Pharmacother. 2020, 127, 110146. [Google Scholar] [CrossRef]
- Kidambi, S.; Patel, S.B. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: A review. Xenobiotica 2008, 38, 1119–1139. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L.Q. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.Y.; Chang, C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 2008, 7, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Calvo, M.; Lisnock, J.M.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick Cl-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [Green Version]
- Temel, R.E.; Tang, W.; Ma, Y.; Rudel, L.L.; Willingham, M.C.; Ioannou, Y.A.; Davies, J.P.; Nilsson, L.M.; Yu, L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Investig. 2007, 117, 1968–1978. [Google Scholar] [CrossRef]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ressaissi, A.; Attia, N.; Pacheco, R.; Falé, P.L.; Serralheiro, M.L.M. Cholesterol transporter proteins in HepG2 cells can be modulated by phenolic compounds present in Opuntia ficus-indica aqueous solutions. J. Funct. Foods 2020, 64, 103674. [Google Scholar] [CrossRef]
- Ge, Z.Z.; Zhu, W.; Peng, J.M.; Deng, X.Y.; Li, C.M. Persimmon tannin regulates the expression of genes critical for cholesterol absorption and cholesterol efflux by LXR alpha independent pathway. J. Funct. Foods 2016, 23, 283–293. [Google Scholar] [CrossRef]
- Ontawong, A.; Pasachan, T.; Trisuwan, K.; Soodvilai, S.; Duangjai, A.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Coffea arabica pulp aqueous extract attenuates oxidative stress and hepatic lipid accumulation in HepG2 cells. J. Herb. Med. 2021, 29, 100465. [Google Scholar] [CrossRef]
- Chiang, J.Y. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J. Hepatol. 2004, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sugiyama, Y. Bile salt export pump (BSEP/ABCB11): Trafficking and sorting disturbances. Curr. Mol. Pharmacol. 2013, 6, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Hoppel, C. The role of carnitine in normal and altered fatty acid metabolism. Am. J. Kidney Dis. 2003, 41, S4–S12. [Google Scholar] [CrossRef]
- Christensen, E.; Woldseth, B.; Hagve, T.A.; Poll-The, B.T.; Wanders, R.J.; Sprecher, H.; Stokke, O.; Christophersen, B.O. Peroxisomal beta-oxidation of polyunsaturated long chain fatty acids in human fibroblasts. The polyunsaturated and the saturated long chain fatty acids are retroconverted by the same acyl-CoA oxidase. Scand. J. Clin. Lab. Investig. Suppl. 1993, 215, 61–74. [Google Scholar] [CrossRef]


| Total Antioxidant Capacity | ||
|---|---|---|
| DPPH (mM VCE/g) | ABTS (mM VCE/g) | FRAP (mM FeSO4/g) |
| 282.6 ± 13.5 | 560.8 ± 62.6 | 4.73 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Bae, M.; Kim, B. Effects of Bilberry Extract on Hepatic Cholesterol Metabolism in HepG2 Cells. Appl. Sci. 2023, 13, 516. https://doi.org/10.3390/app13010516
Hong J, Bae M, Kim B. Effects of Bilberry Extract on Hepatic Cholesterol Metabolism in HepG2 Cells. Applied Sciences. 2023; 13(1):516. https://doi.org/10.3390/app13010516
Chicago/Turabian StyleHong, Jimin, Minkyung Bae, and Bohkyung Kim. 2023. "Effects of Bilberry Extract on Hepatic Cholesterol Metabolism in HepG2 Cells" Applied Sciences 13, no. 1: 516. https://doi.org/10.3390/app13010516






