Radiological Assessment of Extracranial Vertebral Artery Variations: A Computed Tomography Angiography Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CTA Acquisition Protocol
2.3. Data Acquisition
2.4. Statistical Analysis
3. Results
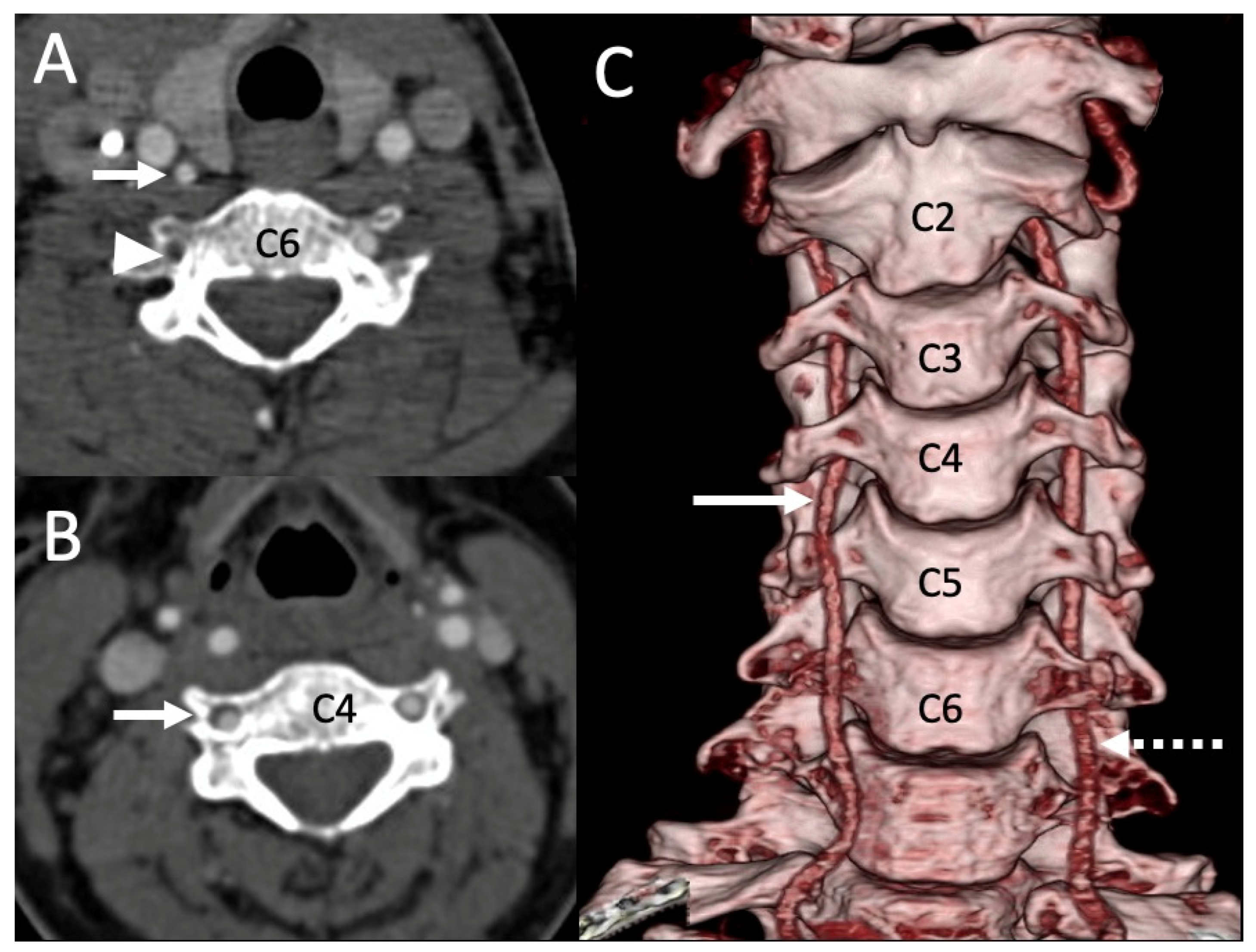
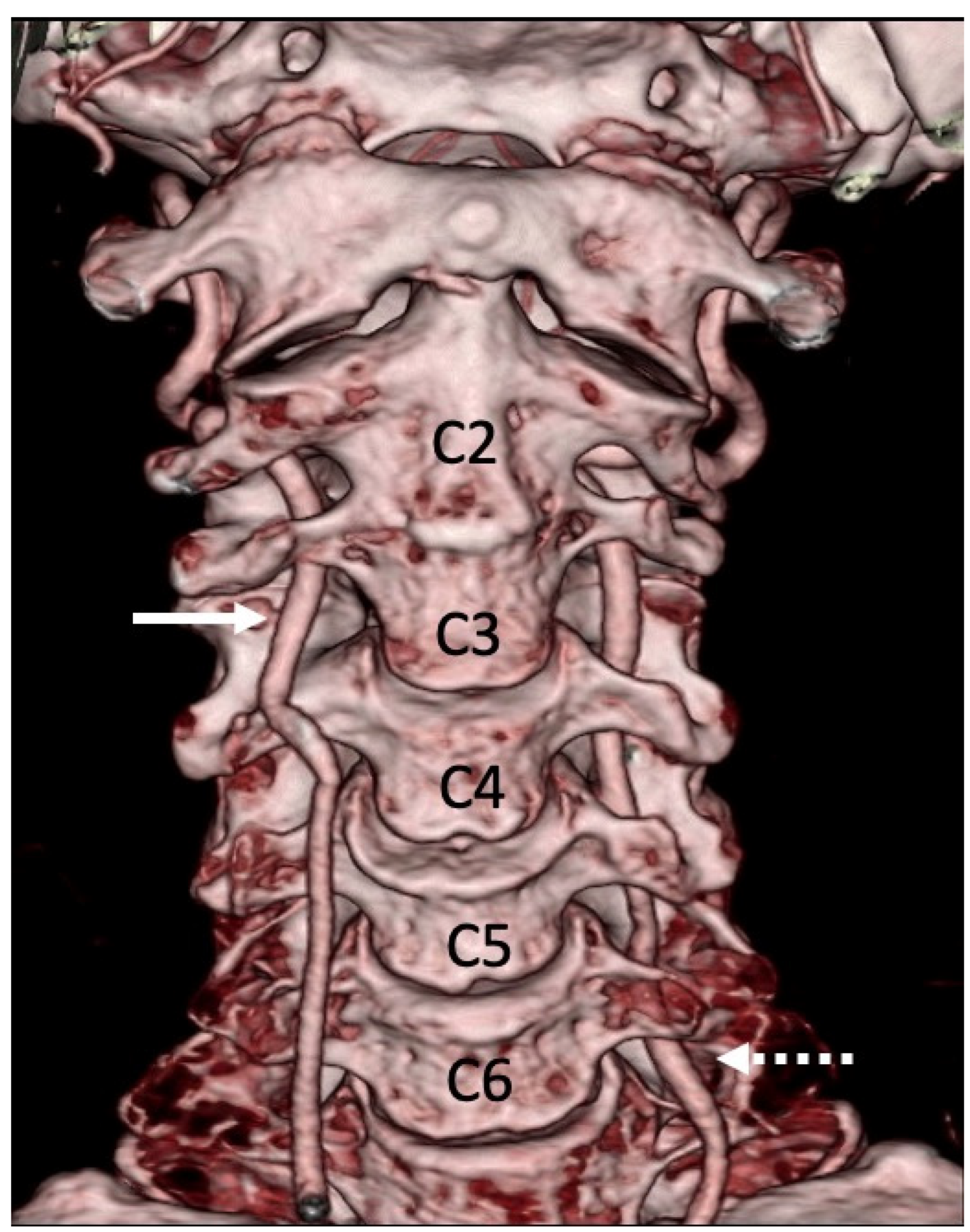
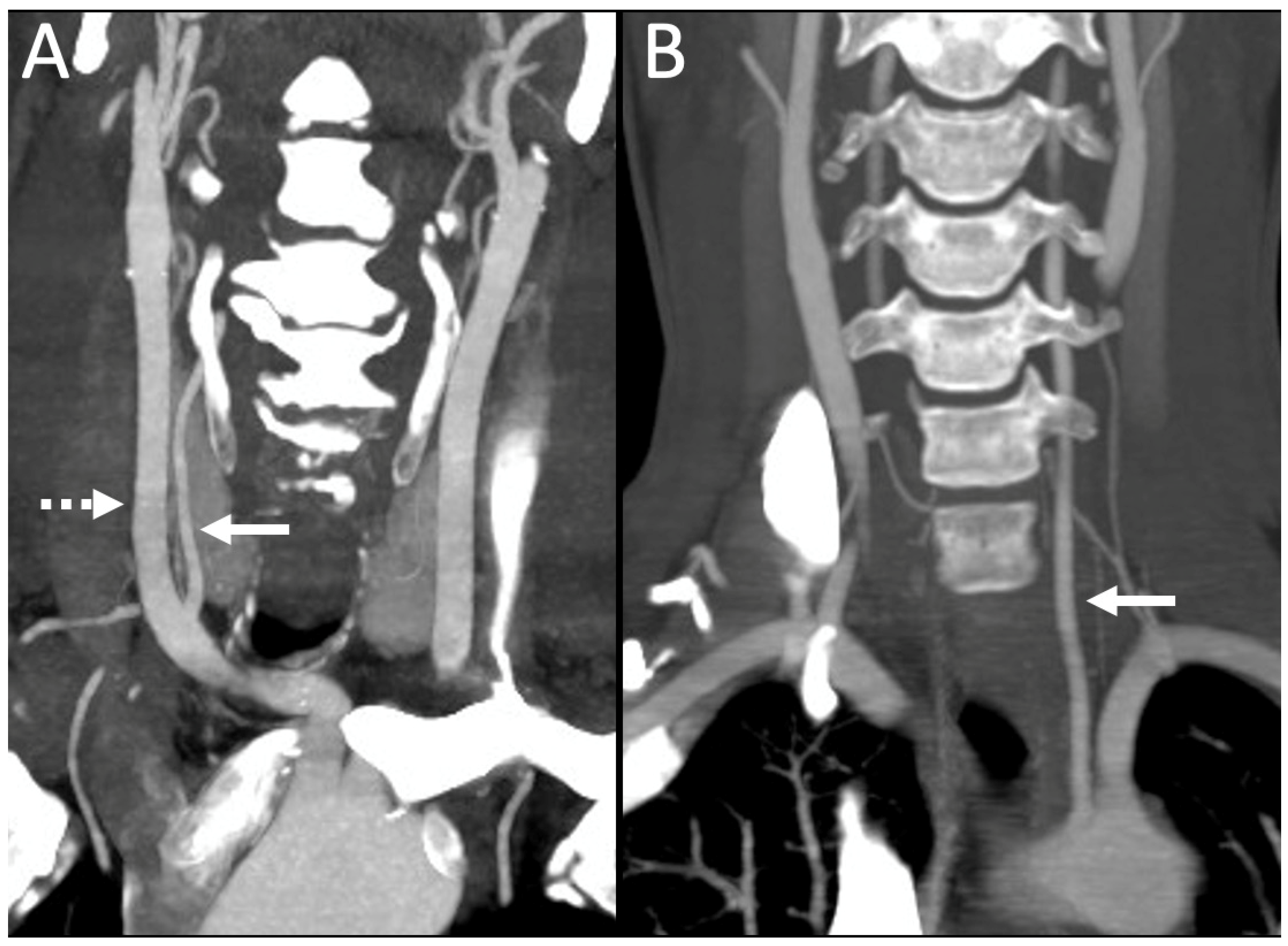
3.1. Variations in the V2 Segment
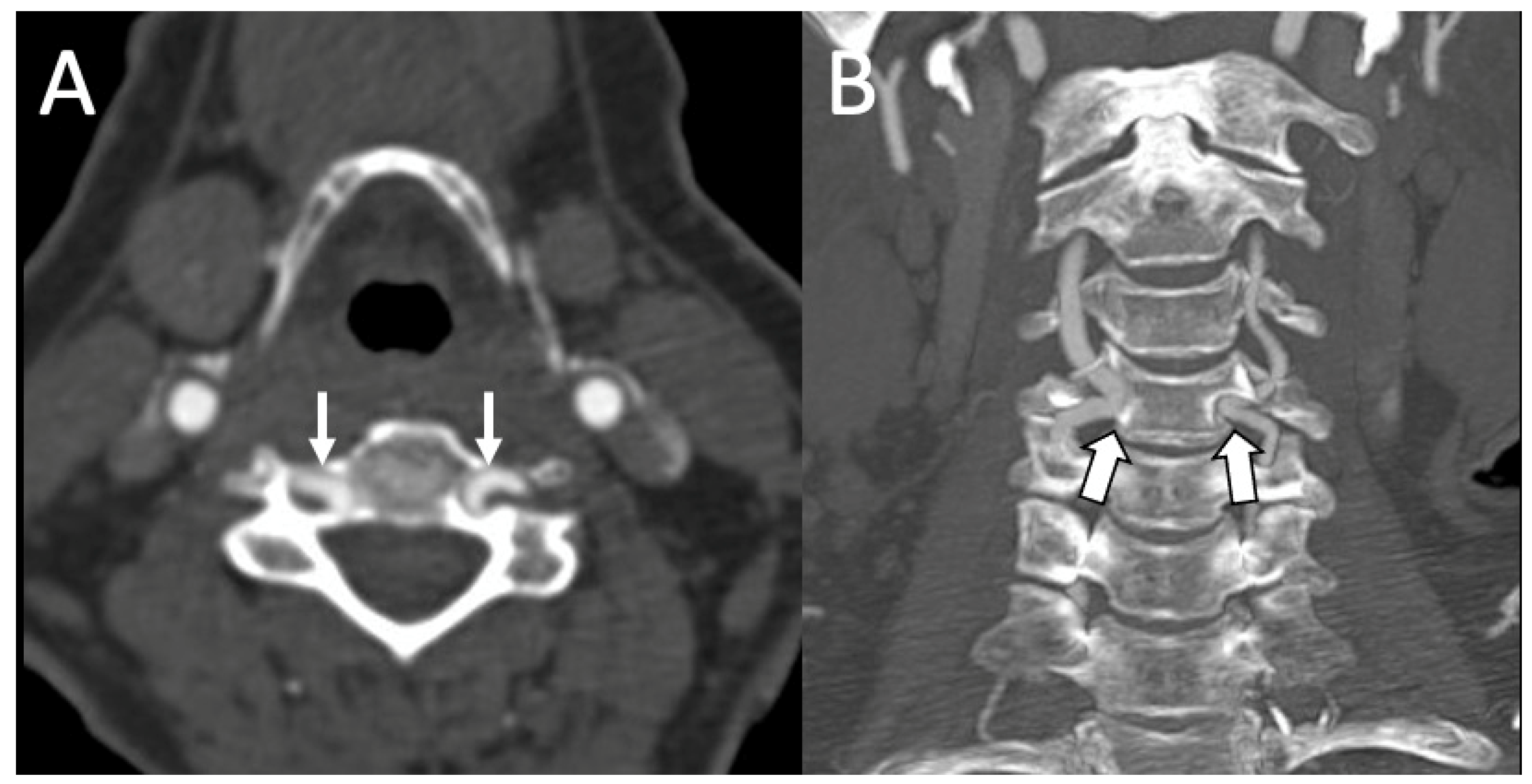
3.2. Variations of the V3 Segment
3.3. Vertebral Artery Dominance (VAD)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, W.K.; Kannan, A.; Mai, H.T.; Fehlings, M.G.; Smith, Z.A.; Traynelis, V.C.; Gokaslan, Z.L.; Hilibrand, A.S.; Nassr, A.; Arnold, P.M.; et al. Epidemiology and Outcomes of Vertebral Artery Injury in 16 582 Cervical Spine Surgery Patients: An AOSpine North America Multicenter Study. Glob. Spine J. 2017, 7, 21S–27S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Y.-F.; Dong, X.-F.; Feng, H.-X.; Zhao, H.-Q.; Liu, C.-F. Study on the correlation of vertebral artery dominance, basilar artery curvature and posterior circulation infarction. Acta Neurol. Belg. 2016, 116, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.; Piagkou, M.; Loukas, M.; Piperaki, E.-T.; Totlis, T.; Noussios, G.; Natsis, K. A systematic classification of the vertebral artery variable origin: Clinical and surgical implications. Surg. Radiol. Anat. 2018, 40, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.J.; Benndorf, G.; Liebig, T.; Felix, R. Anomalous origin of the right vertebral artery: Review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. AJNR Am. J. Neuroradiol. 1999, 20, 1318–1321. [Google Scholar]
- Elnaggar, M.E.; Abduljawad, H.; Assiri, A.; Ebrahim, W.H. Anomalous origin of right vertebral artery from right common carotid artery. Radiol. Case Rep. 2021, 16, 1574–1579. [Google Scholar] [CrossRef]
- Bruneau, M.; Cornelius, J.F.; Marneffe, V.; Triffaux, M.; George, B. Anatomical variations of the V2 segment of the vertebral artery. Neurosurgery 2006, 59, ONS20–ONS24. [Google Scholar] [CrossRef]
- Hong, J.T.; Lee, S.W.; Son, B.C.; Sung, J.H.; Yang, S.H.; Kim, I.S.; Park, C.K. Analysis of anatomical variations of bone and vascular structures around the posterior atlantal arch using three-dimensional computed tomography angiography. J. Neurosurg. Spine 2008, 8, 230–236. [Google Scholar] [CrossRef]
- Wakao, N.; Takeuchi, M.; Nishimura, M.; Riew, K.D.; Kamiya, M.; Hirasawa, A.; Kawanami, K.; Imagama, S.; Sato, K.; Takayasu, M. Vertebral artery variations and osseous anomaly at the C1-2 level diagnosed by 3D CT angiography in normal subjects. Neuroradiology 2014, 56, 843–849. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, H.J.; Kim, J.H.; Hong, J.T. Quantitative analysis of unusual entrance of the vertebral artery into the cervical foramen (V2 segment) and its clinical implications. Eur. Spine J. 2016, 25, 4188–4194. [Google Scholar] [CrossRef]
- Yi, X.; Xie, P.; Zhang, L.; Lu, F.; Chen, H.; Liu, K. Entrance and origin of the extracranial vertebral artery found on computed tomography angiography. Sci. Rep. 2022, 12, 15274. [Google Scholar] [CrossRef]
- Wang, S.; Ren, W.-J.; Zheng, L.; Sun, S.-T.; Zhang, B.-H.; Chen, Y.; Xiang, L.-B. Anatomical Variations of the Vertebral Artery: Analysis by Three-Dimensional Computed Tomography Angiography in Chinese Population. Orthop. Surg. 2021, 13, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Eskander, M.S.; Connolly, P.J.; Eskander, J.P.; Brooks, D.D. Injury of an aberrant vertebral artery during a routine corpectomy: A case report and literature review. Spinal Cord 2009, 47, 773–775. [Google Scholar] [CrossRef]
- Dinç, Y.; Özpar, R.; Emir, B.; Hakyemez, B.; Bakar, M. Vertebral artery hypoplasia as an independent risk factor of posterior circulation atherosclerosis and ischemic stroke. Medicine 2021, 100, e27280. [Google Scholar] [CrossRef]
- Naldemir, I.F.; Unlu, E.N.; Onbas, O. Tortuous vertebral artery triggering vertebral foramen expansion and radiculopathy in a 19-year-old patient: A case report. J. Med. Case Rep. 2020, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, B.R.; Harrichandparsad, R.; Satyapal, K.S.; Moodley, I.G.; Lazarus, L. Radiological anatomy of the intracranial vertebral artery in a select South African cohort of patients. Sci. Rep. 2021, 11, 12138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-W.; Yang, Y.; Liu, Y.-G.; Cao, J.-W.; Li, F. Anatomical features and clinical significance of radiculomuscular artery variants involving the suboccipital segment of vertebral artery: Angiographic and cadaver studies. Clin. Neuroradiol. 2018, 28, 75–80. [Google Scholar] [CrossRef]
- Arslan, D.; Ozer, M.A.; Govsa, F.; Kitis, O. Surgicoanatomical aspect in vascular variations of the V3 segment of vertebral artery as a risk factor for C1 instrumentation. J. Clin. Neurosci. 2019, 68, 243–249. [Google Scholar] [CrossRef]
- Magklara, E.-P.; Pantelia, E.-T.; Solia, E.; Panagouli, E.; Piagkou, M.; Mazarakis, A.; Skandalakis, P.; Troupis, T.; Filippou, D. Vertebral artery variations revised: Origin, course, branches and embryonic development. Folia Morphol. 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Meila, D.; Tysiac, M.; Petersen, M.; Theisen, O.; Wetter, A.; Mangold, A.; Schlunz-Hendann, M.; Papke, K.; Brassel, F.; Berenstein, A. Origin and course of the extracranial vertebral artery: CTA findings and embryologic considerations. Clin. Neuroradiol. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Woraputtaporn, W.; Ananteerakul, T.; Iamsaard, S.; Namking, M. Incidence of vertebral artery of aortic arch origin, its level of entry into transverse foramen, length, diameter and clinical significance. Anat. Sci. Int. 2019, 94, 275–279. [Google Scholar] [CrossRef]
- Zhang, H.; Chai, W.; Wang, S.; Wang, Y.; Li, H. Persistent first intersegmental artery (PFIA) visualized by three-dimensional computed tomography angiography in Chinese population. Int. J. Surg. 2018, 52, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bai, X.; Huan, X.; Wang, T. Paracondylar process combined with persistent first intersegmental vertebral artery: An anatomic case report and literature review. Br. J. Neurosurg. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Habib, A.; Albadr, F.; Ahmed, J.; Aleissa, A.; Al Towim, A. Quantitative assessment of vertebral artery anatomy in relation to cervical pedicles: Surgical considerations based on regional differences. Neurosciences 2018, 23, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Vaiman, M.; Beckerman, I. Importance of preoperative detection of vertebral artery anomalies in neck surgery. ANZ J. Surg. 2011, 81, 164–167. [Google Scholar] [CrossRef]
- Bhatia, K.; Ghabriel, M.N.; Henneberg, M. Anatomical variations in the branches of the human aortic arch: A recent study of a South Australian population. Folia Morphol. 2005, 64, 217–223. [Google Scholar]
- Yaprak, F.; Ozer, M.A.; Govsa, F.; Eraslan, C. Variations of the extracranial segment of vertebral artery as a bleeding risk factor. Surg. Radiol. Anat. 2021, 43, 1735–1743. [Google Scholar] [CrossRef]
- Elgueta, M.F.; Ortiz Jimenez, J.; Wang, N.N.; Pérez Lara, A.; Chankowsky, J.; Charghi, R.; Tran, D.Q.; Finlayson, R.J. Anatomical Variations of the Vertebral Artery in the Upper Cervical Spine: Clinical Relevance for Procedures Targeting the C1/C2 and C2/C3 Joints. Reg. Anesth. Pain Med. 2018, 43, 367–371. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, J.-M.; Roh, J.-K. Hypoplastic vertebral artery: Frequency and associations with ischaemic stroke territory. J. Neurolo. Neurosurg. Psychiatry 2007, 78, 954–958. [Google Scholar] [CrossRef]
- Chuang, Y.-M.; Huang, Y.-C.; Hu, H.-H.; Yang, C.-Y. Toward a further elucidation: Role of vertebral artery hypoplasia in acute ischemic stroke. Eur. Neurol. 2006, 55, 193–197. [Google Scholar] [CrossRef]
- Jeng, J.-S.; Yip, P.-K. Evaluation of vertebral artery hypoplasia and asymmetry by color-coded duplex ultrasonography. Ultrasound Med. Biol. 2004, 30, 605–609. [Google Scholar] [CrossRef]
- Vujmilović, S.; Spasojević, G.; Vujnović, S.; Malobabić, S.; Vujković, Z. Variability of the vertebral artery origin and transverse foramen entrance level-CT angiographic study. Folia Morphol. 2018, 77, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, R.; Cihan, O.F. Multidetector computed tomography evaluation of origin, V2 segment variations and morphology of vertebral artery. Folia Morphol. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Narouze, S. Ultrasound-guided stellate ganglion block: Safety and efficacy. Curr. Pain Headache Rep. 2014, 18, 424. [Google Scholar] [CrossRef] [PubMed]
- Motomura, M.; Watanabe, K.; Tabira, Y.; Iwanaga, J.; Matsuuchi, W.; Yoshida, D.; Saga, T.; Yamaki, K.-I. A Case of Duplicated Right Vertebral Artery. Kurume Med. J. 2018, 64, 69–73. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Liu, Y.-S.; Chen, Y.-C.; Shih, Y.-H.; Chang, C.-C.; Chuang, M.-T. Variations in the origin and course of the extracranial vertebral artery on multidetector computed tomography angiography. Iran. J. Radiol. 2018, 15, e61623. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lee, J.-K.; Moon, K.-S.; Kim, Y.-S.; Kwak, H.-J.; Joo, S.-P.; Kim, J.-H.; Kim, S.-H. Endovascular embolization of iatrogenic vertebral artery injury during anterior cervical spine surgery: Report of two cases and review of the literature. Spine 2006, 31, E891–E894. [Google Scholar] [CrossRef]
- O’Donnell, C.M.; Child, Z.A.; Nguyen, Q.; Anderson, P.A.; Lee, M.J. Vertebral artery anomalies at the craniovertebral junction in the US population. Spine 2014, 39, E1053–E1057. [Google Scholar] [CrossRef]
- Yamazaki, M.; Koda, M.; Aramomi, M.A.; Hashimoto, M.; Masaki, Y.; Okawa, A. Anomalous vertebral artery at the extraosseous and intraosseous regions of the craniovertebral junction: Analysis by three-dimensional computed tomography angiography. Spine 2005, 30, 2452–2457. [Google Scholar] [CrossRef]
- Kaushal, P. Epitransverse process: A rare outgrowth from atlas vertebra. Int. J. Anat. Var. 2010, 3, 108–109. [Google Scholar]
- Taitz, C. Bony observations of some morphological variations and anomalies of the craniovertebral region. Clin. Anat. 2000, 13, 354–360. [Google Scholar] [CrossRef]
- Kaur, J.; Srivastava, D.; Singh, D.; Raheja, S. The study of hyperostosic variants: Significance of hyperostotic variants of human skulls in anthropology. Anat. Cell Biol. 2012, 45, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Murlimanju, B.V.; Chettiar, G.K.; Krishnamurthy, A.; Pai, M.M.; Saralaya, V.V.; Prabhu, L.V.; Vadgaonkar, R. The Paracondylar Skull Base: Anatomical Variants and Their Clinical Implications. Turk. Neurosurg. 2015, 25, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Shankar, B.; Paruthikunnan, S.M.; Kulkarni, C.D. Paracondylar process of the occipital bone of the skull: A rare congenital anatomical variant. BMJ Case Rep. 2014, 2014, bcr2014205315. [Google Scholar] [CrossRef]
- Guebert, G.M.; Rowe, L.J.; Yochum, T.R. Congenital anomalies and normal skeletal variants. In Essentials of Skeletal Radiology, 3rd ed.; Yochum, T.R., Rowe, L.J., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 262–264. [Google Scholar]
- McCall, T.; Coppens, J.; Couldwell, W.; Dailey, A. Symptomatic occipitocervical paracondylar process. J. Neurosurg. Spine 2010, 12, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dayani, F.; Purger, D.A.; Cage, T.; Lee, M.; Patel, M.; Singh, H. Extraforaminal Vertebral Artery Anomalies and Their Associated Surgical Implications: An Epidemiologic and Anatomic Report on 1000 Patients. World Neurosurg. 2020, 141, e971–e975. [Google Scholar] [CrossRef] [PubMed]
- Perren, F.; Poglia, D.; Landis, T.; Sztajzel, R. Vertebral artery hypoplasia: A predisposing factor for posterior circulation stroke? Neurology 2007, 68, 65–67. [Google Scholar] [CrossRef]
- Zhang, D.-P.; Zhang, S.-L.; Zhang, J.-W.; Zhang, H.-T.; Fu, S.-Q.; Yu, M.; Ren, Y.-F.; Ji, P. Basilar artery bending length, vascular risk factors, and pontine infarction. J. Neurol. Sci. 2014, 338, 142–147. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Y.-M.; Xu, P. The Clinical Research Progress of Vertebral Artery Dominance and Posterior Circulation Ischemic Stroke. Cerebrovasc. Dis. 2022, 51, 553–556. [Google Scholar] [CrossRef]
- Ngo, M.T.; Kwak, H.S.; Chung, G.H. Change in basilar artery length and bending according to aging and vertebral artery dominance: A longitudinal study. Sci. Rep. 2020, 10, 8904. [Google Scholar] [CrossRef]
- Hong, J.M.; Chung, C.S.; Bang, O.Y.; Yong, S.W.; Joo, I.S.; Huh, K. Vertebral artery dominance con-tributes to basilar artery curvature and peri-vertebrobasilar junctional infarcts. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1087–1092. [Google Scholar] [CrossRef]
- Liu, Y.-D.; Li, Z.-Q.; Fu, J.-J.; E, Y.-J. A rare anomalous origin of right vertebral artery with double branch: First case report. Interv. Neuroradiol. 2018, 24, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-M. Aberrant Origin of Vertebral Artery and its Clinical Implications. Braz. J. Cardiovasc. Surg. 2016, 31, 52–59. [Google Scholar] [CrossRef] [PubMed]


| Gender | Male (676) | Female (482) | Chi-Square Test | |
| V2 | UE-V2 (%) | 10.35% (70/676) | 10.58% (51/482) | X2 = 0.001 p = 0.979 |
| MM (%) | 1.18% (8/676) | 1.45% (7/482) | X2 = 0.018 p = 0.892 | |
| V3 | PFIA (%) | 0.30% (2/676) | 0% (0/482) | X2 = 0.228 P = 0.541 |
| PP (%) | 0.30% (2/676) | 1.04% (5/482) | X2 = 1.489 p = 0.135 | |
| Side | Right side | Left side | ||
| V2 | UE-V2 (%) | 5.44% (63/1158) | 5.01% (58/1158) | X2 = 0.148 p = 0.701 |
| MM (%) | 0.35% (4/1158) | 0.95% (11/1158) | X2 = 2.431 p = 0.119 | |
| V3 | PFIA (%) | 0.17% (2/1158) | 0 | X2 = 0.501 p = 0.500 |
| PP (%) | 0.17% (2/1158) | 0.43% (5/1158) | X2 = 0.575 p = 0.452 | |
| Author & Year | Bruneau et al., 2006 [6] | Hong et al., 2008 [7] | Wakao et al., 2014 [8] | Kim et al., 2016 [9] | Vujmilovic et al., 2018 [31] | Yi et al., 2022 [10] | Present study |
| Country | France | Korea | Japan | Korea | Republika Srpska | China | Oman |
| Total Patients (n), Modality | 250 (200 MRI, 50 CT) | 350 (CTA) | 919 (CTA) | 2207 (CTA) | 112 (CTA) | 223 (CTA) | 579 (CTA) |
| Overall Unusual Entrance (%) | 7% (35/500) | 5.1% (36/700) | 4.4% (81/1838) | 6.5% (286/4414) | 12.5% (28/224) | 8.2% (37/446) | 10.4% (121/1158) |
| C3% | 2.9% | - | - | 0.3% | - | 2.7% | 0.8% |
| C4% | 14.3% | 30.6% | 12.3% | 18.2% | 3.1% | 29.7% | 20.7% |
| C5% | 71.4% | 63.9% | 70.4% | 74.8% | 8.9% | 56.8% | 71% |
| C7% | 11.4% | 5.5% | 17.3% | 6.7% | 0.4% | 10.8% | 7.4% |
| Right (n)/Left (n) | 17/18 | 16/16 | 40/41 | 150/135 | 16/12 | 22/15 | 72/49 |
| Unilateral (%) vs. Bilateral (%) | 94 vs. 6 | 94 vs. 6 | 91 vs. 9 | 86.5 vs. 13.5 | NA | NA | 85 vs. 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hajri, F.; Al Yahya’ey, B.; Sirasanagandla, S.R.; Mogali, S.R.; Al-Ajmi, E. Radiological Assessment of Extracranial Vertebral Artery Variations: A Computed Tomography Angiography Study. Appl. Sci. 2023, 13, 5822. https://doi.org/10.3390/app13105822
Al Hajri F, Al Yahya’ey B, Sirasanagandla SR, Mogali SR, Al-Ajmi E. Radiological Assessment of Extracranial Vertebral Artery Variations: A Computed Tomography Angiography Study. Applied Sciences. 2023; 13(10):5822. https://doi.org/10.3390/app13105822
Chicago/Turabian StyleAl Hajri, Faiza, Bayan Al Yahya’ey, Srinivasa Rao Sirasanagandla, Sreenivasulu Reddy Mogali, and Eiman Al-Ajmi. 2023. "Radiological Assessment of Extracranial Vertebral Artery Variations: A Computed Tomography Angiography Study" Applied Sciences 13, no. 10: 5822. https://doi.org/10.3390/app13105822






