Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biology Materials
2.2. Chemicals and Reagents
2.3. Preparation of WG, BG, and FBG Extract
2.4. Cell Cultures
2.5. Cytotoxicity Assay
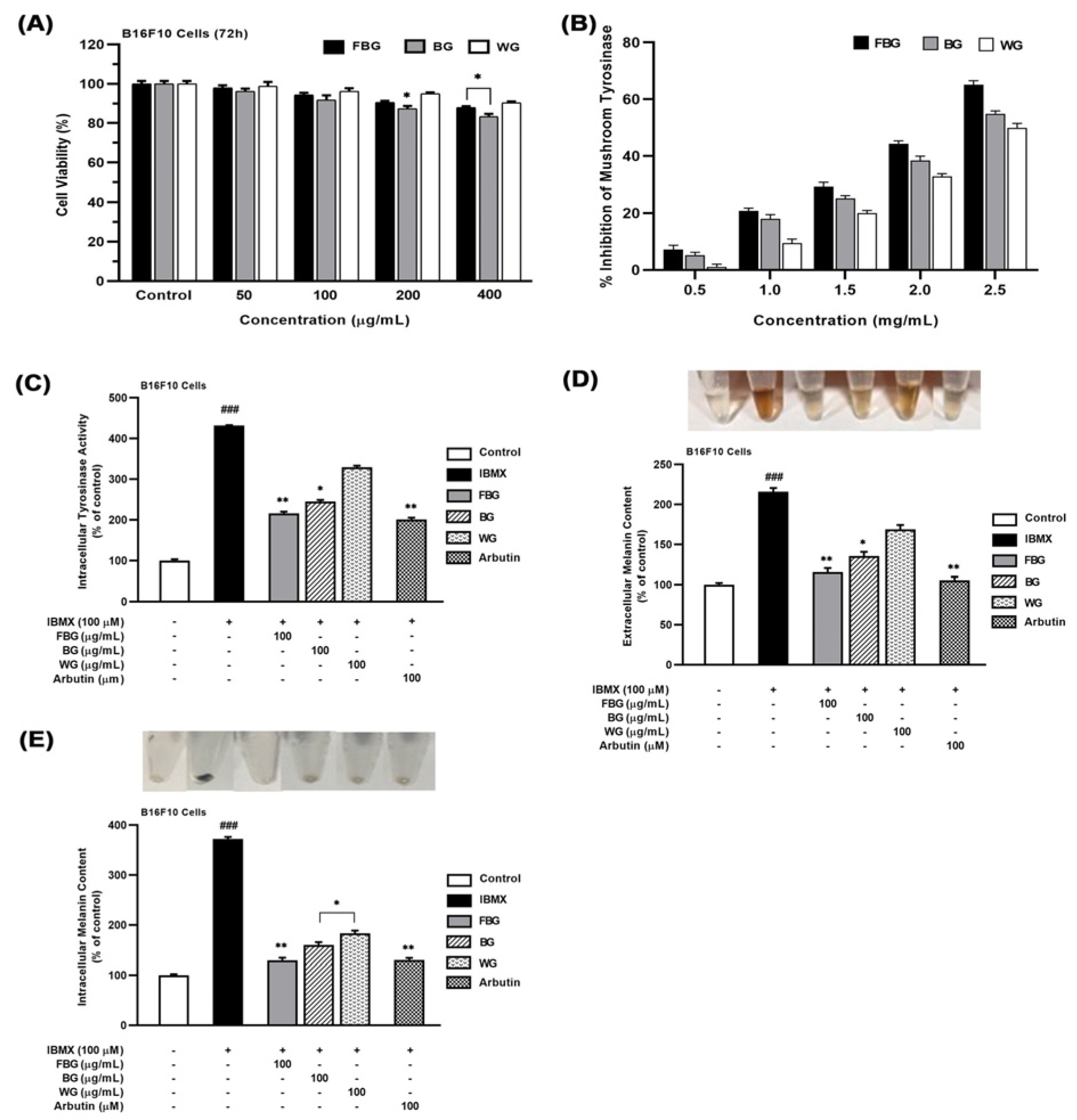
2.6. Anti-Melanogenic Activity Measurements
2.6.1. Determination of Mushroom Tyrosinase Inhibition
2.6.2. Determination of Intracellular Tyrosinase Activity in B16F10 Cells
2.6.3. Determination of Melanin Content
2.7. Anti-Wrinkle Activity Measurements
2.7.1. Determination of Elastase Inhibition
2.7.2. Determination of Elastase Activity in HDF Cells
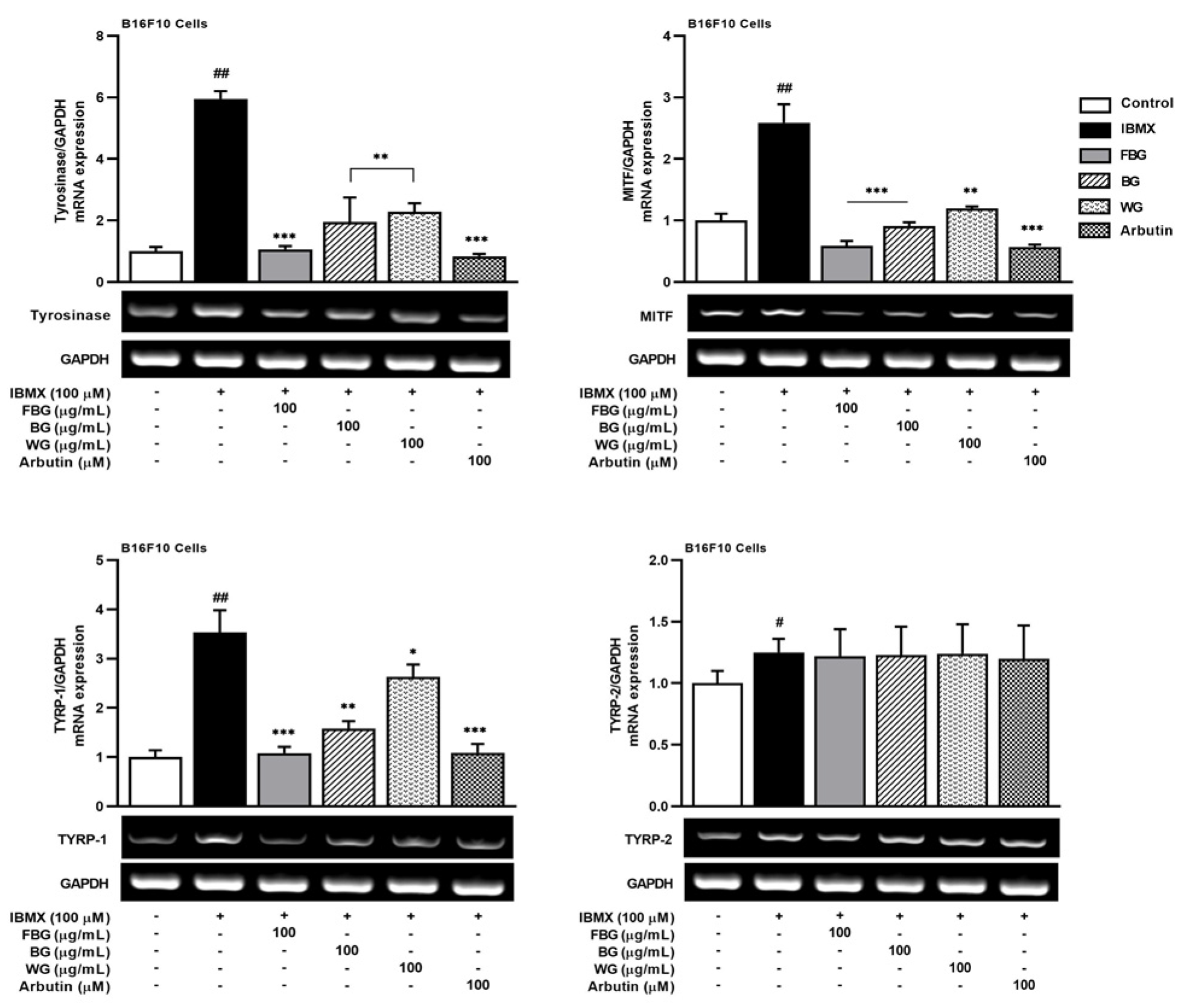
2.8. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Total Phenolic Content (TPC) Measurement
2.10. Total Flavonoid Content (TFC) Measurement
2.11. Antioxidant Properties Measurements
2.11.1. DPPH (2,2-Diphenyl-1-picryl-hydrazyl) Assay
2.11.2. Reducing Power
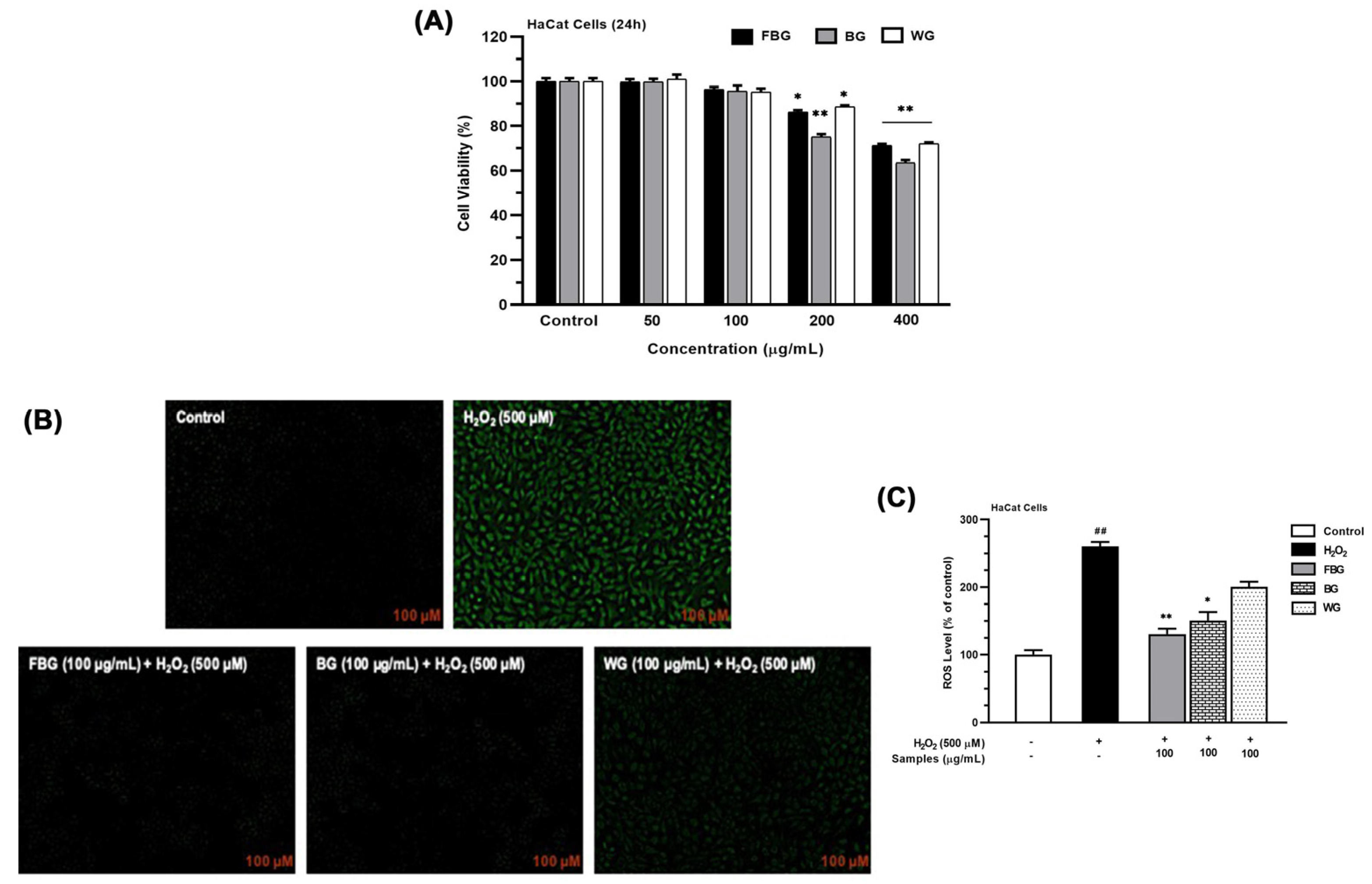
2.11.3. ROS Generation
2.12. Statistical Analysis
3. Results
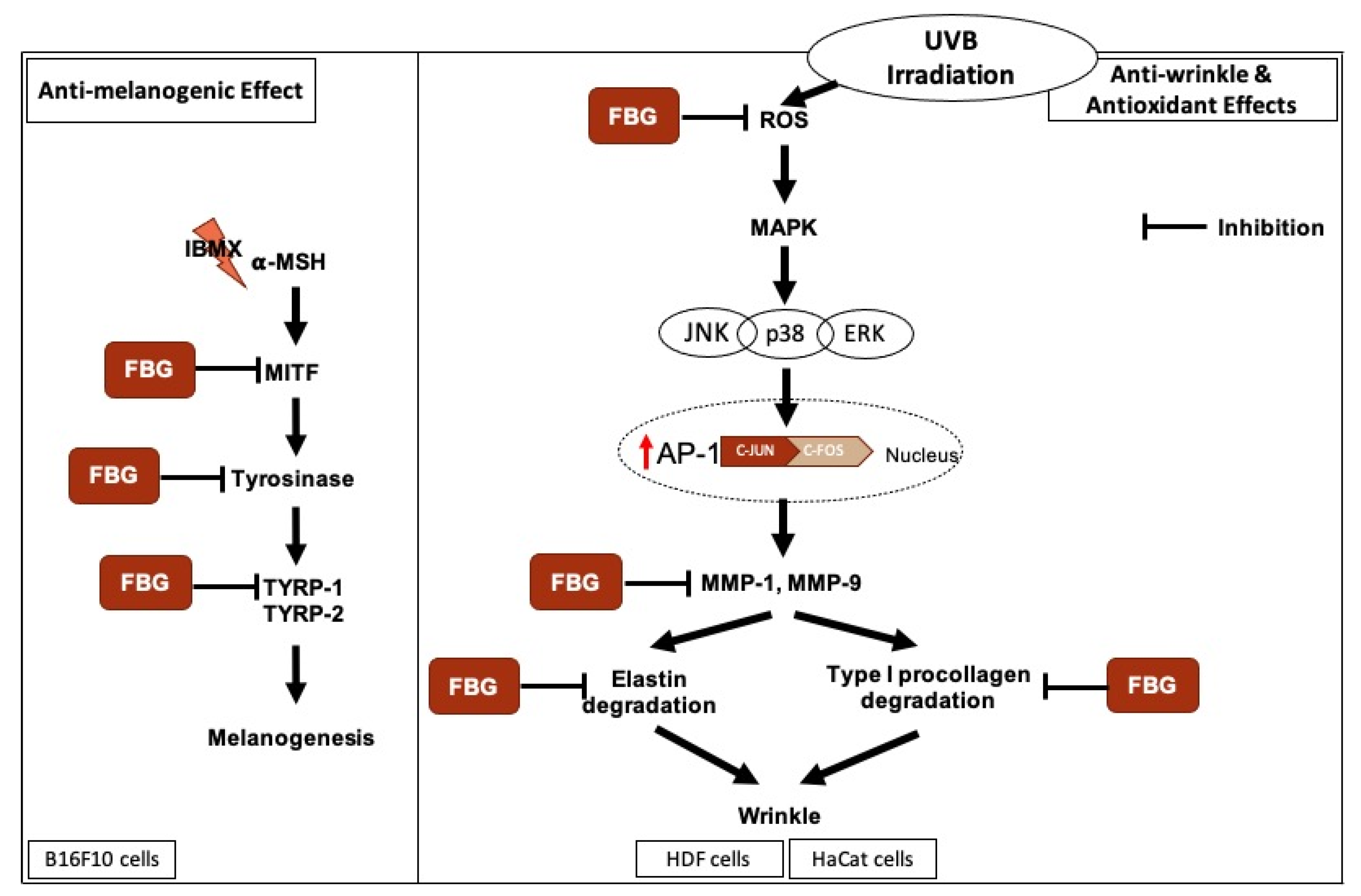
3.1. Anti-Melanogenic Effects of FBG in B16F10 Cells
3.2. Anti-Wrinkle Effects of FBG in HDF Cells
3.3. Antioxidant Effects of FBG
3.3.1. TPC, TFC, and Antioxidant Activities
3.3.2. ROS Generation of FBG in HaCat Cells
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buranasirin, P.; Pongpirul, K.; Meephansan, J. Development of a global subjective skin aging assessment score from the perspective of dermatologists. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Davó, A.P.; Truchuelo, M.T.; Vitale, M.; Gonzalez-Castro, J. Efficacy of an antiaging treatment against environmental factors: Deschampsia antarctica extract and high-tolerance retinoids combination. J. Clin. Aesthetic Dermatol. 2019, 12, E65. [Google Scholar]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and center in the bright side of dark eumelanin and DNA repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [Green Version]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Mishra, S.; Das, S.; Aggarwal, S.; Rani, V. Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ. Toxicol. Pharmacol. 2015, 39, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J. The importance of natural products in cosmetics. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 643–685. [Google Scholar]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, T.-H.; Choi, T.-Y.; Lee, M.S. Ginseng for health care: A systematic review of randomized controlled trials in Korean literature. PLoS ONE 2013, 8, e59978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, O.-J.; Kim, J.-S. Comparison of ginsenoside contents in different parts of Korean ginseng (Panax ginseng CA Meyer). Prev. Nutr. Food Sci. 2016, 21, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks Jr, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Panax spp root-derived ingredients as used in cosmetics. Int. J. Toxicol. 2015, 34, 5S–42S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease. Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef] [Green Version]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.-M.; Lim, J.-J.; Ahn, M.-S.; Jeong, H.-N.; An, T.-J.; Kim, S.-H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016, 40, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.M.; Huo, Y.; Kang, J.P.; Mathiyalagan, R.; Zhang, H.; Yang, D.U.; Kim, M.; Yang, D.C.; Kang, S.C.; Wang, Y.P. Diversity of ginsenoside profiles produced by various processing technologies. Molecules 2020, 25, 4390. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, Y.-J.; Jeon, J.-N.; Wang, C.; Min, J.-W.; Noh, H.-Y.; Yang, D.-C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, L.; Song, J.; Dou, D. A new simple and fast approach to analyze chemical composition on white, red, and black ginseng. Ind. Crops Prod. 2019, 134, 185–194. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, S.-H.; Choi, B.-R.; Choi, D.J.; Lee, Y.-G.; Kim, H.-G.; Kim, G.-S.; Kim, K.; Lee, Y.-H.; Baek, N.-I. UPLC-QTOF/MS-based metabolomics applied for the quality evaluation of four processed Panax ginseng products. Molecules 2018, 23, 2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akao, T.; Kanaoka, M.; Kobashi, K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration: Measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 1998, 21, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H. Metabolism of ginsenosides to bioactive compounds by intestinal microflora and its industrial application. J. Ginseng Res. 2009, 33, 165–176. [Google Scholar]
- Sharma, A.; Lee, H.-J. Ginsenoside compound K: Insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Jeong, E.-B.; Kim, S.-A.; Shin, K.-C.; Oh, D.-K. Biotransformation of protopanaxadiol-type ginsenosides in Korean ginseng extract into food-available compound K by an extracellular enzyme from Aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 1560–1567. [Google Scholar] [CrossRef]
- Park, J.K.; Yang, D.U.; Arunkumar, L.; Han, Y.; Lee, S.J.; Arif, M.H.; Li, J.F.; Huo, Y.; Kang, J.P.; Hoang, V.A. Cumulative Production of Bioactive Rg3, Rg5, Rk1, and CK from Fermented Black Ginseng Using Novel Aspergillus niger KHNT-1 Strain Isolated from Korean Traditional Food. Processes 2021, 9, 227. [Google Scholar] [CrossRef]
- Lim, C.-J.; Choi, W.-Y.; Jung, H.-J. Stereoselective Skin Anti-Photoaging Properties of Ginsenoside Rg3 in UV-B–Irradiated Keratinocytes. Biol. Pharm. Bull. 2014, 37, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Lee, W.J.; Chang, S.E.; Lee, G.-Y. Antimelanogenic effect of ginsenoside Rg3 through extracellular signal-regulated kinase-mediated inhibition of microphthalmia-associated transcription factor. J. Ginseng Res. 2015, 39, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Kim, J.H.; Hong, H.-D.; Kwon, J.; Lee, E.J.; Jang, M.; Lee, S.-Y.; Han, A.-R.; Nam, T.G.; Hong, S.K. Ginsenosides Rg5 and Rk1, the skin-whitening agents in black ginseng. J. Funct. Foods 2018, 45, 67–74. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Yoo, S.; Hong, Y.H.; Han, S.Y.; Jeong, S.; Jeong, D.; Kim, J.-H.; Cho, J.Y.; Park, J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng Res. 2018, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, D.-U.; Huo, Y.; Pu, J.; Lee, S.-J.; Yang, D.-C.; Kang, S.C. In vitro evaluation of anti-lung cancer and anti-COVID-19 effects using fermented black color ginseng extract. Nat. Prod. Commun. 2021, 16, 1934578X211034387. [Google Scholar] [CrossRef]
- Pham, Q.L.; Jang, H.J.; Kim, K.B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int. J. Mol. Med. 2017, 39, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; An, J.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Lee, E.; Ha, J.; Cho, C.H.; Seo, D.-W.; Kim, K.-B. Effects of anti-wrinkle and skin-whitening fermented black ginseng on human subjects and underlying mechanism of action. J. Toxicol. Environ. Health Part A 2020, 83, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, D.-S.; Kim, C.-E.; Shin, M.-S.; Seo, C.-S.; Shin, H.-K.; Hwang, G.S.; An, J.M.; Kim, S.-N.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Song, K.; Zhang, Q.; Guo, J.; Huang, J. Simultaneous determination of six isoflavones from puerariae lobatae radix by cpe-hplc and effect of puerarin on tyrosinase activity. Molecules 2020, 25, 344. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A potent tyrosinase inhibitor,(E)-3-(2, 4-Dihydroxyphenyl)-1-(thiophen-2-yl) prop-2-en-1-one, with anti-melanogenesis properties in α-MSH and IBMX-induced B16F10 melanoma cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, W.J.; Park, S.Y.; Kim, H.; Chung, D.K. In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes 2021, 9, 145. [Google Scholar] [CrossRef]
- Kim, W.S.; Seo, J.H.; Lee, J.-I.; Ko, E.-S.; Cho, S.-M.; Kang, J.-R.; Jeong, J.-H.; Jeong, Y.J.; Kim, C.Y.; Cha, J.-D. The Metabolite Profile in Culture Supernatant of Aster yomena Callus and Its Anti-Photoaging Effect in Skin Cells Exposed to UVB. Plants 2021, 10, 659. [Google Scholar] [CrossRef]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.-G.; Kim, T.-R.; Kim, H.; Lee, J. Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, J.Y.; Ramadhania, Z.M.; Mathiyalagan, R.; Huo, Y.; Han, Y.; Li, J.F.; Ahn, J.C.; Xu, F.J.; Lee, D.W.; Zeng, X.H. Ginsenosides Conversion and Anti-Oxidant Activities in Puffed Cultured Roots of Mountain Ginseng. Processes 2021, 9, 2271. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, Y.J.; Kim, W.; Kim, D.-O.; Kim, B.-Y.; Lee, H.; Baik, M.-Y. Change of ginsenoside profiles in processed ginseng by drying, steaming, and puffing. . Microbiol. Biotechnol. 2019, 29, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.S.; Lee, H.J.; Bae, S.H.; Kim, S.Y.; Park, Y.; Suh, H.J.; Jeong, Y.H. The bioavailability of red ginseng extract fermented by Phellinus linteus. J. Ginseng Res. 2013, 37, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochín-Medina, J.J.; Ramírez, K.; Rangel-Peraza, J.G.; Bustos-Terrones, Y.A. Increase of content and bioactivity of total phenolic compounds from spent coffee grounds through solid state fermentation by Bacillus clausii. J. Food Sci. Technol. 2018, 55, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [Green Version]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-melanogenic effect of ethanolic extract of Sorghum bicolor on IBMX–induced melanogenesis in B16/F10 melanoma cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.-H.; Kim, H.T.; Yoon, H.-Y.; Kwon, K.-B.; Choi, I.-W.; Jung, S.H.; Kim, H.-U.; Park, B.-H.; Park, J.-W. Effect of xanthohumol on melanogenesis in B16 melanoma cells. Exp. Mol. Med. 2008, 40, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A. Skin pigmentation enhancers. Compr. Ser. Photosciences 2001, 3, 637–675. [Google Scholar]
- Jeon, N.-J.; Kim, Y.-S.; Kim, E.-K.; Dong, X.; Lee, J.-W.; Park, J.-S.; Shin, W.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Inhibitory effect of carvacrol on melanin synthesis via suppression of tyrosinase expression. J. Funct. Foods 2018, 45, 199–205. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Lee, J.; Min, D.; Kim, J.; Kim, H.-J.; No, K.T. Tyrosinase-targeting gallacetophenone inhibits melanogenesis in melanocytes and human skin-equivalents. Int. J. Mol. Sci. 2020, 21, 3144. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Mauro, G.D.; Martini, F.; Tognon, M.; Mattei, M.D. microRNAs in the Regulation of Melanogenesis. Int. J. Mol. Sci. 2021, 22, 6104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.P.; Mandal, B.B. Inhibitory role of silk cocoon extract against elastase, hyaluronidase and UV radiation-induced matrix metalloproteinase expression in human dermal fibroblasts and keratinocytes. Photochem. Photobiol. Sci. 2019, 18, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef] [Green Version]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. /Współczesna Onkol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Kim, M.-R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: In vitro and animal study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef] [PubMed]


| Solution | A | B | C | D |
|---|---|---|---|---|
| Phosphate buffer (µL) | 40 | 80 | 0 | 40 |
| L-DOPA (µL) | 40 | 40 | 40 | 40 |
| Sample (µL) | 0 | 0 | 40 | 40 |
| Tyrosinase (µL) | 40 | 0 | 40 | 0 |
| Gene | Primer Sequence (5′-3′) |
|---|---|
| Tyrosinase | F: TTG CCA CTT CATGTC ATC ATA GAA R: TTT ATC AAA GGT GTG ACT GCT ATA |
| TYRP-1 | F: GCT GCA GGA GCC TTC TTT CTC R: AAG ACG CTG CAC TGC TGG TCT |
| TYRP-2 | F: GGA TGA CCG TGA GCA ATG GCC R: CGG TTG TGA CCA ATG GGT GCC |
| MITF | F: CGC CTG ATC TGG TGA ATC G R: CCT GGC TGC AGT TCT CAA GAA |
| MMP-1 | F: ATT CTA CTG ATA TCG GGG CTT TGA R: ATG TCC TTG GGG TAT CCG TGT AG |
| MMP-9 | F: CGT CGT GAT CCC CAC TTA CT R: AGA GTA CTG CTT GCC CAG GA |
| COL-1 | F: TGA CGA GAC CAA GAA CTG R: TAC CAG GGT TTG AGC TCA GC |
| GAPDH | F: CAA GGT CAT CCA TGA CAA CTT TG R: GTC CAC CAC CCT GTT GCT GTA G |
| Samples | TPC (µg GAE/mg Extract *) | TFC (µg RE/mg Extract **) | Antioxidant Activities | |
|---|---|---|---|---|
| DPPH | Reducing Power | |||
| (µg GAE/mg Extract) | (µg GAE/mg Extract) | |||
| FBG | 48.7 ± 0.83 a | 30.24 ± 0.80 a | 7.25 ± 1.10 a | 22.34 ± 0.54 a |
| BG | 39.8 ± 0.65 b | 24.40 ± 0.29 b | 6.01 ± 0.73 b | 18.14 ± 0.76 b |
| WG | 7.4 ± 0.50 c | 6.20 ± 0.15 c | 1.4 ± 1.21 c | 5.82 ± 0.81 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadhania, Z.M.; Yang, D.U.; Moektiwardojo, M.; Han, Y.; Park, J.K.; Rupa, E.J.; Yang, D.C.; Lee, S.J.; Kang, S.C. Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1. Appl. Sci. 2023, 13, 550. https://doi.org/10.3390/app13010550
Ramadhania ZM, Yang DU, Moektiwardojo M, Han Y, Park JK, Rupa EJ, Yang DC, Lee SJ, Kang SC. Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1. Applied Sciences. 2023; 13(1):550. https://doi.org/10.3390/app13010550
Chicago/Turabian StyleRamadhania, Zelika Mega, Dong Uk Yang, Moelyono Moektiwardojo, Yaxi Han, Jin Kyu Park, Esrat Jahan Rupa, Deok Chun Yang, Seung Jin Lee, and Se Chan Kang. 2023. "Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1" Applied Sciences 13, no. 1: 550. https://doi.org/10.3390/app13010550
APA StyleRamadhania, Z. M., Yang, D. U., Moektiwardojo, M., Han, Y., Park, J. K., Rupa, E. J., Yang, D. C., Lee, S. J., & Kang, S. C. (2023). Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1. Applied Sciences, 13(1), 550. https://doi.org/10.3390/app13010550








