Development and Chemico-Physical Characterization of Ovine Milk-Based Ingredients for Infant Formulae
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Samples
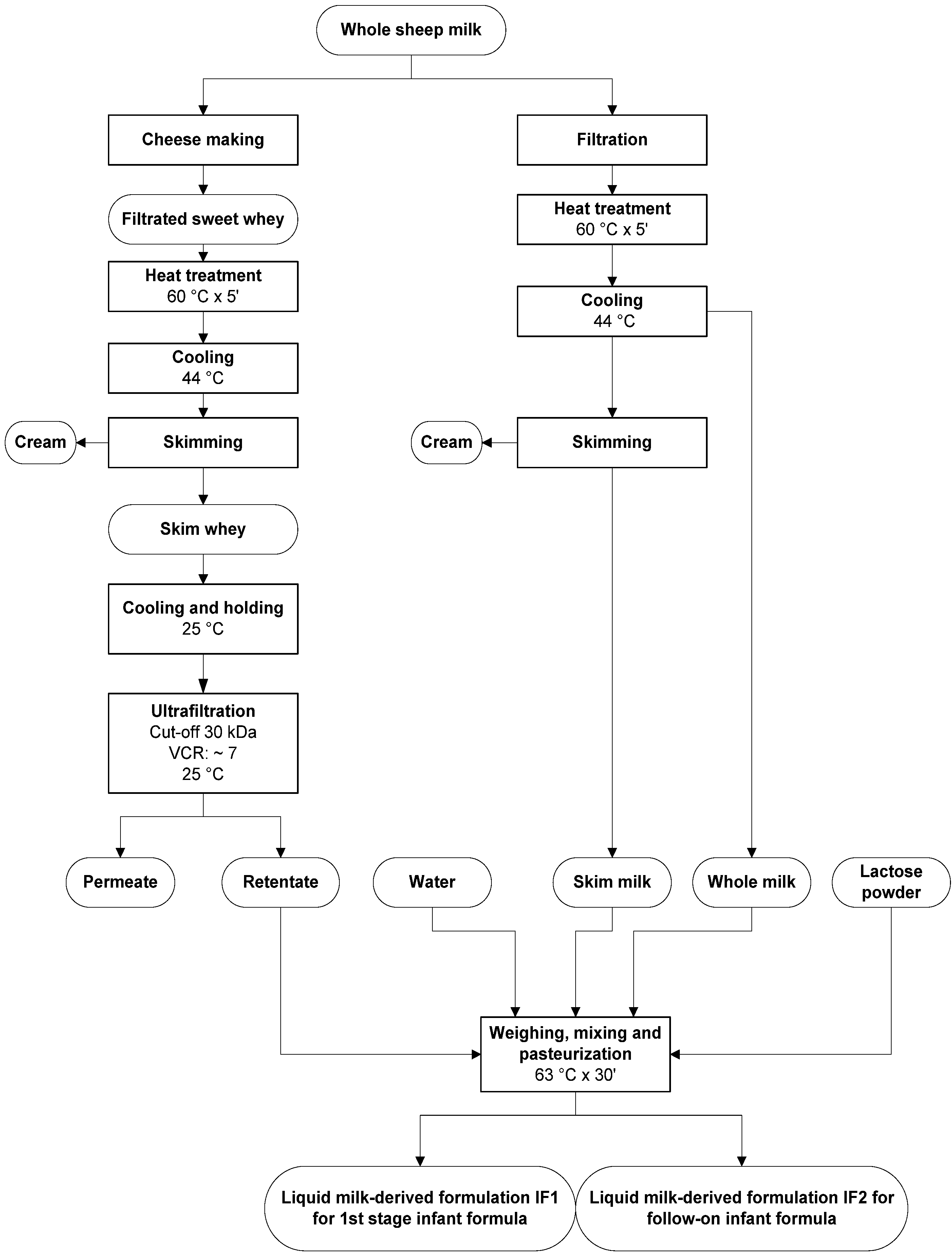
2.2. Ultrafiltered Whey Retentate Production
2.3. Liquid Milk-Derived Formulations (IF) Manufacturing
2.4. Chemico-Physical Composition
2.5. Retinol, α-Tocopherol and Cholesterol Determination
2.6. Fatty Acids Composition
2.7. NMR Spectroscopy
2.8. Statistical Analysis
3. Results and Discussion
3.1. Milk, Retentate, and IF Chemical-Physical Composition
3.2. Milk, Retentate, and IF Mineral Composition
3.3. Milk and IF Fatty Fraction
3.4. 1H NMR Low Molecular Weight Water Soluble Metabolites of IF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendricks, G.M.; Guo, M. Bioactive components in human milk. In Human Milk Biochemistry and Infant Formula Manufacturing; Guo, M., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 33–54. [Google Scholar]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bøgh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, N.A.; Kelly, A.; O’Mahony, J.A.; Hickey, D.K.; Chaurin, V.; Fenelon, M.A. Effect of protein content on emulsion stability of a model infant formula. Int. Dairy J. 2012, 25, 80–86. [Google Scholar] [CrossRef]
- Crowley, S.V.; Dowling, A.P.; Caldeo, V.; Kelly, A.L.; O’Mahony, J.A. Impact of α-lactalbumin:β-lactoglobulin ratio on the heat stability of model infant milk formula protein systems. Food Chem. 2016, 194, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Flis, Z.; Molik, E. Importance of Bioactive Substances in Sheep’s Milk in Human Health. Int. J. Mol. Sci. 2021, 22, 4364. [Google Scholar] [CrossRef]
- Flis, Z.; Szczecina, J.; Molik, E. The role of sheep’s milk bioactive substances in the prevention of metabolic and viral diseases. J. Anim. Feed Sci. 2022, 31, 211–216. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Moatsou, G.; Sakkas, L. Sheep milk components: Focus on nutritional advantages and biofunctional potential. Small Rumin. Res. 2019, 180, 86–99. [Google Scholar] [CrossRef]
- Gill, B.D.; Indyk, H.E.; Manley-Harris, M. Determination of total potentially available nucleosides in bovine, caprine, and ovine milk. Int. Dairy J. 2012, 24, 40–43. [Google Scholar] [CrossRef]
- Daniloski, D.; McCarthy, N.A.; Vasiljevic, T. Bovine β-Casomorphins: Friends or Foes? A comprehensive assessment of evidence from in vitro and ex vivo studies. Trends Food Sci. Technol. 2021, 116, 681–700. [Google Scholar] [CrossRef]
- Lai, G.; Pes, M.; Addis, M.; Pirisi, A. A Cluster Project Approach to Develop New Functional Dairy Products from Sheep and Goat Milk. Dairy 2020, 1, 10. [Google Scholar] [CrossRef]
- ISO 6731 (IDF 21:2010); Milk, Cream and Evaporated Milk—Determination of Total Solids Content (Reference Method). International Organisation for Standardization: Geneva, Switzerland, 2010.
- AOAC. Ash of milk—Gravimetric method. Method 945.56. In Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- ISO 15151 (IDF 229: 2018); Milk, Milk Products, Infant Formula and Adult Nutritionals—Determination of Minerals and Trace elements—Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) Method. International Organisation for Standardization: Geneva, Switzerland, 2018.
- Panfili, G.; Manzi, P.; Pizzoferrato, L. High-performance liquid chromatographic method for the simultaneous determination of tocopherols, carotenes, and retinol and its geometric isomers in Italian cheeses. Analyst 1994, 119, 1161–1165. [Google Scholar] [CrossRef]
- Manzi, P.; Panfili, G.; Pizzoferrato, L. Normal and Reversed-Phase HPLC for more complete evaluation of tocopherols, retinols, carotenes and sterols in dairy products. Chromatographia 1996, 43, 89–93. [Google Scholar] [CrossRef]
- ISO Methods 14156: 2001 (IDF 172: 2001); Milk and Milk Products—Extraction Methods for Lipids and Liposoluble Compounds. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO Methods 15884: 2002 (IDF 182: 2002); Milk Fat—Preparation of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2002.
- Kramer, J.; Cruz-Hernandez, K.G.; Deng, C.; Zhou, Z.; Jahreis, J.G.; Dugan, M.E.R. Analysis of conjugated linoleic acid and trans 18:1 isomers in synthetic and animal products. Am. J. Clin. Nutr. 2004, 79, 1137S–1145S. [Google Scholar] [CrossRef]
- Tari, N.R.; Fan, M.; Archbold, T.; Kristo, E.; Guri, A.; Arranz, E.; Corredig, M. Effect of milk protein composition of a model infant formula on the physicochemical properties of in vivo gastric digestates. J. Dairy Sci. 2018, 101, 2851–2861. [Google Scholar] [CrossRef]
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Whey Proteins in Infant Formula. In Whey Proteins: From Milk to Medicine; Hilton, C., Deeth, N.B., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 439–494. [Google Scholar]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar] [CrossRef]
- Grewal, M.K.; Vasiljevic, T.; Huppertz, T. Influence of calcium and magnesium on the secondary structure in solutions of individual caseins and binary casein mixtures. Int. Dairy J. 2021, 112, 104879. [Google Scholar] [CrossRef]
- Li, S.; Delger, M.; Dave, A.; Singh, H.; Ye, A. Seasonal Variations in the Composition and Physicochemical Characteristics of Sheep and Goat Milks. Foods 2022, 11, 1737. [Google Scholar] [CrossRef]
- Molska, A.; Gutowska, I.; Baranowska-Bosiacka, I.; Noceń, I.; Chlubek, D. The Content of Elements in Infant Formulas and Drinks Against Mineral Requirements of Children. Biol. Trace Elem. Res. 2014, 158, 422–427. [Google Scholar] [CrossRef][Green Version]
- Kwiecień, M.; Winiarska-Mieczan, A.; Samolińska, W.; Kiczorowska, B.; Rusinek-Prystupa, E. The content of magnesium, calcium, sodium and potassium in infant formulas. J. Elem. 2017, 22, 339–347. [Google Scholar] [CrossRef]
- Baldi, A.; Pinotti, L. Lipophilic Microconstituents of Milk. In Advances in Experimental Medicine and Biology; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; Volume 606, pp. 109–125. [Google Scholar]
- Redel, H.; Polsky, B. Nutrition, Immunity, and Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennet, J., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 132–141. [Google Scholar]
- Guo, M.; Ahmad, S. Formulation guidelines for infant formula. In Human Milk Biochemistry and Infant Formula Manufacturing; Guo, M., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 141–171. [Google Scholar]
- lvarez-Sala, A.; Garcia-Llatas, G.; Barberá, R.; Lagarda, M.J. Determination of cholesterol in human milk: An alternative to chromatographic methods. Nutr. Hosp. 2015, 32, 1535–1540. [Google Scholar] [CrossRef]
- Mollica, M.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.; Cabiddu, A.; Pinna, G.; Decandia, M.; Piredda, G.; Pirisi, A.; Molle, G. Milk and Cheese Fatty Acid Composition in Sheep Fed Mediterranean Forages with Reference to Conjugated Linoleic Acid cis-9,trans-11. J. Dairy Sci. 2005, 88, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, A.; Delgadillo-Puga, C.; Decandia, M.; Molle, G. Extensive Ruminant Production Systems and Milk Quality with Emphasis on Unsaturated Fatty Acids, Volatile Compounds, Antioxidant Protection Degree and Phenol Content. Animals 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Danielsen, M.; Nieuwenhuizen, A.G.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Cabiddu, A.; Decandia, M.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Managing Mediterranean pastures in order to enhance the level of beneficial fatty acids in sheep milk. Small Rumin. Res. 2005, 59, 169–180. [Google Scholar] [CrossRef]
- EU Commission. Regulations. Commission delegated regulation (EU) 2016/127. Off. J. Eur. Union 2016, L25, 1–29. [Google Scholar]
- GB 10765–2021; National Health Commission of the People’s Republic of China and State Administration for Market Regulation. National Food Safety Standard—Infant Formula. The National Standards of Food Safety of People’s Republic of China: Beijing, China, 2021.
- Besle, J.M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdagué, J.L.; Fraisse, D.; Lamaison, J.L.; Coulon, J.B. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef]
- Agostoni, C.; Carratù, B.; Boniglia, C.; Riva, E.; Sanzini, E. Free Amino Acid Content in Standard Infant Formulas: Comparison with Human Milk. J. Am. Coll. Nutr. 2000, 19, 434–438. [Google Scholar] [CrossRef]
- Neelima; Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Mineguchi, Y.; Goto, K.; Sudo, Y.; Hirayama, K.; Kashiwagi, H.; Sasagase, I.; Kitazawa, H.; Asakuma, S.; Fukuda, K.; Urashima, T. Characterisation of sugar nucleotides in colostrum of dairy domestic farms animals. Int. Dairy J. 2021, 113, 104897. [Google Scholar] [CrossRef]
- Scano, P.; Murgia, A.; Demuru, M.; Consonni, R.; Caboni, P. Metabolite profiles of formula milk compared to breast milk. Food Res. Int. 2016, 87, 76–82. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Orotic acid salts as sources of orotic acid and various minerals added for nutritional purposes to food supplements. EFSA J. 2009, 1187, 1–25. [Google Scholar] [CrossRef]
- Choi, S.S.; Lynch, B.S.; Baldwin, N.; Dakoulas, E.W.; Roy, S.; Moore, C.; Thorsrud, B.A.; Röhrig, C.H. Safety evaluation of the human-identical milk monosaccharide, l-fucose. Regul. Toxicol. Pharmacol. 2015, 72, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Dietary Intake of Creatine in Children Aged 0–24 Months. Ann. Nutr. Metab. 2021, 77, 185–188. [Google Scholar] [CrossRef]
- Woollard, D.C.; Indyk, H.E.; Christiansen, S.K. The analysis of pantothenic acid in milk and infant formulas by HPLC. Food Chem. 2000, 69, 201–208. [Google Scholar] [CrossRef]
| Whole Milk (n = 6) | Skimmed Milk (n = 6) | Retentate * (n = 6) | |
|---|---|---|---|
| pH (UpH) | 6.61 ± 0.03 | 6.63 ± 0.04 | 6.57 ± 0.13 |
| Dry matter (%) | 16.8 ± 0.4 a | 11.7 ± 0.2 c | 13.9 ± 1.7 b |
| Fat (%) | 5.9 ± 0.2 a | 0.4 ± 0.1 b | 0.6 ± 0.1 b |
| Protein (%) | 5.4 ± 0.2 b | 5.7 ± 0.2 b | 8.0 ± 1.9 a |
| Fat/Protein (-) | 1.10 ± 0.04 a | 0.06 ± 0.01 b | 0.08 ± 0.01 b |
| Whey protein (%) | 0.9 ± 0.1 b | 0.9 ± 0.1 b | 7.7 ± 1.8 a |
| Lactose (%) | 4.4 ± 0.1 b | 4.8 ± 0.1 a | 4.5 ± 0.1 ab |
| Ash (%) | 0.88 ± 0.15 ab | 0.96 ± 0.08 a | 0.70 ± 0.01 b |
| IF1 (n = 6) | IF2 (n = 6) | |
|---|---|---|
| Retentate * (%) | 31 ± 6 | 9 ± 1 |
| Whole milk (%) | 23 ± 1 | 52 ± 1 |
| Skim milk (%) | 3 ± 1 | 13 ± 1 |
| Lactose powder (%) | 8.1 ± 0.3 | 4.8 ± 0.2 |
| Water (%) | 34 ± 5 | 21 ± 2 |
| IF1 (n = 6) | IF2 (n = 6) | |
|---|---|---|
| pH (UpH) | 6.74 ± 0.10 | 6.71 ± 0.03 |
| Dry matter (%) | 16.2 ± 0.1 | 16.1 ± 0.1 |
| Fat (%) | 1.5 ± 0.1 b | 3.1 ± 0.1 a |
| Protein (%) | 3.82 ± 0.08 b | 4.32 ± 0.02 a |
| Fat/Protein (-) | 0.38 ± 0.01 b | 0.72 ± 0.01 a |
| Casein (%) | 1.3 ± 0.1 b | 2.8 ± 0.1 a |
| Whey protein (%) | 2.6 ± 0.1 a | 1.6 ± 0.1 b |
| Casein/Whey protein (-) | 0.49 ± 0.06 b | 1.67 ± 0.05 a |
| Lactose (%) | 10.16 ± 0.03 a | 7.88 ± 0.04 b |
| Ash (%) | 0.46 ± 0.03 b | 0.69 ± 0.11 a |
| Retentate * (n = 6) | Whole Milk (n = 6) | Skimmed Milk (n = 6) | IF1 (n = 6) | IF2 (n = 6) | |
|---|---|---|---|---|---|
| Ca | 44.1 ± 0.3 e | 180.6 ± 6.6 b | 192.3 ± 7.8 a | 64.0 ± 1.6 d | 122.8 ± 7.9 c |
| Mg | 11 ± 2 b | 17 ± 1 a | 19 ± 1 a | 8 ± 1 c | 12 ± 1 b |
| Na | 57 ± 9 ab | 60 ± 8 a | 60 ± 3 a | 47 ± 4 b | 49 ± 5 b |
| K | 137 ± 16 a | 114 ± 4 b | 119 ± 5 b | 79 ± 7 c | 86 ± 6 c |
| P | 57.6 ± 0.3 c | 123.2 ± 8.3 a | 128.6 ± 7.7 a | 49.3 ± 4.8 c | 86.7 ± 5.4 b |
| S | 22 ± 2 | 21 ± 7 | 23 ± 6 | 17 ± 4 | 19 ± 4 |
| B | <0.05 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Zn | 0.30 ± 0.07 bc | 0.50 ± 0.13 ab | 0.64 ± 0.14 a | 0.28 ± 0.07 c | 0.44 ± 0.14 bc |
| Cu | 0.05 ± 0.00 | 0.08 ± 0.06 | 0.09 ± 0.06 | 0.10 ± 0.04 | 0.09 ± 0.06 |
| Mn | 0.06 ± 0.08 | 0.06 ± 0.05 | 0.05 ± 0.06 | <0.05 | 0.09 ± 0.10 |
| Fe | 0.09 ± 0.04 | 0.14 ± 0.06 | 0.18 ± 0.06 | 0.12 ± 0.11 | 0.15 ± 0.06 |
| Whole Milk (n = 6) | IF1 (n = 6) | IF2 (n = 6) | |
|---|---|---|---|
| Retinol | 0.84 ± 0.21 a | 0.17 ± 0.02 c | 0.42 ± 0.10 b |
| α-Tocopherol | 1.72 ± 0.17 a | 0.47 ± 0.05 c | 1.01 ± 0.07 b |
| Cholesterol | 202 ± 13 a | 112 ± 9 c | 139 ± 8 b |
| C4:0 | 4.0 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.2 |
| C6:0 | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.2 ± 0.1 |
| C8:0 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 |
| C10:0 | 9.1 ± 0.3 | 9.1 ± 0.4 | 9.0 ± 0. 2 |
| C12:0 | 5.2 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 |
| C14:0 | 14.0 ± 0.4 | 14.0 ± 0.3 | 13.7 ± 0.5 |
| C16:0 | 30 ± 1 | 30 ± 1 | 30 ± 1 |
| C18:0 | 5.5 ± 0.2 | 5.7 ± 0.2 | 5.5 ± 0.3 |
| C18:1 11 trans | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 |
| C18:1 9 cis | 10.7 ± 0.7 | 11.1 ± 0.9 | 10.7 ± 0.3 |
| C18:2 9 cis 12 cis | 1.73 ± 0.15 | 1.77 ± 0.15 | 1.81 ± 0.11 |
| C18:3 9 cis 12 cis 15 cis | 1.6 ± 0.4 | 1.5 ± 0.3 | 1.8 ± 0.6 |
| CLA 9 cis 11 trans | 0.56 ± 0.05 | 0.60 ± 0.05 | 0.58 ± 0.06 |
| SFAs | 79 ± 1 | 78 ± 1 | 78 ± 1 |
| MUFAs | 15.6 ± 0.6 | 16.1 ± 0.9 | 15.9 ± 0.1 |
| PUFAs | 5.6 ± 0.7 | 5.5 ± 0.3 | 5.9 ± 0.9 |
| Omega-6 | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.4 ± 0.1 |
| Omega-3 | 2.3 ± 0.6 | 2.1 ± 0.4 | 2.5 ± 0.7 |
| TFAs | 3.2 ± 0.5 | 3.3 ± 0.3 | 3.4 ± 0.6 |
| IF1 (n = 3) | IF2 (n = 3) | |||
|---|---|---|---|---|
| Metabolites | Mean SD | Mean% | Mean SD | Mean% |
| Amino acids | ||||
| Alanine | 0.09 ± 0.01 a | 2.7 | 0.032 ± 0.009 b | 1.5 |
| Anserine | 0.10 ± 0.02 a | 3.0 | 0.031 ± 0.002 b | 1.5 |
| Glutamate | 0.28 ± 0.01 a | 8.1 | 0.11 ± 0.04 b | 5.3 |
| Isoleucine | 0.26 ± 0.03 a | 7.5 | 0.08 ± 0.01 b | 3.8 |
| Leucine | 0.287 ± 0.004 a | 8.3 | 0.102 ± 0.041 b | 4.9 |
| Lysine | 0.208 ± 0.007 a | 6.0 | 0.087 ± 0.031 b | 4.2 |
| Proline | 0.44 ± 0.13 | 12.5 | 0.29 ± 0.04 | 14.2 |
| Threonine | 0.044 ± 0.003 | 1.3 | 0.038 ± 0.012 | 1.8 |
| Valine | 0.030 ± 0.005 a | 0.9 | 0.019 ± 0.003 b | 0.9 |
| Organic acids | ||||
| Acetate | 0.069 ± 0.009 a | 2.0 | 0.035 ± 0.003 b | 1.7 |
| Citrate | 0.7 ± 0.1 | 21.6 | 0.6 ± 0.1 | 30.8 |
| Formate | 0.024 ± 0.001 a | 0.7 | 0.015 ± 0.002 b | 0.7 |
| Hippurate | 0.046 ± 0.003 a | 1.3 | 0.031 ± 0.002 b | 1.5 |
| Nucleotides | ||||
| UDP-N-Acetylglucosamine | 0.018 ± 0.001 a | 0.5 | 0.025 ± 0.010 b | 1.2 |
| UDP-galactose | 0.08 ± 0.01 a | 2.4 | 0.06 ± 0.01 b | 3.1 |
| UDP-glucose | 0.067 ± 0.007 a | 1.9 | 0.062 ± 0.015 b | 3.0 |
| Uridine | 0.040 ± 0.012 a | 1.2 | 0.041 ± 0.007 b | 2.0 |
| Others | ||||
| Caprylate | 0.113 ± 0.007 a | 3.3 | 0.042 ± 0.001 b | 2.0 |
| Choline | 0.020 ± 0.002 | 0.6 | 0.018 ± 0.004 | 0.9 |
| O-Phosphocholine | 0.027 ± 0.004 | 0.8 | 0.023 ± 0.006 | 1.1 |
| sn-Glycero-3-phosphocholine | 0.14 ± 0.02 | 4.1 | 0.12 ± 0.01 | 5.8 |
| Creatine | 0.035 ± 0.002 a | 1.0 | 0.024 ± 0.003 b | 1.2 |
| Pantothenate | 0.066 ± 0.008 | 1.9 | 0.055 ± 0.015 | 2.7 |
| Fucose | 0.223 ± 0.007 a | 6.4 | 0.087 ± 0.020 b | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, G.; Caboni, P.; Piras, C.; Pes, M.; Sitzia, M.; Addis, M.; Pirisi, A.; Scano, P. Development and Chemico-Physical Characterization of Ovine Milk-Based Ingredients for Infant Formulae. Appl. Sci. 2023, 13, 653. https://doi.org/10.3390/app13010653
Lai G, Caboni P, Piras C, Pes M, Sitzia M, Addis M, Pirisi A, Scano P. Development and Chemico-Physical Characterization of Ovine Milk-Based Ingredients for Infant Formulae. Applied Sciences. 2023; 13(1):653. https://doi.org/10.3390/app13010653
Chicago/Turabian StyleLai, Giacomo, Pierluigi Caboni, Cristina Piras, Massimo Pes, Maria Sitzia, Margherita Addis, Antonio Pirisi, and Paola Scano. 2023. "Development and Chemico-Physical Characterization of Ovine Milk-Based Ingredients for Infant Formulae" Applied Sciences 13, no. 1: 653. https://doi.org/10.3390/app13010653
APA StyleLai, G., Caboni, P., Piras, C., Pes, M., Sitzia, M., Addis, M., Pirisi, A., & Scano, P. (2023). Development and Chemico-Physical Characterization of Ovine Milk-Based Ingredients for Infant Formulae. Applied Sciences, 13(1), 653. https://doi.org/10.3390/app13010653








