Portable Prototype of Hydrogen Fuel Cells for Educational Training
Abstract
:1. Introduction
2. Theoretical Background
2.1. Applications of Fuel Cells
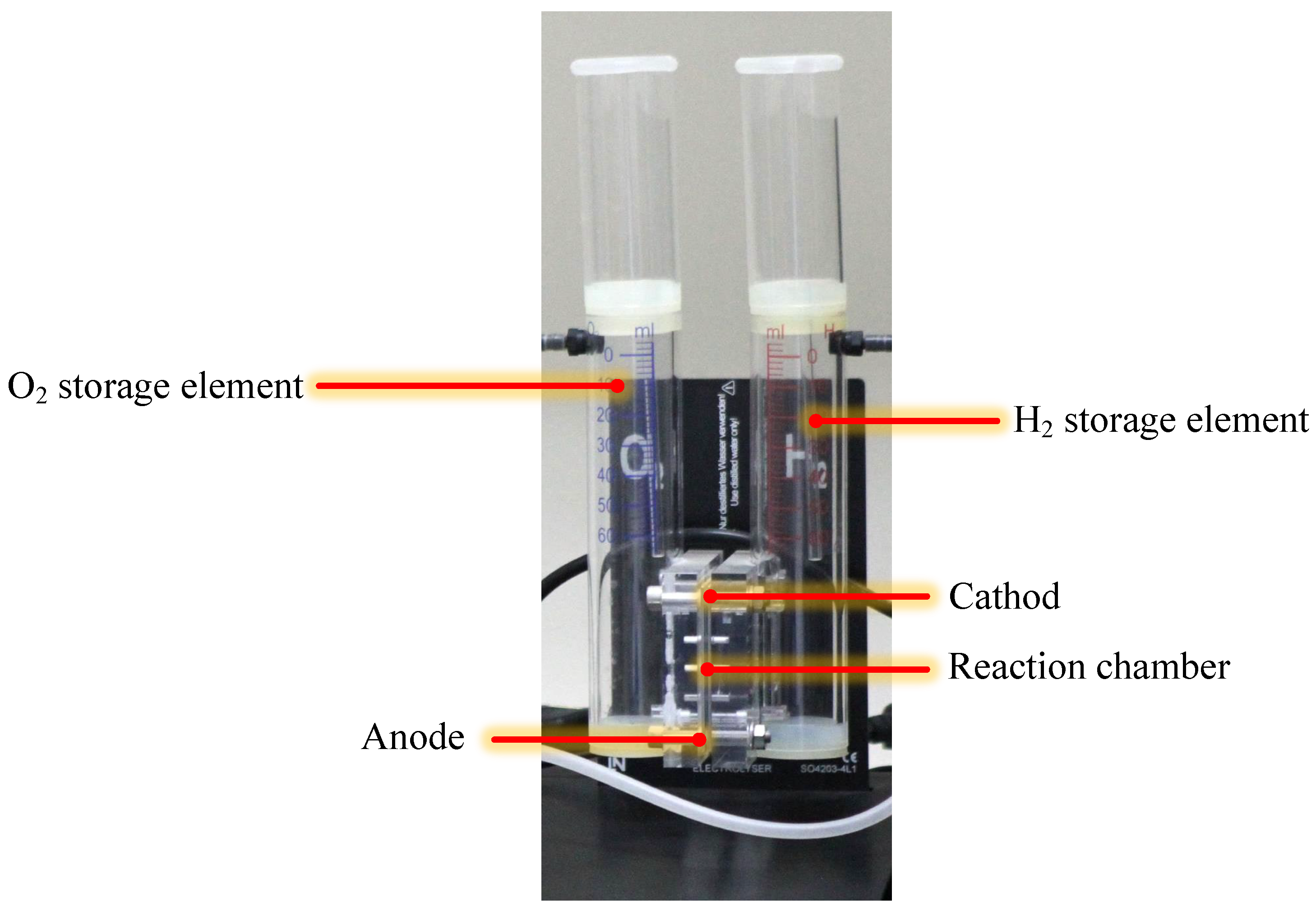
2.2. Electrolyzer
2.3. Cell and the Stack
2.3.1. Operating Principles
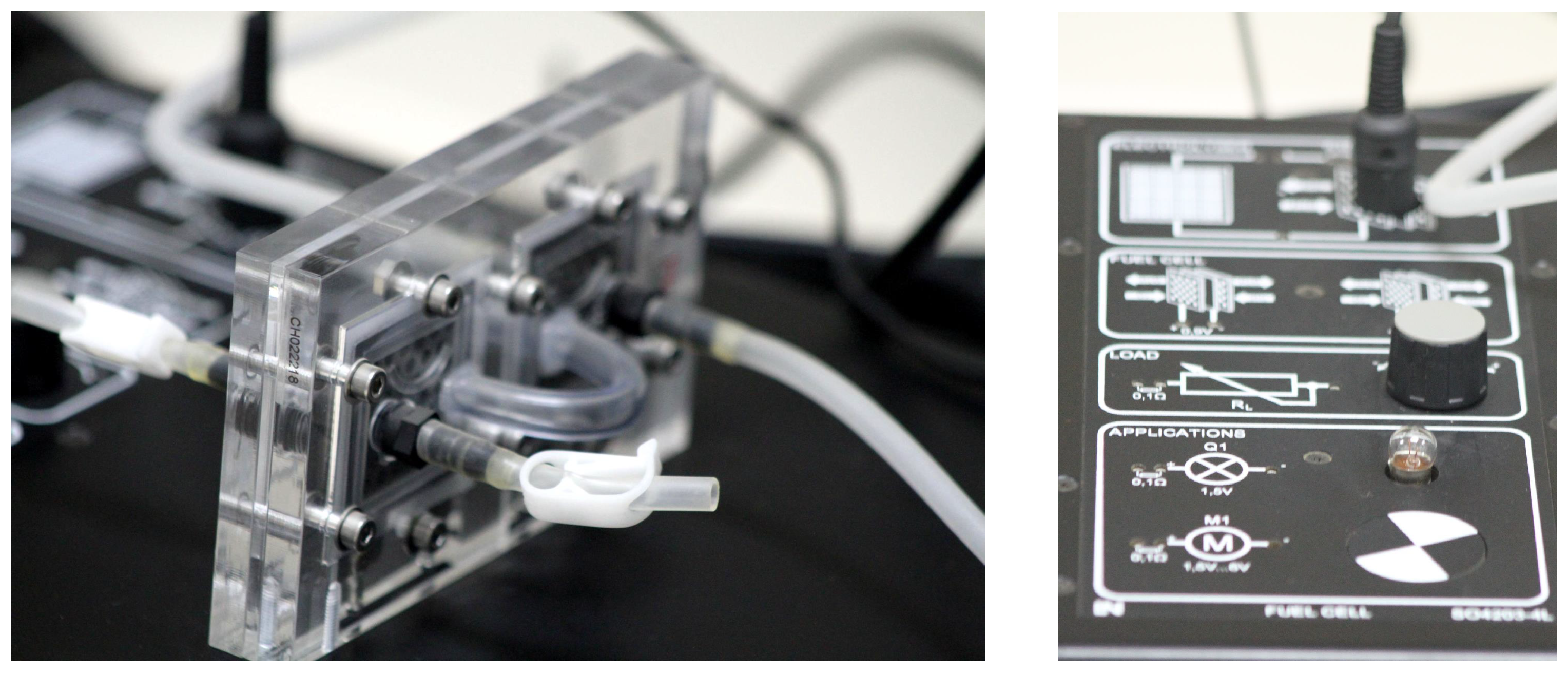
2.3.2. Fuel Cell Stack and Components
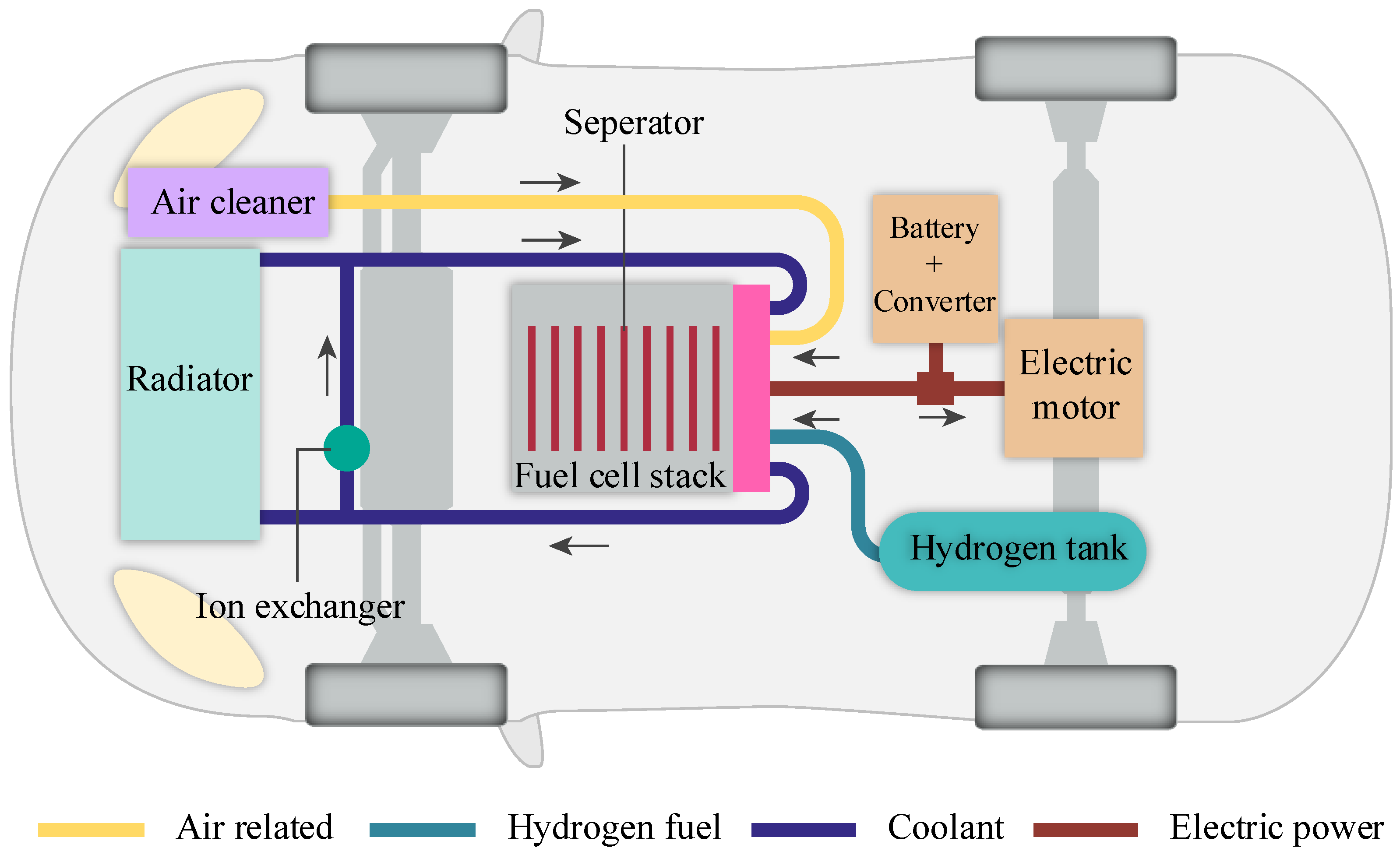
2.4. Material Flow
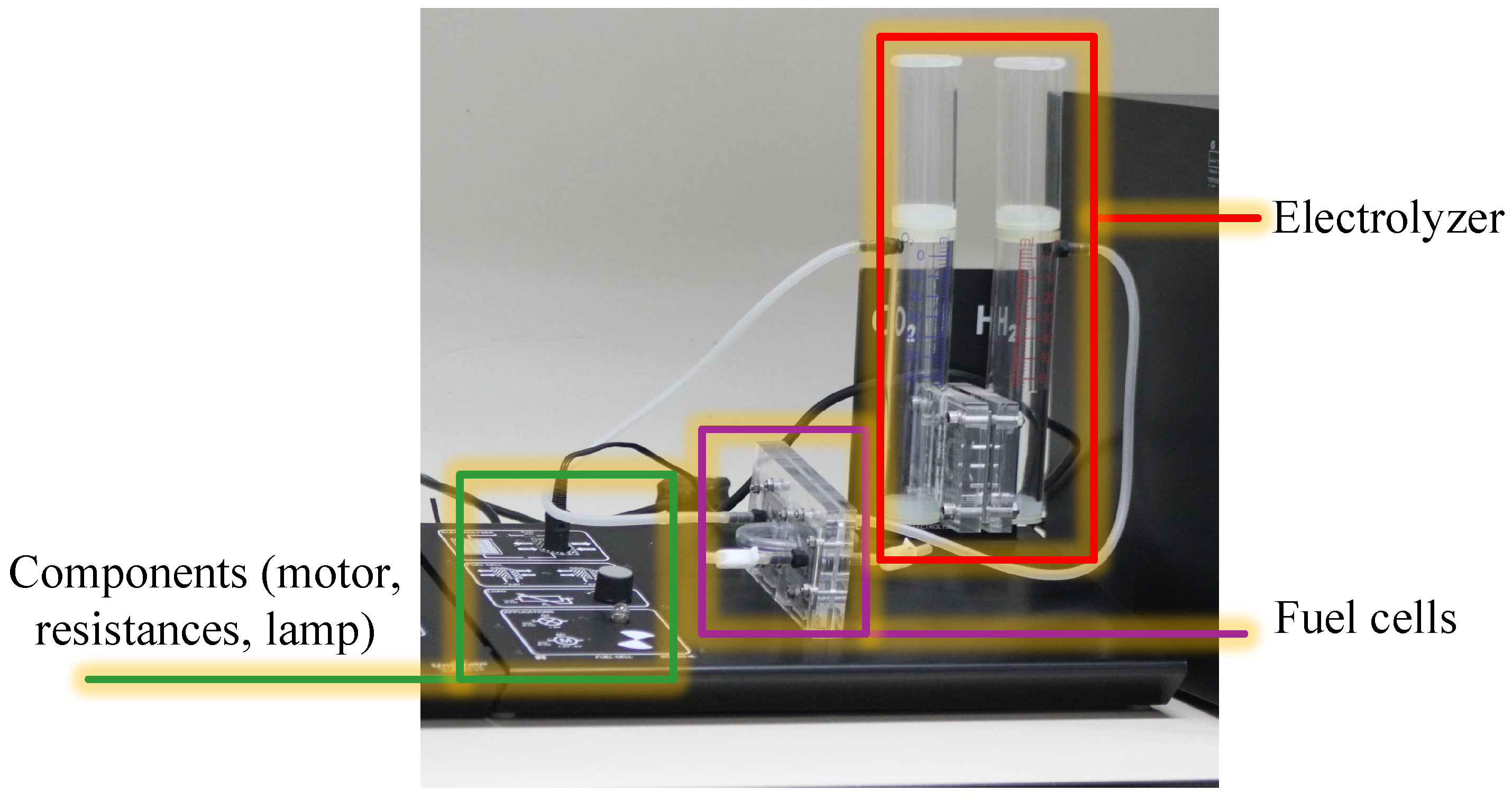
3. The Portable Educational Prototype of Hydrogen Fuel Cell
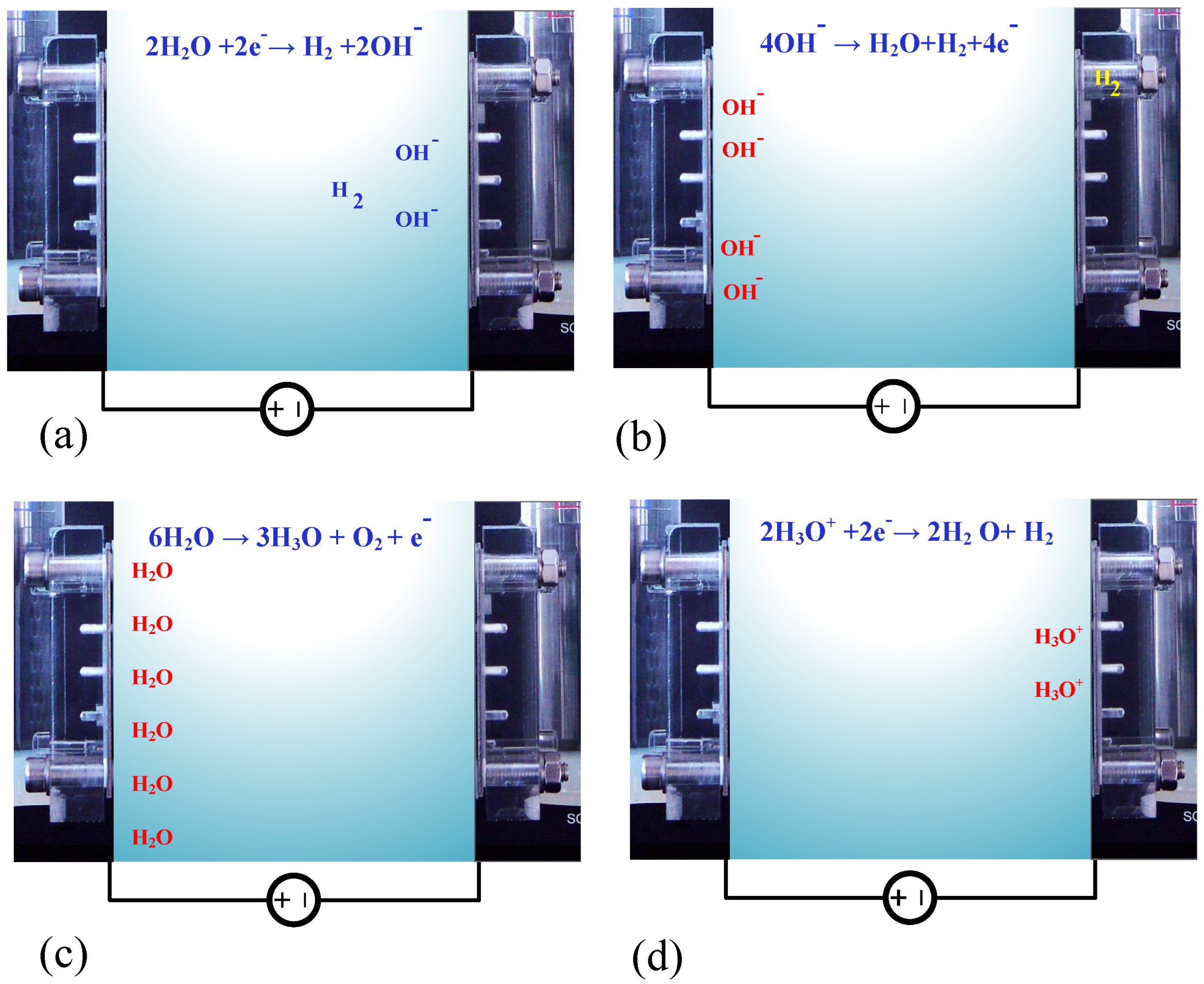
The Principle of the Electrolyzer Prototype Board
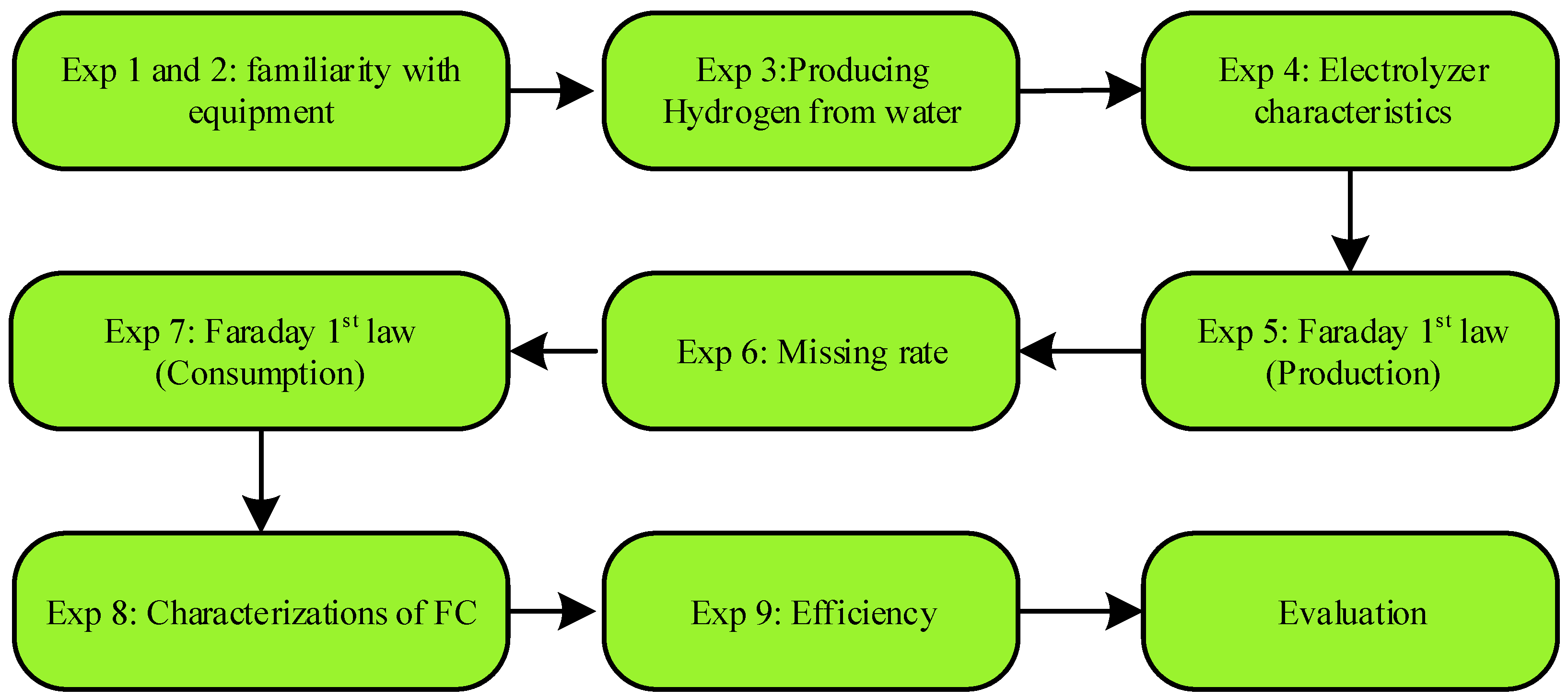
4. Experimental Setup
4.1. First and Second Experiment: Familiarity with the Equipment
4.2. Third Experiment: Water =
4.3. Fourth Experiment: Electrolyzer Characteristics
4.4. Fifth Experiment: Faraday’s First Law: Production of
4.5. Sixth Experiment: Missing Rate
4.6. Seventh Experiment: Faraday’s First Law (Consumption)
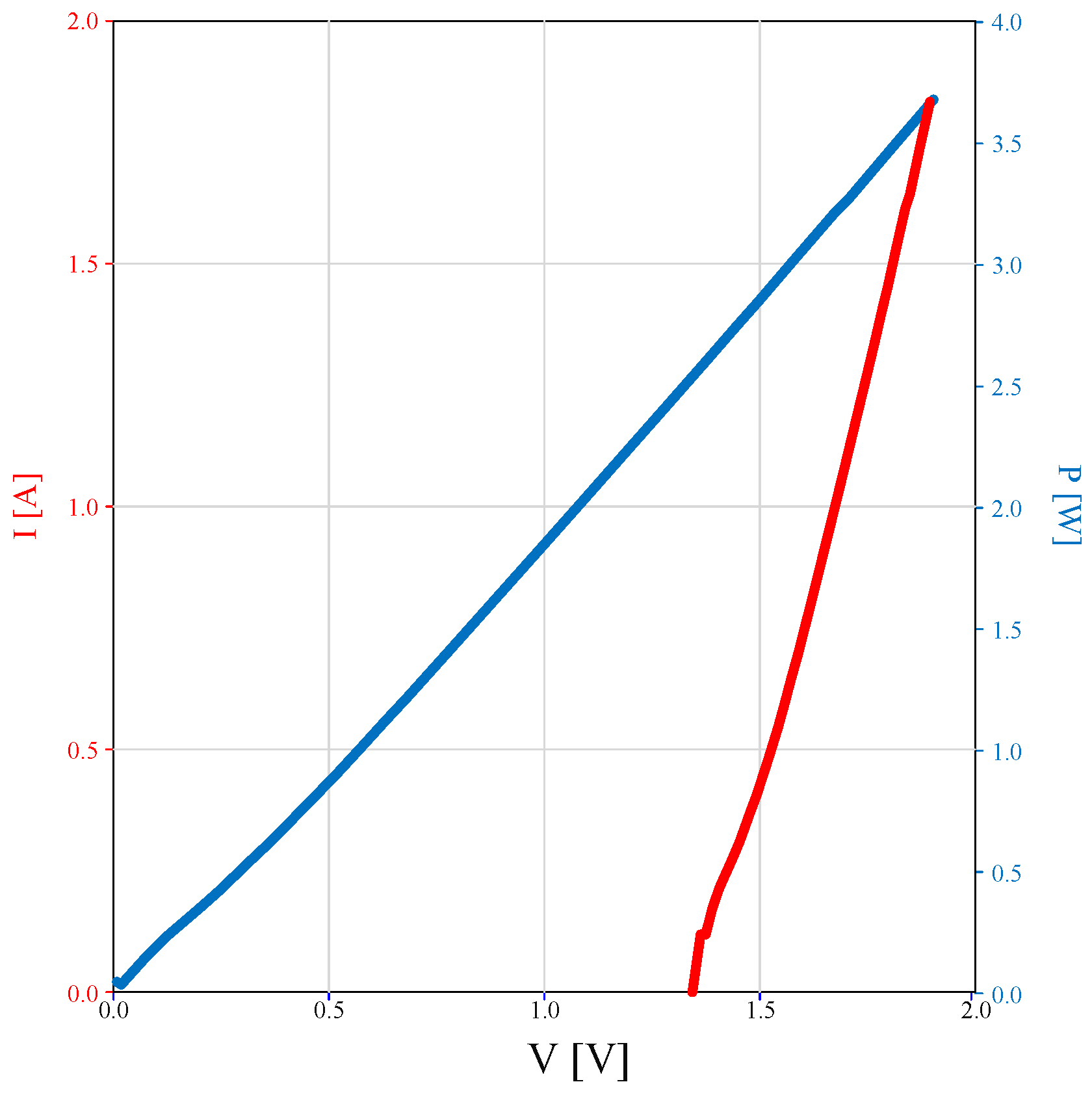
4.7. Experiment 8: Characterization of Fuel Cells
4.8. Efficiency of the Fuel Cell
4.9. Evaluation of the Characterization Results
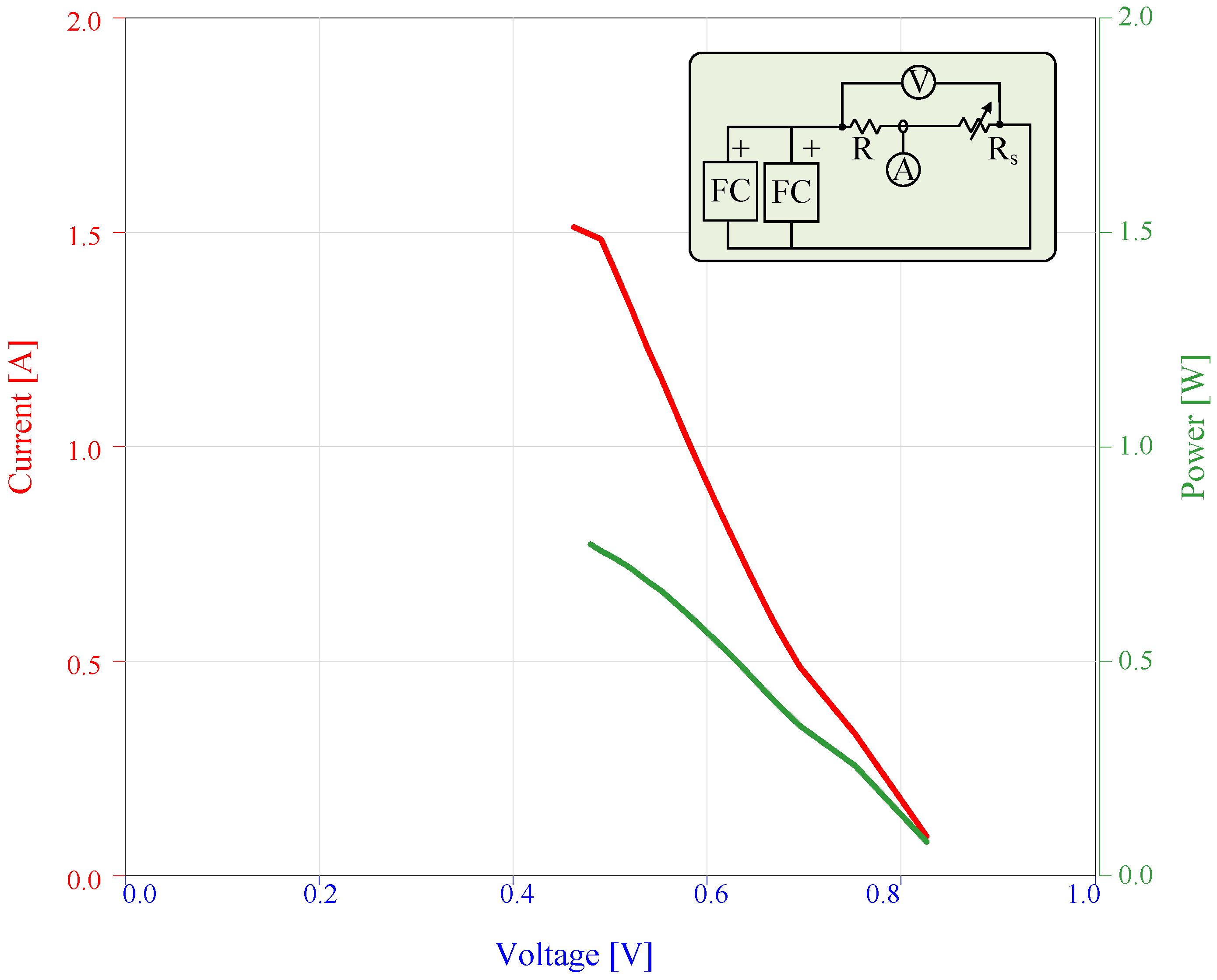
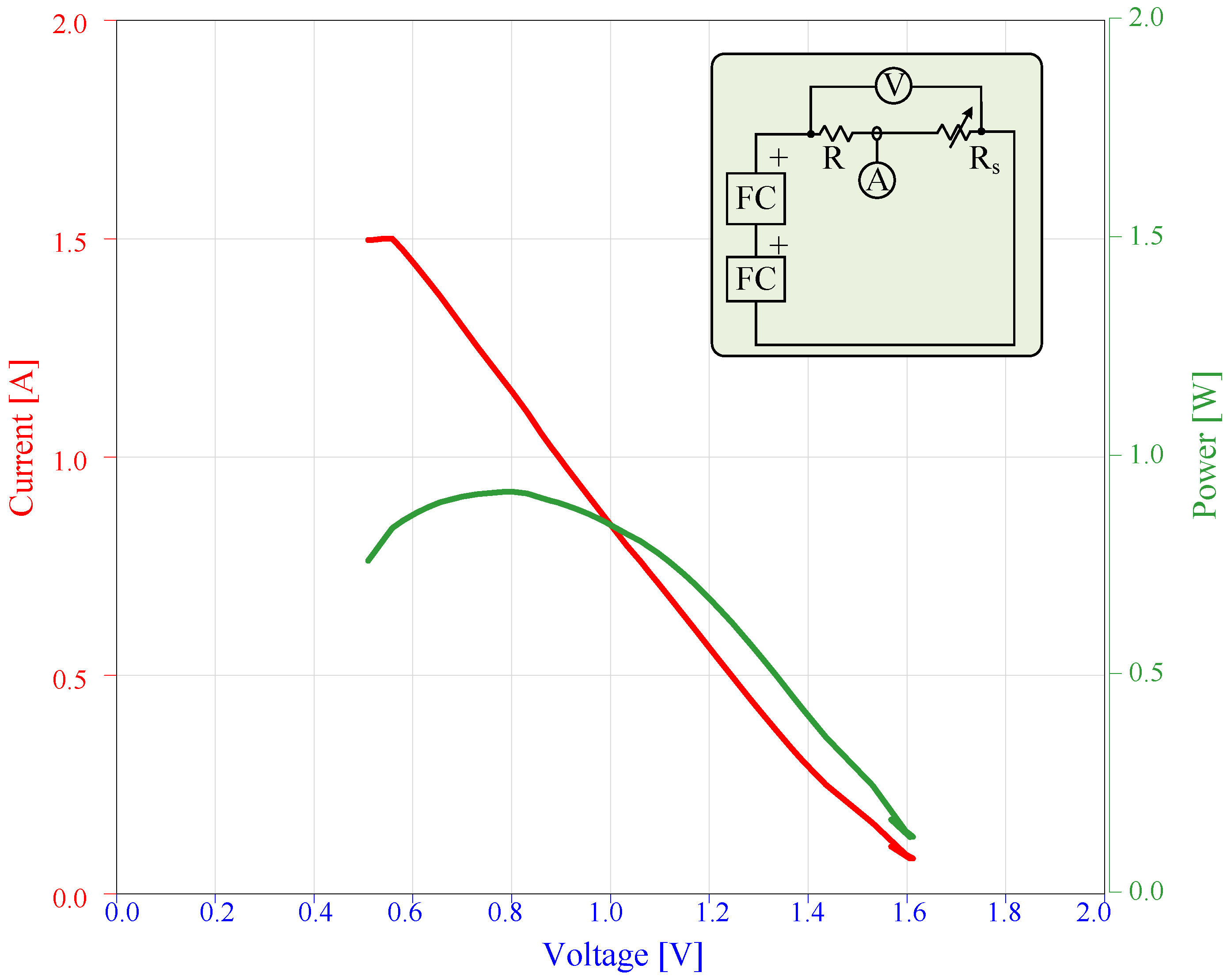
4.9.1. Serially-Connected FC
4.9.2. Parallel-Connected FC
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IEA | International Energy Agency |
| MCFC | Molten Carbonate Fuel Cell |
| SOFC | Solid oxide fuel cell |
| FCV | Fuel cell vehicles |
| CHP | Combined heat and power |
| PSO | Particle swarm optimization |
| AFC | Alkaline fuel cells |
| PAFC | Phosphoric acid fuel cells |
| Zirconium dioxide |
References
- Andújar, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar] [CrossRef]
- Grove, W.R. VIII. On the gas voltaic battery.—Experiments made with a view of ascertaining the rationale of its action and its application to eudiometry. Philos. Trans. R. Soc. Lond. 1843, 133, 91–112. [Google Scholar]
- Ortiz-Rivera, E.; Salazar-Llinas, A.; Velez-Delgado, J. An enriched undergraduate research experience based on the simulation, experiments, and theory of fuel cells. In Proceedings of the 2009 39th IEEE Frontiers in Education Conference, San Antonio, TX, USA, 18–21 October 2009; pp. 1–6. [Google Scholar]
- Katz, E.; Bollella, P. Fuel cells and biofuel cells: From past to perspectives. Isr. J. Chem. 2021, 61, 68–84. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Demirbas, A. Biohydrogen; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wright, C.R.A.; Thompson, C. XVII. Note on the development of voltaic electricity by atmospheric oxidation of combustible gases and other substances. Proc. R. Soc. Lond. 1890, 46, 372–376. [Google Scholar]
- Spiegel, C. PEM Fuel Cell Modeling and Simulation Using MATLAB; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lorca Martínez, J.M. Optimización del contenido de catalizador en una pila de combustible tipo PEM. 2021. Available online: https://repositorio.upct.es/bitstream/handle/10317/9892/tfg-lor-opt.pdf?sequence=1 (accessed on 22 November 2022).
- Stambouli, A.B. Fuel cells: The expectations for an environmental-friendly and sustainable source of energy. Renew. Sustain. Energy Rev. 2011, 15, 4507–4520. [Google Scholar] [CrossRef]
- Turco, M.; Ausiello, A.; Micoli, L. Fuel cells operating and structural features of MCFCs and SOFCs. In Treatment of Biogas for Feeding High Temperature Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–76. [Google Scholar]
- Arto, I.; Capellán-Pérez, I.; Lago, R.; Bueno, G.; Bermejo, R. The energy requirements of a developed world. Energy Sustain. Dev. 2016, 33, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.; Kopp, R.J.; Portney, P.R. Energy resources and global development. Science 2003, 302, 1528–1531. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Cader, J.; Koneczna, R.; Olczak, P. The Impact of Economic, Energy, and Environmental Factors on the Development of the Hydrogen Economy. Energies 2021, 14, 4811. [Google Scholar] [CrossRef]
- Burke, A.F. Batteries and ultracapacitors for electric, hybrid, and fuel cell vehicles. Proc. IEEE 2007, 95, 806–820. [Google Scholar] [CrossRef]
- Mench, M.M. Fuel Cell Engines; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Agbossou, K.; Kolhe, M.; Hamelin, J.; Bose, T.K. Performance of a stand-alone renewable energy system based on energy storage as hydrogen. IEEE Trans. Energy Convers. 2004, 19, 633–640. [Google Scholar] [CrossRef]
- Lucas Nülle, G. Electrical Power Engineering, Renewable Energies, EPH 2 Advanced Photovoltaics Trainer (PV). Available online: https://www.4test.no/cosmoshop/default/artikelpdf/LN-5677-EPH_en.pdf (accessed on 22 November 2022).
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrog. Energy 2021, 46, 10049–10058. [Google Scholar] [CrossRef]
- Pei, P.; Chen, H. Main factors affecting the lifetime of Proton Exchange Membrane fuel cells in vehicle applications: A review. Appl. Energy 2014, 125, 60–75. [Google Scholar] [CrossRef]
- Muthukumar, M.; Rengarajan, N.; Velliyangiri, B.; Omprakas, M.; Rohit, C.; Raja, U.K. The development of fuel cell electric vehicles–A review. Mater. Today Proc. 2021, 45, 1181–1187. [Google Scholar] [CrossRef]
- Boettner, D.D.; Paganelli, G.; Guezennec, Y.G.; Rizzoni, G.; Moran, M.J. Proton exchange membrane fuel cell system model for automotive vehicle simulation and control. J. Energy Resour. Technol. 2002, 124, 20–27. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Rousseau, A.; Kumar, R. Fuel economy of hydrogen fuel cell vehicles. J. Power Sources 2004, 130, 192–201. [Google Scholar] [CrossRef]
- Maleki, A. Optimal operation of a grid-connected fuel cell based combined heat and power systems using particle swarm optimisation for residential sector. Int. J. Ambient. Energy 2021, 42, 550–557. [Google Scholar] [CrossRef]
- Gencoglu, M.T.; Ural, Z. Design of a PEM fuel cell system for residential application. Int. J. Hydrog. Energy 2009, 34, 5242–5248. [Google Scholar] [CrossRef]
- Rahim, A.A.; Tijani, A.S.; Kamarudin, S.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Ulleberg, Ø. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrog. Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Stempien, J.P.; Sun, Q.; Chan, S.H. Solid Oxide Electrolyzer Cell Modeling: A Review. J. Power Technol. 2013, 93. [Google Scholar]
- Hall, J. Cell Components; Liskins, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 238–399. [Google Scholar]
- O’hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 1–23. [Google Scholar] [CrossRef]
- Ottman, N.; Ruokolainen, L.; Suomalainen, A.; Sinkko, H.; Karisola, P.; Lehtimäki, J.; Lehto, M.; Hanski, I.; Alenius, H.; Fyhrquist, N. Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model. J. Allergy Clin. Immunol. 2019, 143, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrog. Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Taccani, R.; Zuliani, N. Effect of flow field design on performances of high temperature PEM fuel cells: Experimental analysis. Int. J. Hydrogen Energy 2011, 36, 10282–10287. [Google Scholar] [CrossRef]
- Meyers, R.A. Encyclopedia of Physical Science and Technology; Academic: Cambridge, MA, USA, 2002. [Google Scholar]
- Xu, J.; Zhang, C.; Wan, Z.; Chen, X.; Chan, S.H.; Tu, Z. Progress and perspectives of integrated thermal management systems in PEM fuel cell vehicles: A review. Renew. Sustain. Energy Rev. 2022, 155, 111908. [Google Scholar] [CrossRef]
- Carlsson, G.A.; Ljungberg, M. Basic Atomic and Nuclear Physics. In Handbook of Nuclear Medicine and Molecular Imaging for Physicists; CRC Press: Boca Raton, FL, USA, 2022; pp. 15–37. [Google Scholar]
- Bethe, H.A.; Bacher, R.F. Nuclear physics A. Stationary states of nuclei. Rev. Mod. Phys. 1936, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Whittle, K. Nuclear Materials Science; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- McQuarrie, D.A. Quantum Chemistry; University Science Books: Sausalito, CA, USA, 1983. [Google Scholar]
- Bland, W.J.; Rolls, D. Weathering: An Introduction to the Scientific Principles; Routledge: London, UK, 2016. [Google Scholar]
- Hao, D.; Wang, X.; Zhang, Y.; Wang, R.; Chen, G.; Li, J. Experimental study on hydrogen leakage and emission of fuel cell vehicles in confined spaces. Automot. Innov. 2020, 3, 111–122. [Google Scholar] [CrossRef]
- Sakamoto, J.; Misono, H.; Nakayama, J.; Kasai, N.; Shibutani, T.; Miyake, A. Evaluation of safety measures of a hydrogen fueling station using physical modeling. Sustainability 2018, 10, 3846. [Google Scholar] [CrossRef] [Green Version]
- Dyantyi, N.; Parsons, A.; Sita, C.; Pasupathi, S. PEMFC for aeronautic applications: A review on the durability aspects. Open Eng. 2017, 7, 287–302. [Google Scholar] [CrossRef]






| Alkaline | PEM | Solid Oxide | |
|---|---|---|---|
| Electrolyte | 15–35% NaOH or KOH | Perfluorosulfonic acid | ZrO doped |
| Cell seperator | Diaphragm | Electrolyte membrane | Electrolyte membrane |
| Anode reaction | |||
| Cathode reaction | 2 H H | O 0.5 O +2 | |
| Typical Temperature | 50–20 °C | 80–100 °C | 750–1000 °C |
| Durability | 100,000 h | 10,000–50,000 h | 500 –2000 h |
| Capacity | 1–700 Nm per hour | 1–100 Nm per hour | 1–10 Nm per hour |
| Cost | 1300$ per kWh | 2000$ per kWh | 2300$ per kWh |
| Advantages | - Commercialized technology - Cost effective - High nominal output power - Abundance of raw material | - Commercialized technology - Fast dynamic characteristics - Fast start operation - Pure hydrogen output | - High efficiency - Direct synthesis gas generation |
| Disadvantages | - Slow cold start - vulnerability to impurities in | - Electrodes are made of valuable electrodes - High cost | - Still in development phase - High cost - Slow start |
| Time [s] | 60.0 | 90.0 | 120.0 | 150.0 | 180.0 | 210.0 | 240.0 |
|---|---|---|---|---|---|---|---|
| Production H2, V [mL] | 7.0 | 11.0 | 14.0 | 18.0 | 21.0 | 24.0 | 28.0 |
| I [A] | 0.40 | 0.80 | 1.20 | 1.60 | 20 |
|---|---|---|---|---|---|
| Production H2, V [mL] | 9.0 | 16.0 | 26.0 | 35.0 | 44.0 |
| Time [s] | 60.0 | 120.0 | 180.0 | 240.0 |
|---|---|---|---|---|
| Volume of [mL] | 44.0 | 38.0 | 34.0 | 28.0 |
| loss [mL] | 0.80 | 1.60 | 2.40 | 3.20 |
| consumed [mL] | 15.20 | 20.40 | 23.60 | 28.80 |
| I [mA] | 400 | 800 | 1200 |
|---|---|---|---|
| Volume of [mL] | 52 | 46 | 42 |
| loss [mL] | 1.6 | 1.6 | 1.6 |
| consumed [mL] | 6.4 | 12.4 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A. Portable Prototype of Hydrogen Fuel Cells for Educational Training. Appl. Sci. 2023, 13, 608. https://doi.org/10.3390/app13010608
Alzahrani A. Portable Prototype of Hydrogen Fuel Cells for Educational Training. Applied Sciences. 2023; 13(1):608. https://doi.org/10.3390/app13010608
Chicago/Turabian StyleAlzahrani, Ahmad. 2023. "Portable Prototype of Hydrogen Fuel Cells for Educational Training" Applied Sciences 13, no. 1: 608. https://doi.org/10.3390/app13010608
APA StyleAlzahrani, A. (2023). Portable Prototype of Hydrogen Fuel Cells for Educational Training. Applied Sciences, 13(1), 608. https://doi.org/10.3390/app13010608





