Ophthalmic Manifestation in Neurofibromatosis Type 2
Abstract
:1. Introduction
- Bilateral vestibular schwannomas;
- First-degree relative with NF2 and unilateral vestibular schwannomas;
- First-degree relative with NF2 or unilateral vestibular schwannomas and two among meningioma, cataract, glioma, neurofibroma, schwannoma, cerebral calcification;
- Multiple meningiomas and two among unilateral vestibular schwannomas, cataract, glioma, neurofibroma, schwannoma, and cerebral calcification.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Recklinghausen, F. Ueber die Multiplen Fibrome der Haut und Ihre Beziehung zu den Multiplen Neuromen: Festschrift zur Feier des Fünfundzwanzigjährigen Bestehens des Pathologischen Instituts zu Berlin Herrn Rudolf Virchow; A Hirschwald: Berlin, Germany, 1882. [Google Scholar]
- Gardner, W.J.; Frazier, C.H. Bilateral acoustic neurofibromas: A clinical study and field survey of a family of five generations with bilateral deafness in thirty-eight members. Arch. NeurPsych. 1930, 23, 266–302. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. A 2010, 152A, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Trofatter, J.A.; MacCollin, M.M.; Rutter, J.L.; Murrell, J.R.; Duyao, M.P.; Parry, D.M.; Eldridge, R.; Kley, N.; Menon, A.G.; Pulaski, K.; et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 1993, 72, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.M.; Eldridge, R.; Kaiser-Kupfer, M.I.; Bouzas, E.A.; Pikus, A.; Patronas, N. Neurofibromatosis 2 (NF2): Clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am. J. Med. Genet. 1994, 52, 450–461. [Google Scholar] [CrossRef]
- Coy, S.; Rashid, R.; Stemmer-Rachamimov, A.; Santagata, S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020, 139, 643–665. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2022, 24, 1967–1977. [Google Scholar] [CrossRef]
- Halliday, D.; Emmanouil, B.; Pretorius, P.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Genetic Severity Score predicts clinical phenotype in NF2. J. Med. Genet. 2017, 54, 657–664. [Google Scholar] [CrossRef]
- Bosch, M.M.; Boltshauser, E.; Harpes, P.; Landau, K. Ophthalmologic findings and long-term course in patients with neurofibromatosis type 2. Am. J. Ophthalmol. 2006, 141, 1068–1077. [Google Scholar] [CrossRef]
- Painter, S.L.; Sipkova, Z.; Emmanouil, B.; Halliday, D.; Parry, A.; Elston, J.S. Neurofibromatosis Type 2-Related Eye Disease Correlated With Genetic Severity Type. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2019, 39, 44–49. [Google Scholar] [CrossRef]
- Smith, M.J.; Bowers, N.L.; Bulman, M.; Gokhale, C.; Wallace, A.J.; King, A.T.; Lloyd, S.K.; Rutherford, S.A.; Hammerbeck-Ward, C.L.; Freeman, S.R.; et al. Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology 2017, 88, 87–92. [Google Scholar] [CrossRef]
- Evans, D.G.; Huson, S.M.; Donnai, D.; Neary, W.; Blair, V.; Newton, V.; Strachan, T.; Harris, R. A genetic study of type 2 neurofibromatosis in the United Kingdom. II. Guidelines for genetic counselling. J. Med. Genet. 1992, 29, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Baser, M.E.; Friedman, J.M.; Aeschliman, D.; Joe, H.; Wallace, A.J.; Ramsden, R.T.; Evans, D.G.R. Predictors of the risk of mortality in neurofibromatosis 2. Am. J. Hum. Genet. 2002, 71, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Baser, M.E.; Kuramoto, L.; Joe, H.; Friedman, J.; Wallace, A.J.; Gillespie, J.E.; Ramsden, R.T.; Evans, G. Genotype-phenotype correlations for nervous system tumors in neurofibromatosis 2: A population-based study. Am. J. Hum. Genet. 2004, 75, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, E.A.; Freidlin, V.; Parry, D.M.; Eldridge, R.; Kaiser-Kupfer, M.I. Lens opacities in neurofibromatosis 2: Further significant correlations. Br. J. Ophthalmol. 1993, 77, 354–357. [Google Scholar] [CrossRef]
- Sisk, R.A.; Berrocal, A.M.; Schefler, A.C.; Dubovy, S.R.; Bauer, M.S. Epiretinal membranes indicate a severe phenotype of neurofibromatosis type 2. Retina 2010, 30 (Suppl. S4), S51–S58. [Google Scholar] [CrossRef]
- Waisberg, V.; Rodrigues, L.O.C.; Nehemy, M.B.; Bastos-Rodrigues, L.; de Miranda, D.M. Ocular alterations, molecular findings, and three novel pathological mutations in a series of NF2 patients. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht. Graefes. Arch. Klin. Exp. Ophthalmol. 2019, 257, 1453–1458. [Google Scholar] [CrossRef]
- Schefler, A.C.; Dubovy, S.R.; Berrocal, A.M. Optical coherence tomography characteristics of epiretinal membranes in neurofibromatosis 2. Ophthalmic. Surg. Lasers Imaging Off. J. Int. Soc. Imaging Eye 2008, 39, 73–77. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Pepin, S.M.; Maccollin, M.; Choopong, P.; Lessell, S. Ocular pathologic findings of neurofibromatosis type 2. Arch. Ophthalmol. 2007, 125, 389–394. [Google Scholar] [CrossRef]
- Landau, K.; Yaşargil, G.M. Ocular fundus in neurofibromatosis type 2. Br. J. Ophthalmol. 1993, 77, 646–649. [Google Scholar] [CrossRef]
- Emmanouil, B.; Wasik, M.; Charbel Issa, P.; Halliday, D.; Parry, A.; Sharma, S.M. Structural Abnormalities of the Central Retina in Neurofibromatosis Type 2. Ophthalmic. Res. 2022, 65, 77–85. [Google Scholar] [CrossRef]
- Waisberg, V.; Rodrigues, L.O.C.; Nehemy, M.B.; Frasson, M.; de Miranda, D.M. Spectral-Domain Optical Coherence Tomography Findings in Neurofibromatosis Type 2. Investig. Ophthalmol. Vis. Sci. 2016, 57, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Mallone, F.; Lucchino, L.; Giustini, S.; Lambiase, A.; Moramarco, A. An update on choroidal abnormalities and retinal microvascular changes in neurofibromatosis type 1. Orphanet. J. Rare Dis. 2022, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.A.; Thompson, C.R.; MacCollin, M.; Lessell, S. Monocular elevator paresis in neurofibromatosis type 2. Neurology 2001, 56, 1222–1224. [Google Scholar] [CrossRef]
- Harada, T.; Sawamura, Y.; Ohashi, T.; Harada, C.; Shinmei, Y.; Yoshida, K.; Matsuda, H. Severe optic disc edema without hydrocephalus in neurofibromatosis 2. Jpn. J. Ophthalmol. 1998, 42, 381–384. [Google Scholar] [CrossRef]
- Asthagiri, A.R.; Parry, D.M.; Butman, J.A.; Kim, H.J.; Tsilou, E.T.; Zhuang, Z.; Lonser, R.R. Neurofibromatosis type 2. Lancet 2009, 373, 1974–1986. [Google Scholar] [CrossRef]
- Grant, E.A.; Trzupek, K.M.; Reiss, J.; Crow, K.; Messiaen, L.; Weleber, R.G. Combined retinal hamartomas leading to the diagnosis of neurofibromatosis type 2. Ophthalmic. Genet. 2008, 29, 133–138. [Google Scholar] [CrossRef]
- Bouzas, E.A.; Parry, D.M.; Eldridge, R.; Kaiser-Kupfer, M.I. Familial occurrence of combined pigment epithelial and retinal hamartomas associated with neurofibromatosis 2. Retina 1992, 12, 103–107. [Google Scholar] [CrossRef]
- Feucht, M.; Griffiths, B.; Niemüller, I.; Haase, W.; Richard, G.; Mautner, V.F. Neurofibromatosis 2 leads to higher incidence of strabismological and neuro-ophthalmological disorders. Acta Ophthalmol. 2008, 86, 882–886. [Google Scholar] [CrossRef]
- Ragge, N.K.; Baser, M.E.; Klein, J.; Nechiporuk, A.; Sainz, J.; Pulst, S.-M.; Riccardi, V.M. Ocular abnormalities in neurofibromatosis 2. Am. J. Ophthalmol. 1995, 120, 634–641. [Google Scholar] [CrossRef]
- Barrett, V.J.M.; Tan, M.H.; Elston, J.S. Recurrent third nerve palsy as the presenting feature of neurofibromatosis 2. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2012, 32, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Sokwala, A.; Knapp, C.; Gottlob, I. Neurofibromatosis type II presenting as vertical diplopia. Strabismus 2004, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kaye, L.D.; Rothner, A.D.; Beauchamp, G.R.; Meyers, S.M.; Estes, M.L. Ocular findings associated with neurofibromatosis type II. Ophthalmology 1992, 99, 1424–1429. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age | Age at Diagnosis | VA RE | VA LE | NF2-Variant | Main Ocular Findings |
|---|---|---|---|---|---|---|---|
| 1 | F | 55 | 51 | 1.00 | 1.00 | Gardner variant | Lagophthalmos (LE) |
| 2 | F | 58 | 37 | 0.90 | 0.80 | Gardner variant | Epiretinal membrane grade 0, ptosis (LE), and exotropia |
| 3 | F | 68 | 55 | 1.00 | 0.90 | Gardner variant | Ptosis (LE) and exotropia |
| 4 | F | 48 | 46 | 1.00 | 1.00 | Gardner variant | Microstrabismus |
| 5 | F | 63 | 50 | 1.00 | 1.00 | Gardner variant | Lagophthalmos and superficial punctate keratitis (RE) |
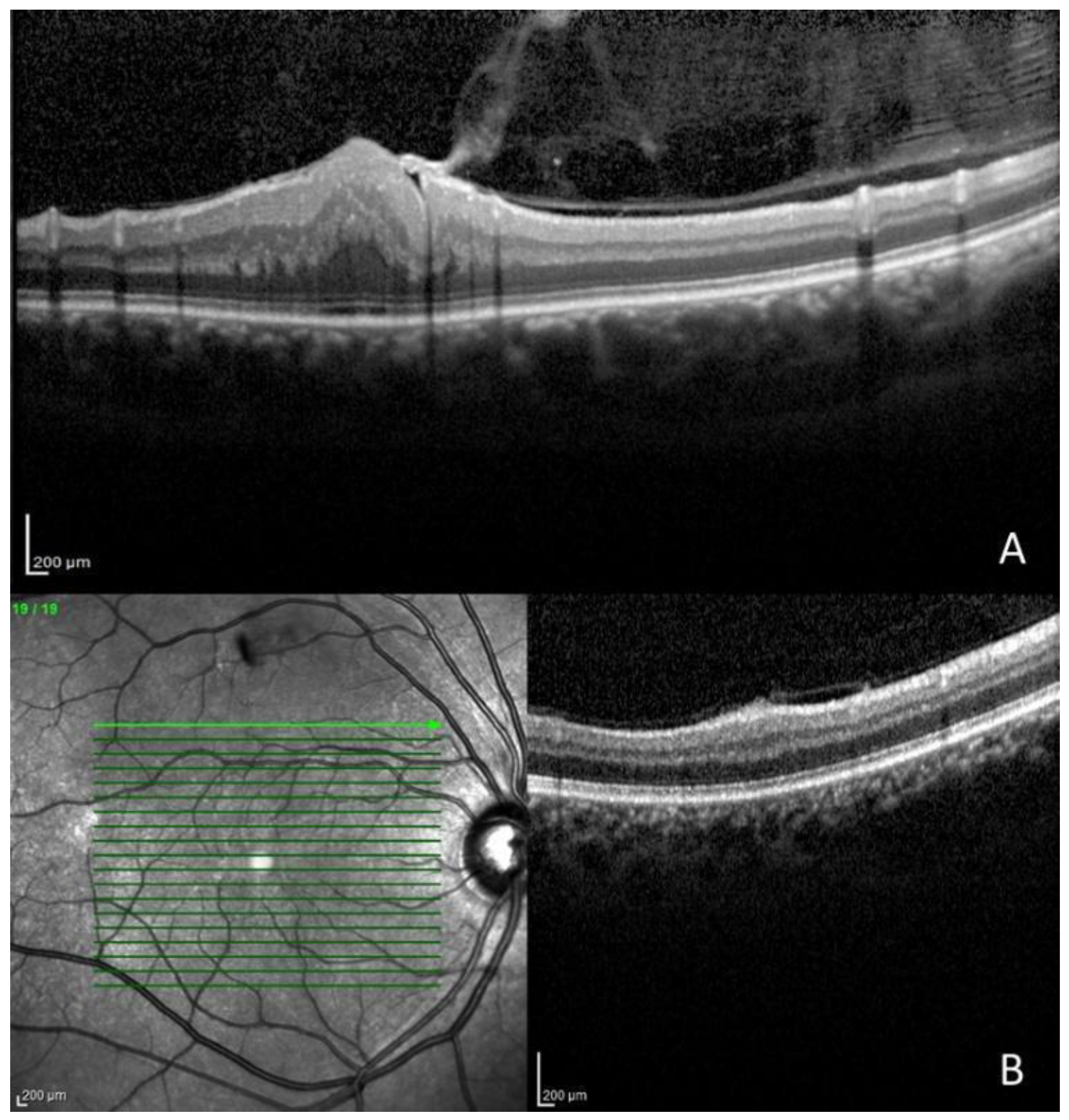
| 6 | F | 37 | 30 | 0.90 | 0.90 | Gardner variant | Cataract (RE), paramacular epiretinal membrane grade 2 and optic nerve edema (RE and LE), and exotropia |
| 7 | F | 62 | 28 | 1.00 | 1.00 | Gardner variant | - |
| 8 | F | 78 | 46 | 0.63 | 0.32 | Gardner variant | Cataract (RE and LE) and retinal hamartoma (LE) |
| 9 | M | 52 | 46 | No light perception | 0.50 | Gardner variant | Corneal pannus and esotropia (RE) and corneal leukoma (LE) |
| 10 | M | 59 | 59 | 1.00 | 1.00 | Gardner variant | Lagophthalmos and superficial punctate keratitis (LE) |
| 11 | M | 30 | 24 | 1.00 | 1.00 | Gardner variant | Epiretinal membrane grade 1 and pseudophakia (RE), corneal leukoma and cataract (LE), and Duane syndrome type 1 |
| 12 | M | 73 | 71 | 1.00 | 0.32 | Gardner variant | Cataract (LE) and microstrabismus |
| 13 | F | 44 | 44 | 1.00 | 1.00 | Gardner variant | Lagophthalmos (RE) and pseudophakia (LE) |
| 14 | M | 16 | 10 | 1.00 | 0.03 | Wishart variant | Cataract (LE), epiretinal membrane grade 2 (RE and LE), and microstrabismus |
| Ocular Findings | Number of Patients | % | Number of Eyes | % |
|---|---|---|---|---|
| Lagophthalmos | 4 | 28.6% | 4 | 14.3% |
| Ptosis of eyelid | 2 | 14.3% | 2 | 7.1% |
| Duane syndrome type 1 | 1 | 7.1% | ||
| Pseudophakia | 2 | 14.3% | 2 | 7.1% |
| Cataract | 5 | 35.7% | 6 | 21.4% |
| Superficial punctate keratitis | 2 | 14.3% | 2 | 7.1% |
| Leukoma | 2 | 14.3% | 2 | 7.1% |
| Corneal pannus | 1 | 7.1% | 1 | 3.6% |
| Ocular Findings | Number of Patients | % | Number of Eyes | % |
|---|---|---|---|---|
| Epiretinal membrane | 4 | 28.6% | 6 | 21.4% |
| Vitreo retinal Tuft | 3 | 21.4% | 3 | 10.7% |
| Retinal hamartoma | 1 | 7.1% | 1 | 3.6% |
| Optic nerve edema | 1 | 7.1% | 2 | 7.1% |
| Patient | LCR Near | LCR Distance | CT Near | CT Distance |
|---|---|---|---|---|
| 2 | −8° | −12° | −18 PD | −25 PD |
| 3 | −6° | −10° | −15 PD | −20 PD |
| 4 | symm | symm | +6 PD | +6 PD |
| 6 | −20° | −15° | −40 DP | −35 DP |
| 9 | +20° | +20° | +40 PD (Krimsky test) | +40 PD (Krimsky test) |
| 12 | symm | symm | +7 PD | +5 PD |
| 14 | symm | symm | +5 PD | +4 PD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armentano, M.; Lucchino, L.; Alisi, L.; Chicca, A.V.; Di Martino, V.; Miraglia, E.; Iannetti, L.; Comberiati, A.M.; Giustini, S.; Lambiase, A.; et al. Ophthalmic Manifestation in Neurofibromatosis Type 2. Appl. Sci. 2023, 13, 6304. https://doi.org/10.3390/app13106304
Armentano M, Lucchino L, Alisi L, Chicca AV, Di Martino V, Miraglia E, Iannetti L, Comberiati AM, Giustini S, Lambiase A, et al. Ophthalmic Manifestation in Neurofibromatosis Type 2. Applied Sciences. 2023; 13(10):6304. https://doi.org/10.3390/app13106304
Chicago/Turabian StyleArmentano, Marta, Luca Lucchino, Ludovico Alisi, Antonio Valerio Chicca, Valerio Di Martino, Emanuele Miraglia, Ludovico Iannetti, Anna Maria Comberiati, Sandra Giustini, Alessandro Lambiase, and et al. 2023. "Ophthalmic Manifestation in Neurofibromatosis Type 2" Applied Sciences 13, no. 10: 6304. https://doi.org/10.3390/app13106304
APA StyleArmentano, M., Lucchino, L., Alisi, L., Chicca, A. V., Di Martino, V., Miraglia, E., Iannetti, L., Comberiati, A. M., Giustini, S., Lambiase, A., & Moramarco, A. (2023). Ophthalmic Manifestation in Neurofibromatosis Type 2. Applied Sciences, 13(10), 6304. https://doi.org/10.3390/app13106304







