Predicting Adherence to Home-Based Cardiac Rehabilitation with Data-Driven Methods
Abstract
:1. Introduction
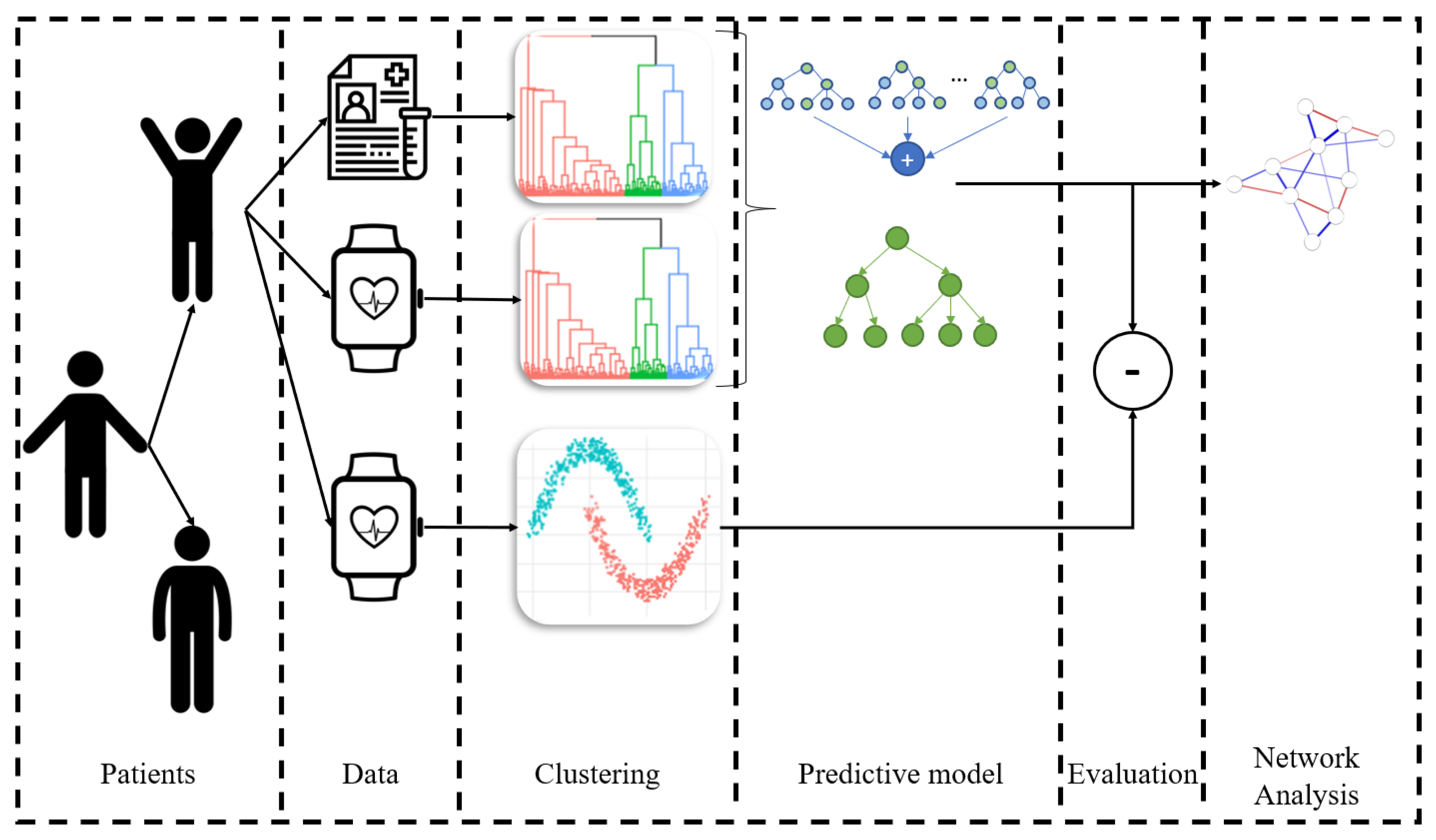
2. Materials and Methods
2.1. Data Description
2.1.1. Data from Baseline and Periodic Clinical Evaluation
- Cardiovascular Risk Profile: These markers were collected through blood sampling and anthropometric measurements. The Framingham cardiovascular risk score was calculated as described in [32].
- Health-Related Physical Fitness: These data represent the findings from a maximally graded cardiopulmonary exercise test (CPET) on a bicycle, along with muscle strength testing including maximal isometric and isokinetic quadriceps strength, handgrip strength, and a 30 s sit-to-stand test.
- Psychological wellbeing and intervention effectiveness: This subjective information was collected using standardized questionnaires assessing QoL [33], physical activity behavior [34,35,36], smoking, alcohol consumption [37], diet [38], stress [39], medication adherence [40], mental wellbeing [41], social support [42], self-efficacy [43], and perceived health status [44,45].
2.1.2. Exercise Session Data
Exercise Adherence Metric
Exercise Performance Metric
2.2. Investigation of Different Patient Clusters at Baseline
2.2.1. Clustering Baseline Profiles
2.2.2. Clustering Familiarization Adherence Behavior
2.2.3. Clustering whole-Program Adherence Behavior
2.3. Predictive Modeling for Whole-Program Adherence Prediction
2.4. Statistical Differences between the Groups
2.5. Network Analysis Per Group
3. Results
3.1. Absent Versus Present Patients during Familiarization
3.2. Patient Profile Clusters
3.2.1. Clusters Based on Clinical Baseline Characteristics
3.2.2. Clusters of Patient Adherence during Familiarization
3.3. Program Adherence Clusters
3.4. Predicting Program Adherence
- A patient that is recruited for a home- and exercise-based rehabilitation program has a 58.5 ± 2.5% probability to be adherent without any additional knowledge.
- If the patient is adherent during the familiarization phase, then the probability of being adherent for the whole program reaches 92.3 ± 3.5%.
- For a patient that is non-adherent or has a transient exercise behavior in the familiarization phase, the possibility to be non-adherent for the rest of the program is 55.6 ± 3%.
- If those patients are of high risk, based on the baseline characteristics, then the probability of being non-adherent increases to 82.4 ± 3.9%.
- If those patients are of low risk or are included in the average-baseline cluster, then the probability of being adherent is 63.3 ± 3.3%.
3.5. Network Analysis and Detection of Structure in Patient Profiles
- In the high-risk and fit group, the risk was correlated with glucose and SBP, while in the high-risk and sedentary group, the risk was correlated with the level of MVPA.
- In the average group, STS and SBP were positively correlated, while in the high-risk and sedentary group they were negatively correlated.
- In the high-risk and fit group, the main connections included peak HR–MVPA–STS–SBP (physical/cardiovascular condition), while in the X group a glucose–SBP–STS link prevailed.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- WHO. Adherence to Long-Term Therapies: Evidence for Action; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Livitckaia, K.; Koutkias, V.; Maglaveras, N.; Kouidi, E.; Van Gils, M.; Chouvarda, I. Adherence to physical activity in patients with heart disease: Types, settings and evaluation instruments. In Proceedings of the International Conference on Biomedical and Health Informatics, Thessaloniki, Greece, 18–21 November 2017; pp. 255–259. [Google Scholar]
- Naderi, S.H.; Bestwick, J.P.; Wald, D.S. Adherence to drugs that prevent cardiovascular disease: Meta-analysis on 376,162 patients. Am. J. Med. 2012, 125, 882–887.e1. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason-Wehrens, B.; McGee, H.; Zwisler, A.D.; Piepoli, M.F.; Benzer, W.; Schmid, J.P.; Dendale, P.; Pogosova, N.G.V.; Zdrenghea, D.; Niebauer, J.; et al. Cardiac rehabilitation in Europe: Results from the European Cardiac Rehabilitation Inventory Survey. Eur. J. Prev. Cardiol. 2010, 17, 410–418. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef]
- Chindhy, S.; Taub, P.R.; Lavie, C.J.J.; Shen, J. Current Challenges in Cardiac Rehabilitation: Strategies to Overcome Social Factors and Attendance Barriers. Expert Rev. Cardiovasc. Ther. 2020, 18, 777–789. [Google Scholar] [CrossRef]
- Rose, K.; Eldridge, S.; Chapin, L. The Internet of Things (IoT): An Overview. Int. J. Eng. Res. Appl. 2015, 5, 71–82. [Google Scholar]
- Cavalheiro, A.H.; Silva Cardoso, J.; Rocha, A.; Moreira, E.; Azevedo, L.F. Effectiveness of Tele-rehabilitation Programs in Heart Failure: A Systematic Review and Meta-analysis. Health Serv. Insights 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Claes, J.; Buys, R.; Budts, W.; Smart, N.; Cornelissen, V.A. Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 244–256. [Google Scholar] [CrossRef]
- Rawstorn, J.C.; Gant, N.; Direito, A.; Beckmann, C.; Maddison, R. Telehealth exercise-based cardiac rehabilitation: A systematic review and meta-analysis. Heart 2016, 102, 1183–1192. [Google Scholar] [CrossRef]
- Frederix, I.; Hansen, D.; Coninx, K.; Vandervoort, P.; Vandijck, D.; Hens, N.; Van Craenenbroeck, E.; Van Driessche, N.; Dendale, P. Medium-term effectiveness of a comprehensive internet-based and patient-specific telerehabilitation program with text messaging support for cardiac patients: Randomized controlled trial. J. Med. Internet Res. 2015, 17, e185. [Google Scholar] [CrossRef]
- Pinto, B.M.; Goldstein, M.G.; Papandonatos, G.D.; Farrell, N.; Tilkemeier, P.; Marcus, B.H.; Todaro, J.F. Maintenance of exercise after phase II cardiac rehabilitation: A randomized controlled trial. Am. J. Prev. Med. 2011, 41, 274–283. [Google Scholar] [CrossRef]
- Claes, J.; Buys, R.; Woods, C.; Briggs, A.; Geue, C.; Aitken, M.; Moyna, N.; Moran, K.; McCaffrey, N.; Chouvarda, I.; et al. PATHway I: Design and rationale for the investigation of the feasibility, clinical effectiveness and cost-effectiveness of a technology-enabled cardiac rehabilitation platform. BMJ Open 2017, 7, e016781. [Google Scholar] [CrossRef]
- Anderson, L.; Sharp, G.A.; Norton, R.J.; Dalal, H.; Dean, S.G.; Jolly, K.; Cowie, A.; Zawada, A.; Taylor, R.S. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Pfaeffli Dale, L.; Whittaker, R.; Dixon, R.; Stewart, R.; Jiang, Y.; Carter, K.; Maddison, R. Acceptability of a Mobile Health Exercise-Based Cardiac Rehabilitation Intervention. J. Cardiopulm. Rehabil. Prev. 2015, 35, 312–319. [Google Scholar] [CrossRef]
- Hannan, A.L.; Harders, M.P.; Hing, W.; Climstein, M.; Coombes, J.S.; Furness, J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: Systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2019, 11, 14. [Google Scholar] [CrossRef]
- Hamilton, S.J.; Mills, B.; Birch, E.M.; Thompson, S.C. Smartphones in the secondary prevention of cardiovascular disease: A systematic review. BMC Cardiovasc. Disord. 2018, 18, 25. [Google Scholar] [CrossRef]
- Essery, R.; Geraghty, A.W.A.; Kirby, S.; Yardley, L. Predictors of adherence to home-based physical therapies: A systematic review. Disabil. Rehabil. 2017, 39, 519–534. [Google Scholar] [CrossRef]
- Beinart, N.A.; Goodchild, C.E.; Weinman, J.A.; Ayis, S.; Godfrey, E.L. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: A systematic review. Spine J. 2013, 13, 1940–1950. [Google Scholar] [CrossRef]
- Picorelli, A.M.A.; Pereira, L.S.M.; Pereira, D.S.; Felício, D.; Sherrington, C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Filos, D.; Buys, R.; Claes, J.; Cornelissen, V.; Kouidi, E.; Chatzitofis, A.; Zarpalas, D.; Daras, P.; Walsh, D.; et al. Computerized decision support for beneficial home-based exercise rehabilitation in patients with cardiovascular disease. Comput. Methods Programs Biomed. 2018, 162, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fukuoka, Y.; Goldberg, K.; Vittinghoff, E.; Aswani, A. Applying machine learning to predict future adherence to physical activity programs. BMC Med. Inform. Decis. Mak. 2019, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, O.J.; Zahia, S.; Fuente-Vidal, A.; Férez, N.S.; Noguera, O.R.; Montane, J.; Garcia-Zapirain, B. Predicting physical exercise adherence in fitness apps using a deep learning approach. Int. J. Environ. Res. Public Health 2021, 18, 10769. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Chung, K. Prediction model of user physical activity using data characteristics-based long short-term memory recurrent neural networks. KSII Trans. Internet Inf. Syst. 2019, 13, 2060–2077. [Google Scholar] [CrossRef]
- Claes, J.; Filos, D.; Cornelissen, V.; Chouvarda, I. Prediction of the Adherence to a Home-Based Cardiac Rehabilitation Program. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription; ACSM: Indianapolis, IN, USA, 2013; Volume 9, ISBN 978-1-6091-3955-1. [Google Scholar]
- Shcherbina, A.; Mikael Mattsson, C.; Waggott, D.; Salisbury, H.; Christle, J.W.; Hastie, T.; Wheeler, M.T.; Ashley, E.A. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J. Pers. Med. 2017, 7, 3. [Google Scholar] [CrossRef]
- Walsh, D.M.J.; Kieran, M.; Cornelissen, V.; Buys, R.; Claes, J.; Zampognaro, P.; Melillo, F.; Maglaveras, N.; Chouvarda, I.; Triantafyllidis, A.; et al. The development and codesign of the PATHway intervention: A theory-driven eHealth platform for the self-management of cardiovascular disease. Transl. Behav. Med. 2019, 9, 76–98. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Cruz, L.N.; Camey, S.A.; Fleck, M.P.; Polanczyk, C.A. World Health Organization quality of life instrument-brief and Short Form-36 in patients with coronary artery disease: Do they measure similar quality of life concepts? Psychol. Health Med. 2009, 14, 619–628. [Google Scholar] [CrossRef]
- Hardie Murphy, M.; Rowe, D.A.; Belton, S.; Woods, C.B. Validity of a two-item physical activity questionnaire for assessing attainment of physical activity guidelines in youth. BMC Public Health 2015, 15, 1080. [Google Scholar] [CrossRef]
- McAuley, E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J. Behav. Med. 1992, 15, 65–88. [Google Scholar] [CrossRef]
- Sniehotta, F.F.; Schwarzer, R.; Scholz, U.; Schuz, B. Action planning and coping planning for long-term lifestyle change theory and.pdf. Eur. J. Soc. Psychol. 2005, 35, 565–579. [Google Scholar] [CrossRef]
- Lawford, B.R.; Barnes, M.; Connor, J.P.; Heslop, K.; Nyst, P.; Young, R.M.D. Alcohol use disorders identification test (AUDIT) scores are elevated in antipsychotic-induced hyperprolactinaemia. J. Psychopharmacol. 2012, 26, 324–329. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef]
- Ng Fat, L.; Scholes, S.; Boniface, S.; Mindell, J.; Stewart-Brown, S. Evaluating and establishing national norms for mental wellbeing using the short Warwick–Edinburgh Mental Well-being Scale (SWEMWBS): Findings from the Health Survey for England. Qual. Life Res. 2017, 26, 1129–1144. [Google Scholar] [CrossRef]
- Vaglio, J.; Conard, M.; Poston, W.S.; O’Keefe, J.; Haddock, C.K.; House, J.; Spertus, J.A. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual. Life Outcomes 2004, 2, 24. [Google Scholar] [CrossRef]
- Shields, C.A.; Brawley, L.R. Preferring proxy-agency: Impact on self-efficacy for exercise. J. Health Psychol. 2006, 11, 904–914. [Google Scholar] [CrossRef]
- Razykov, I.; Ziegelstein, R.C.; Whooley, M.A.; Thombs, B.D. The PHQ-9 versus the PHQ-8—Is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J. Psychosom. Res. 2012, 73, 163–168. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The Brief Illness Perception Questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef]
- Claes, J.; Cornelissen, V.; McDermott, C.; Moyna, N.; Pattyn, N.; Cornelis, N.; Gallagher, A.; McCormack, C.; Newton, H.; Gillain, A.; et al. Feasibility, Acceptability, and Clinical Effectiveness of a Technology-Enabled Cardiac Rehabilitation Platform (Physical Activity Toward Health-I): Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e14221. [Google Scholar] [CrossRef]
- Nielsen, F. Hierarchical Clustering. In Introduction to HPC with MPI for Data Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 195–211. ISBN 9789811305535. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- John, C.R.; Watson, D.; Barnes, M.R.; Pitzalis, C.; Lewis, M.J. Spectrum: Fast density-aware spectral clustering for single and multi-omic data. Bioinformatics 2020, 36, 1159–1166. [Google Scholar] [CrossRef]
- Lihi, Z.-M.; Perona, P. Self-Tuning Spectral Clustering. In Advances in Neural Information Processing Systems; Saul, L., Weiss, Y., Bottou, L., Eds.; MIT Press: Cambridge, MA, USA, 2004; Volume 17. [Google Scholar]
- Vens, C.; Struyf, J.; Schietgat, L.; Džeroski, S.; Blockeel, H. Decision trees for hierarchical multi-label classification. Mach. Learn. 2008, 73, 185–214. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B. rpart: Recursive Partitioning and Regression Trees; Scientific Research Publishing: Wuhan, China, 2019. [Google Scholar]
- Mahdi Abdulkareem, N.; Mohsin Abdulazeez, A. Machine Learning Classification Based on Radom Forest Algorithm: A Review. Int. J. Sci. Bus. 2021, 5, 128–142. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Borsboom, D.; Deserno, M.K.; Rhemtulla, M.; Epskamp, S.; Fried, E.I.; McNally, R.J.; Robinaugh, D.J.; Perugini, M.; Dalege, J.; Costantini, G.; et al. Network analysis of multivariate data in psychological science. Nat. Rev. Methods Prim. 2021, 1, 58. [Google Scholar] [CrossRef]
- Zanin, M.; Aitya, N.A.A.; Basilio, J.; Baumbach, J.; Benis, A.; Behera, C.K.; Bucholc, M.; Castiglione, F.; Chouvarda, I.; Comte, B.; et al. An Early Stage Researcher’s Primer on Systems Medicine Terminology. Netw. Syst. Med. 2021, 4, 2–50. [Google Scholar] [CrossRef] [PubMed]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. glasso: Graphical Lasso: Estimation of Gaussian Graphical Models. 2019. Available online: https://CRAN.R-project.org/package=glasso (accessed on 6 April 2023).
- Friedman, J.; Hastie, T.; Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 2008, 9, 432–441. [Google Scholar] [CrossRef]
- Epskamp, S.; Waldorp, L.J.; Mõttus, R.; Borsboom, D. The Gaussian Graphical Model in Cross-Sectional and Time-Series Data. Multivar. Behav. Res. 2018, 53, 453–480. [Google Scholar] [CrossRef]
- Epskamp, S. Brief Report on Estimating Regularized Gaussian Networks from Continuous and Ordinal Data. arXiv 2016, arXiv:1606.05771. [Google Scholar]
- Epskamp, S. graphicalVAR: Graphical VAR for Experience Sampling Data. 2021. Available online: https://cran.r-project.org/web/packages/graphicalVAR/graphicalVAR.pdf (accessed on 6 April 2023).
- Ge, C.; Ma, J.; Xu, Y.; Shi, Y.J.; Zhao, C.H.; Gao, L.; Bai, J.; Wang, Y.; Sun, Z.J.; Guo, J.; et al. Predictors of adherence to home-based cardiac rehabilitation program among coronary artery disease outpatients in China. J. Geriatr. Cardiol. 2019, 16, 749–755. [Google Scholar] [CrossRef]
- Shaw, J.F.; Pilon, S.; Vierula, M.; McIsaac, D.I. Predictors of adherence to prescribed exercise programs for older adults with medical or surgical indications for exercise: A systematic review. Syst. Rev. 2022, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Heindl, B.; Ramirez, L.; Joseph, L.; Clarkson, S.; Thomas, R.; Bittner, V. Hybrid cardiac rehabilitation—The state of the science and the way forward. Prog. Cardiovasc. Dis. 2022, 70, 175–182. [Google Scholar] [CrossRef]
- Tang, L.H.; Harrison, A.; Skou, S.T.; Taylor, R.S.; Dalal, H.; Doherty, P. Are patient characteristics and modes of delivery associated with completion of cardiac rehabilitation? A national registry analysis. Int. J. Cardiol. 2022, 361, 7–13. [Google Scholar] [CrossRef]











| Present | Absent | p-Value | |
|---|---|---|---|
| Baseline | |||
| BARSE | 67.361 ± 22.5 | 53.93 ± 16.6 | 0.043 |
| Sedentary time (min) | 752.53 ± 98.3 | 677.89 ± 55.1 | 0.016 |
| Light activity time (min/day) | 559.88 ± 80.87 | 620.78 ± 50.54 | 0.035 |
| Baseline–6 months | |||
| BMI (kg/m2) | 0.037 ± 1.08 | 1.21 ± 1.84 | 0.022 |
| Waist circumference (cm) | −2.06 ± 5.25 | 2.77 ± 4.24 | 0.014 |
| Triglycerides (mmol/L) | −0.029 ± 0.67 | 0.48 ± 0.72 | 0.028 |
| pLoad (Watt) | −0.122 ± 25.4 | −14.44 ± 18.1 | 0.028 |
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| Low-Risk & Active | High-Risk & Sedentary | High-Risk & Fit | |
| Glucose (mmol/L) | 4.71 ± 0.5 | 6.04 ± 1.81 | 5.81 ± 1.04 |
| Risk score (%) | 6.16 ± 5.9 | 18.65 ± 10.43 | 16.79 ± 10.035 |
| BARSE | 81.23 ± 11.1 | 75.13 ± 17.63 | 58.43 ± 24.39 |
| PSS | 13 ± 7.5 | 7.73 ± 5.34 | 12.48 ± 6.129 |
| PACE | 4.5 ± 1.7 | 4.5 ± 1.85 | 3.12 ± 1.387 |
| Illness perception | 38.2 ± 13.74 | 24.13 ± 12.69 | 34.1 ± 13.37 |
| SF-36 mental | 78.19 ± 15.14 | 83.36 ± 14.65 | 74.18 ± 14.17 |
| EE (kcal) | 1576 ± 639.62 | 1115.4 ± 187.27 | 1583.62 ± 395.35 |
| MVPA (min) | 229.4 ± 46.39 | 77.2 ± 29.46 | 132.43 ± 41.3 |
| Steps (n) | 16720 ± 767.8 | 8994.93 ± 903.62 | 13,173.14 ± 1475.88 |
| 30 s STS (n) | 23 ± 3.24 | 16.53 ± 4.29 | 19.29 ± 4.69 |
| Sedentary time (min) | 655.6 ± 35.84 | 840.93 ± 67.96 | 712.48 ± 76.99 |
| Quadriceps isokinetic (J) | 1636.38 ± 314.61 | 1885.51 ± 849.03 | 2378.82 ± 576.13 |
| Quadriceps isometric (Nm) | 107.2 ± 26.33 | 142.4 ± 51.32 | 156.79 ± 38.1 |
| Week | Cluster 1 Adherent-6w | Cluster 2 Non-Adherent-6w | Cluster 3 Transient-6w | p-Value |
|---|---|---|---|---|
| 1 | 9.43± 1.6 | 8.94 ± 2.4 | 9.4 ± 1.3 | 0.87 |
| 2 | 10.07 ± 5.7 | 9.69 ± 7.3 | 8.93 ± 13.4 | 0.72 |
| 3 | 16 ± 7.9 | 11.27 ± 8.6 | 20.6 ± 19 | 0.35 |
| 4 | 18.75 ± 11.2 | 13.16 ± 9 | 9.78 ± 16.3 | 0.99 |
| 5 | 22.88 ± 9.8 | 10 ± 9 | 28.3 ± 16.8 | 0.00054 |
| 6 | 28.28 ± 12.6 | 5.67 ± 7.3 | 29.16 ± 2.4 | <0.0001 |
| Week | Adherent-6w | Non-Adherent-6w | Transient-6w | p-Value | Adherent-6w | Non-Adherent-6w | Transient-6w | p-Value |
|---|---|---|---|---|---|---|---|---|
| 1 | 81.01 ± 15.5 | 63.44 ± 25.6 | 61.41 ± 37.8 | 0.171 | 84.62 ± 12.78 | 78.91 ± 15.69 | 77.74 ± 18.22 | 0.455 |
| 2 | 82.74 ± 18.4 | 74.12 ± 18 | 83.8 ± 20.9 | 0.256 | 83.1 ± 11.92 | 83.71 ± 15.99 | 91.83 ± 11.51 | 0.5 |
| 3 | 72.72 ± 30.2 | 77.71 ± 14.7 | 82.87 ± 21.3 | 0.838 | 78.03 ± 14.65 | 87.93 ± 14.34 | 91.42 ± 19.63 | 0.161 |
| 4 | 79.38 ± 24.1 | 80.31 ± 14.7 | 95.37 ± 0.9 | 0.181 | 80.76 ± 12.32 | 86.67 ± 14.49 | 93.18 ± 19.94 | 0.508 |
| 5 | 76.9 ± 27.7 | 70.77 ± 24.4 | 73.6 ± 25.3 | 0.492 | 81.77 ± 14.59 | 79.98 ± 14.28 | 83.44 ± 13.24 | 0.959 |
| 6 | 80.7 ± 28 | 77.44 ± 10.4 | 82.81 ± 15.8 | 0.36 | 82.72 ± 16.5 | 86.63 ± 14.44 | 88.22 ± 13.83 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filos, D.; Claes, J.; Cornelissen, V.; Kouidi, E.; Chouvarda, I. Predicting Adherence to Home-Based Cardiac Rehabilitation with Data-Driven Methods. Appl. Sci. 2023, 13, 6120. https://doi.org/10.3390/app13106120
Filos D, Claes J, Cornelissen V, Kouidi E, Chouvarda I. Predicting Adherence to Home-Based Cardiac Rehabilitation with Data-Driven Methods. Applied Sciences. 2023; 13(10):6120. https://doi.org/10.3390/app13106120
Chicago/Turabian StyleFilos, Dimitris, Jomme Claes, Véronique Cornelissen, Evangelia Kouidi, and Ioanna Chouvarda. 2023. "Predicting Adherence to Home-Based Cardiac Rehabilitation with Data-Driven Methods" Applied Sciences 13, no. 10: 6120. https://doi.org/10.3390/app13106120









