Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture
Abstract
:1. Introduction
2. Systematics and Characteristics of Trichoderma Fungi
3. Occurrence and Abundance of Trichoderma Fungi in Different Environments
| Types of Environments | Species of Trichoderma | Reference |
|---|---|---|
| desert soil | T. afroharzianum, T. atrobrunneum, T. longibrachiatum | [44] |
| forest soil | T. afroharzianum, T. asperellum, T. atroviride, T. citrinoviride, T. fertile, T. harzianum, T. longibrachiatum, T. oblongisporum, T. pararogersonii, T. paraviridescens, T. pleurotum, T. piluliferum, T. polysporum, T. rossicum, T. saturnisporum, T. viridescens | [39] |
| steppe soil | T. afroharzianum, T. asperellum, T. atroviride, T. brevicompactum, T. caerulescens, T. gamsii, T. ghanense, T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, T. oblongisporum, T. paraviridescens, T. pleurotum, T. polysporum, T. rossicum, T. saturnisporum, T. semiorbis, T. viridescens | [39] |
| wetland soil | T. asperellum, T. atroviride, T. aureoviride, T. harzianum, T. koningii, T. koningiopsis, T. velutinum, T. virens | [41] |
| soil from garlic crops | T. asperelloides, T. azevedoi, T. hamatum, T. koningiopsis, T. longibrachiatum, T. peberdyi | [46] |
| soil from maize crops | T. asperellum, T. brevicompactum, T. fertile, T. hamatum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. pleuroticola, T. virens | [45] |
| soil from onion crops | T. afroharzianum, T. asperelloides, T. asperellum, T. azevedoi, T. erinaceum, T. lentiforme, T. longibrachiatum, T. peberdyi | [46] |
| soil from rice crops | T. asperellum, T. atroviride, T. brevicompactum, T. capillare, T. erinaceum, T. fertile, T. hamatum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. polysporum, T. saturnisporum, T. spirale, T. velutinum, T. virens | [45] |
| soil from wheat crops | T. asperellum, T. atroviride, T. brevicompactum, T. erinaceum, T. hamatum, T. harzianum, T. koningiopsis, T. virens | [45] |
| root of plant | T. afroharzianum, T. asperelloides, T. asperellum, T. guizhouense, T. harzianum, T. reesei, T. strigosum, T. virens | [42] |
| bark of tree | T. atroviride, T. erinaceum, T. harzianum, T. hebeiensis, T. longibrachiatum, T. parareesei, T. reesei | [52] |
| decaying woods | T. atroviride, T. citrinoviride, T. cremeum, T. gamsii, T. harzianum, T. koningii, T. koningiopsis, T. longibrachiatum, T. longipile, T. paraviridescens, T. trixiae, T. viride, T. viridescens | [40] |
| lignocellulosic compost | T. asperellum, T. citrinoviridae, T. harzianum, T. lixii | [49] |
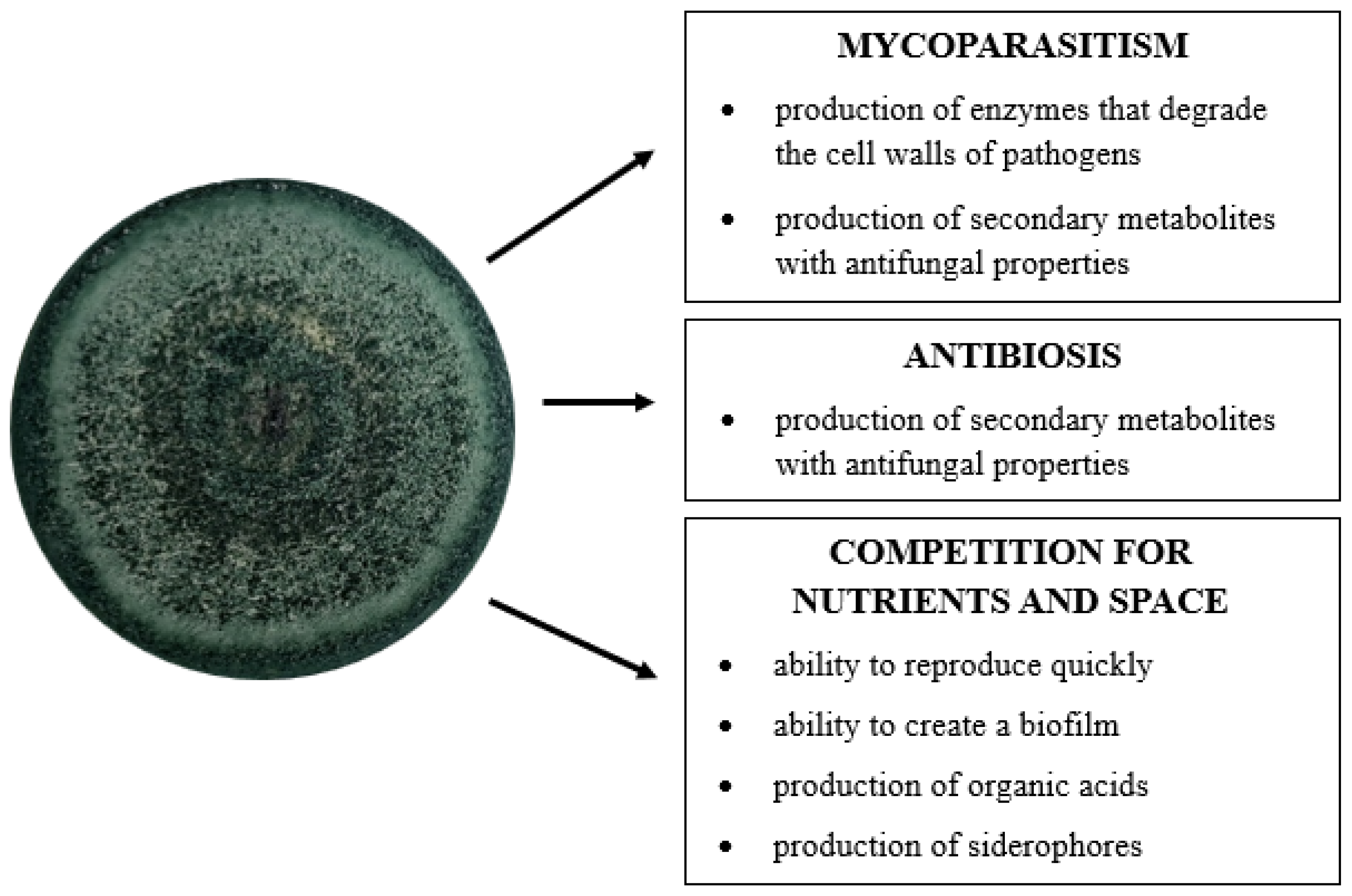
4. Application of Trichoderma Fungi in the Control of Fungal Plant Pathogens
4.1. Antibiosis
4.2. Mycoparasitism
4.3. Competition for Nutrients and Space
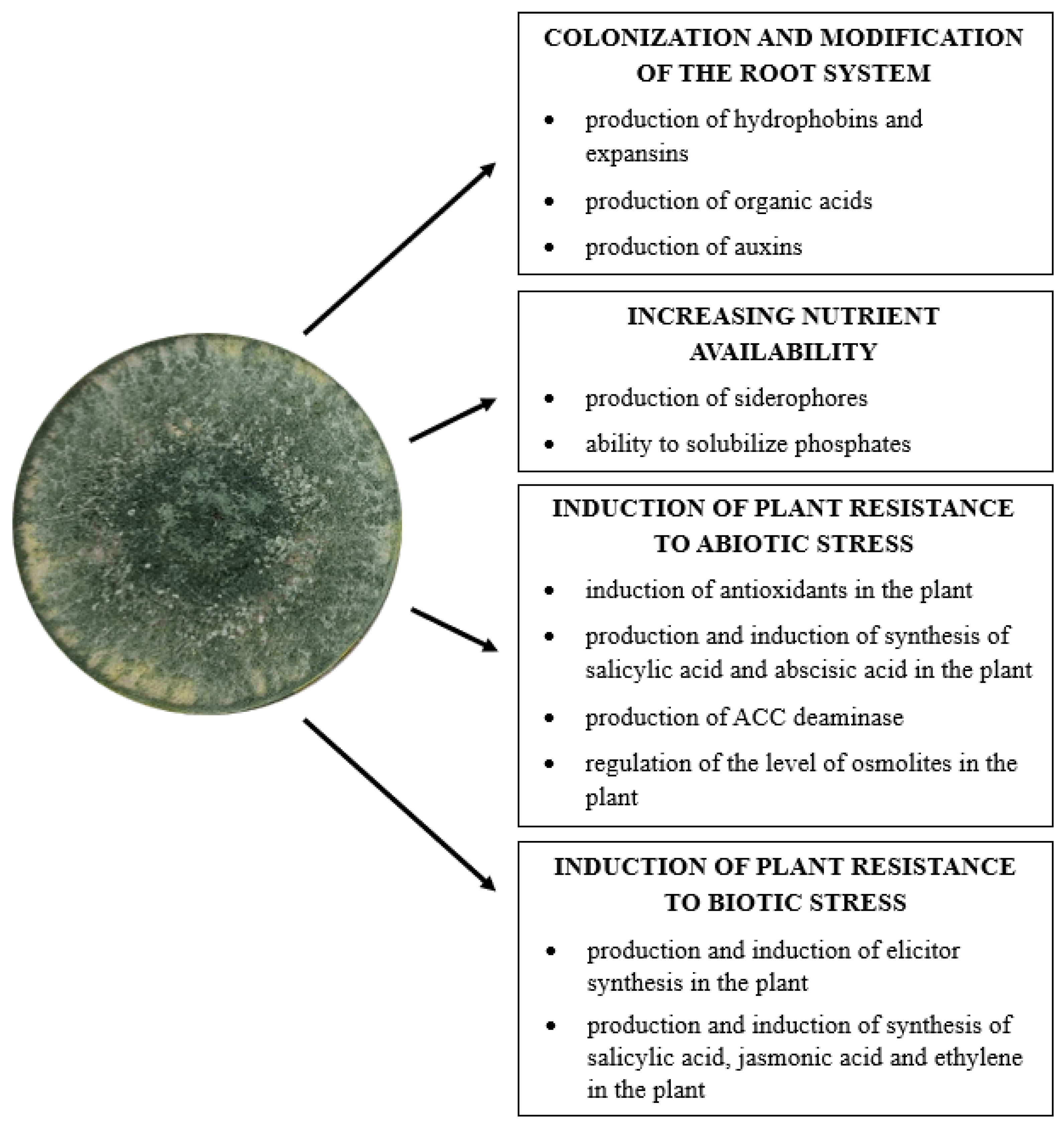
5. Application of Trichoderma Fungi in Promoting Plant Growth
5.1. Colonization and Modification of the Root System of Plants
5.2. Increasing the Availability of Nutrients
5.3. Induction of Plant Resistance to Biotic Stress
| Types of Pathogens | Species of Trichoderma | Crops | Mechanisms of Action | Reference |
|---|---|---|---|---|
| Rhizoctonia solani | Trichoderma harzianum | bean |
| [157] |
| Rhizoctonia solani | Trichoderma atroviride | cucumber |
| [158] |
| Botrytis cinerea | Trichoderma longibrachiatum | cucumber |
| [159] |
| Botrytis cinerea | Trichoderma asperellum | tomato |
| [160] |
| Colletotrichum graminicola | Trichoderma asperellum | sorghum |
| [161] |
| Colletotrichum truncatum | Trichoderma asperellum | chilli |
| [162] |
5.4. Induction of Plant Resistance to Abiotic Stress
5.5. Regulation of Growth, Physiological and Biochemical Processes of Plants
6. Impact of Environmental Conditions on Growth, Spores, and Phytosanitary Properties of Trichoderma Fungi
6.1. Temperature
6.2. PH
6.3. Salinity
7. Fungicides and Plant Growth Stimulants Based on Fungi of the Genus Trichoderma
7.1. Market of Available Fungicides and Plant Growth Stimulants Based on Fungi of the Genus Trichoderma
7.2. Advantages of Using Plant Protection Products Based on Fungi of the Genus Trichoderma
7.3. Problems of Introducing Biological Plant Protection Products Based on Trichoderma sp. to the Commercial Market
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. How to feed the World in 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050, Rome, Italy, 12–13 October 2009. [Google Scholar]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Summary Version; FAO: Rome, Italy, 2018; p. 60. ISBN 9789251309896. [Google Scholar]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol Spectr. 2017, 5, 5.1.14. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT (European Statistical Office). Available online: https://ec.europa.eu/eurostat (accessed on 10 April 2023).
- Hollomon, D.W. Fungicide resistance: Facing the challenge—A review. Plant. Prot. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. [Google Scholar] [CrossRef]
- Ishii, H.; Hollomon, D.W. Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Shefali; Kumar, R.; Sankhla, M.S.; Kumar, R.; Sonone, S.S. Impact of Pesticide Toxicity in Aquatic Environment. Biointerface Res. Appl. Chem. 2020, 11, 10131–10140. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of Fungicides. In Veterinary Toxicology; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 569–580. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commmission to the European Parliament, the European Council, the Council, the European Economic and Social Commmittee and the Commmittee of the Regions, the European Green Deal; European Commission: Brussels, Belgium, 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 10 April 2023).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, EU Biodiversity Strategy for 2030, Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0380 (accessed on 10 April 2023).
- European Commission. Communication from the Commmission to the European Parliament, the European Council, the Council, the European Economic and Social Commmittee and the Commmittee of the Regions, Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0381 (accessed on 10 April 2023).
- European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and91/414/EEC. Official Journal of the European Union. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 10 April 2023).
- European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides, Text with EEA Relevance. Official Journal of the European Union. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 10 April 2023).
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Lu, G.D. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Japanis, F.G.; Vetaryan, S.; Raja, N.K.K.; Mokhtar, M.A.A.; Mohd Fishal, E.M. The Impact of Trichoderma spp. on Agriculture and Their Identification. MAB 2022, 51, 1–15. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Sharma, P.; Nath Patel, A.; Kumar Saini, M.; Deep, S. Field demonstration of Trichoderma harzianum as a plant growth promoter in wheat (Triticum aestivum L.). J. Agric. Sci. 2012, 4, 65–73. [Google Scholar] [CrossRef]
- Mahato, S.; Bhuju, S.; Shrestha, J. Effect of Trichoderma viride as biofertilizer on growth and yield of wheat. Malays. J. Sustain. Agric. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Li, M.; Ma, G.; Lian, H.; SU, X.; Tian, Y.; Huang, W.; Mie, J.; Jiang, X. The effects of Trichoderma on preventing cucumber Fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef]
- Hamden, S.; Shalaby, M.E.; Elkady, E.M.; Elfahar, S.A.; El-Emary, S.A. Potential Impacts of Eminent Fungicide, Certain Bacterial and Fungal Antagonists for Controlling Cercospora Leaf Spot Disease in Sugar Beet. J. Sustain. Agric. Sci. 2023, 49, 1–12. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Nascimento, V.C.; Rodrigues-Santos, K.C.; Carvalho-Alencar, K.L.; Castro, M.B.; Kruger, R.H.; Lopes, F.A.C. Trichoderma: Biological control efficiency and perspectives for the Brazilian Midwest states and Tocantins. Braz. J. Biol. 2022, 82, e260161. [Google Scholar] [CrossRef]
- MycoBank Database. Available online: http://www.MycoBank.org (accessed on 10 April 2023).
- CABI Databases. Available online: https://www.speciesfungorum.org/Names/fundic.asp (accessed on 10 April 2023).
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.—Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Siddiquee, S. Morphology-based characterization of Trichoderma species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions; Springer: Cham, Switzerland, 2017; pp. 41–73. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, A.; Pandey, S.; Singh, J.; Chauhan, S.S.; Srivastava, M. Morphomolecular Identification of Trichoderma sp. and their Mycoparasitic Activity Against Soil Borne Pathogens. Int. J. Bio-Resour. Stress Manag. 2021, 11, 613–627. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Sharma, S.; Kour, D.; Rana, K.L.; Dhiman, A.; Thakur, S.; Thakur, P.; Thakur, S.; Thakur, N.; Sudheer, S.; Yadav, N.; et al. Trichoderma: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 85–120. [Google Scholar] [CrossRef]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Strakowska, J.; Chełkowski, J.; Gąbka-Buszek, A.; Kaczmarek, J. Trichoderma species occurring on wood with decay symptoms in mountain forests in Central Europe: Genetic and enzymatic characterization. J. Appl. Genet. 2016, 57, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Wang, M.H. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiol. Mol. Plant Pathol. 2020, 109, 101458. [Google Scholar] [CrossRef]
- Cummings, N.; Ambrose, A.; Braithwaite, M.; Bissett, J.; Roslan, H.A.; Abdullah, J.; Stewart, A.; Agbayani, F.V.; Steyaert, J.; Hill, R.A. Diversity of root-endophytic Trichoderma from Malaysian Borneo. Mycol. Progr. 2016, 15, 50. [Google Scholar] [CrossRef]
- Kamala, T.; Devi, S.I.; Sharma, K.C.; Kennedy, K. Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed Res. Int. 2015, 2015, 285261. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three New Reports of Trichoderma in Algeria: T. atrobrunneum (South), T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.L.; Chen, J.; Mao, L.J.; Feng, X.X.; Zhang, C.L.; Lin, F.C. Trichoderma biodiversity of agricultural fields in east China reveals a gradient distribution of species. PLoS ONE 2016, 11, e0160613. [Google Scholar] [CrossRef]
- Inglis, P.W.; Mello, S.C.M.; Martins, I.; Silva, J.B.T.; Macedo, K.; Sifuentes, D.N.; Valadares-Inglis, M.C. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PLoS ONE 2020, 15, e0228485. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, A.M.; Al-Oweisi, F.A.; Edwards, S.G.; Al-Nadabi, H.; Al-Fahdi, A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, I.L.; Druzhinina, I.S.; Atanasova, L.; Yarden, O.; Jacobs, K. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 2018, 110, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Modification of post-industrial lignin by fungal strains of the genus Trichoderma isolated from different composting stages. J. Environ. Manag. 2020, 266, 110573. [Google Scholar] [CrossRef] [PubMed]
- McMullin, D.R.; Renaud, J.B.; Barasubiye, T.; Sumarah, M.W.; Miller, J.D. Metabolites of Trichoderma species isolated from damp building materials. Can. J. Microbiol. 2017, 63, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, E.; Marik, T.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018, 125, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradation capacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Sotang, N.; Limbu, A.K.; Joshi, A. Impact of Trichoderma sp. in Agriculture: A Mini-Review. J. Biol. Today’s World 2020, 9, 227. [Google Scholar]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanisms of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Kumar, N.; Khurana, S.M.P. Trichoderma-plant-pathogen interactions for benefit of agriculture and environment. In Biocontrol Agents and Secondary Metabolites; Elsevier: Sawston, UK, 2021; pp. 41–63. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic Fungal Volatile Compounds as Solution for Sustainable Agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Monfil, V.O.; Casas-Flores, S. Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 32; pp. 429–453. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary Metabolism in Trichoderma–Chemistry Meets Genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Bioactive secondary metabolites of Trichoderma spp. for efficient management of phytopathogens. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., Garcia-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 125–143. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.K.; Adusumilli, N. Trichoderma—Its paramount role in agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–83. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Barua, J.; McCormick, S.P.; Izquierdo-Bueno, I.; Cardoza, R.E.; Alexander, N.J.; Hermosa, R.; Collado, I.G.; Monte, E.; Gutierrez, S. Novel aspinolide production by Trichoderma arundinaceum with a potential role in Botrytis cinerea antagonistic activity and plant defense priming. Environ. Microbiol. 2014, 17, 1103–1118. [Google Scholar] [CrossRef]
- Alfaro-Vargas, P.; Bastos-Salas, A.; Munoz-Arrieta, R.; Pereira-Reyes, R.; Redondo-Solano, M.; Fernandez, J.; Mora-Villalobos, A.; Lopez-Gomez, J.P. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. J. Fungi 2022, 8, 1037. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; Islam, M.S.; Mahmud, S.; Polash, S.A.; Sultana, R.; Hasan, M.A.; Wang, C.; Jiang, C. In vitro and in silico approach of fungal growth inhibition by Trichoderma asperellum HbGT6-07 derived volatile organic compounds. Arab. J. Chem. 2021, 14, 103290. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.I.; Sunpapao, A. Volatile Organic Compounds Emitted from Endophytic Fungus Trichoderma asperellum T1 Mediate Antifungal Activity, Defense Response and Promote Plant Growth in Lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef]
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis maydis, the Maize Late Wilt Disease Agent. Biology 2021, 10, 897. [Google Scholar] [CrossRef]
- Srinivasa, N.; Sriram, S.; Singh, C.; Shivashankar, K.S. Secondary Metabolites Approach to study the bio-efficacy of Trichoderma asperellum isolates in India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1105–1123. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Wonglom, P.; Suwannarach, N.; Kumla, J.; Thaochan, N.; Chomnunti, P.; Pitija, K.; Sunpapao, A. Volatile Organic Compound from Trichoderma asperelloides TSU1: Impact on Plant Pathogenic Fungi. J. Fungi 2021, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.; Zhan, X.; Ma, Z.; Yu, X.; Zhang, C. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz. J. Microbiol. 2014, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a new antifungal, 10-member lactone from Trichoderma cremeum with plant growth promotion activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef]
- Jeerapong, C.; Phupong, W.; Bangrak, P.; Intana, W.; Tuchinda, P. Trichoharzianol, a new antifungal from Trichoderma harzianum F031. J. Agric. Food Chem. 2015, 63, 3704–3708. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a Novel Plant Growth Regulator and Systemic Resistance Elicitor from Trichoderma harzianum. Plant. Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 2614. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Contreras-Cornejo, X.A.; Macias-Rodriguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological Control and Plant Growth Promotion by Volatile Organic Compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef]
- Hu, M.; Li, Q.L.; Yang, Y.B.; Liu, K.; Miao, C.P.; Zhao, L.X.; Ding, Z.T. Koninginins R-S from the endophytic fungus Trichoderma koningiopsis. Nat. Prod. Res. 2017, 31, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Sahar, L.; Doustmorad, Z. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zeng, H.; He, L.; Jiang, Q.; Ye, P.; Liu, Y.; Sun, X.; Zhang, M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium Rolfsii of Trichoderma virens T23. Phytopathology 2021, 111, 1720–1725. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spp. Genes Involved in the Biocontrol Activity Against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef]
- Hirpara, D.G.; Gajera, H.P.; Hirpara, H.Z.; Golakiya, B.A. Antipathy of Trichoderma against Sclerotium rolfsii Sacc.: Evaluation of Cell Wall-Degrading Enzymatic Activities and Molecular Diversity Analysis of Antagonists. Microb. Physiol. 2017, 27, 22–28. [Google Scholar] [CrossRef]
- El_Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Baiyee, B.; Ito, S.I.; Sunpapao, A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Baiyee, B.; Pornsuriya, C.; Ito, S.; Sunapapo, A. Trichoderma spirale T76-1 displays biocontrol activity on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Control. 2019, 129, 195–200. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.S.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Khatri, D.K.; Tiwari, D.N.; Bariya, H.S. Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to phytopathological fungi. J. App. Biol. Biotech. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Khare, E.; Kumar, S.; Kim, K. Role of peptaibols and lytic enzymes of Trichoderma cerinum Gur1 in biocontrol of Fusarium oxysporum and chickpea wilt. Environ. Sustain. 2018, 1, 39–47. [Google Scholar] [CrossRef]
- Aoki, Y.; Haga, S.; Suzuki, S. Direct antagonistic activity of chitinase produced by Trichoderma sp. SANA20 as biological control agent for gray mould caused by Botrytis cinerea. Cogent Biol. 2020, 6, 1747903. [Google Scholar] [CrossRef]
- Xue, M.; Wang, R.; Zhang, C.; Wang, W.; Zhang, F.; Chen, D.; Ren, S.; Manman, Z.; Hou, J.; Liu, T. Screening and Identification of Trichoderma Strains isolated from Natural Habitats in China with Potential Agricultural Applications. BioMed Res. Int. 2021, 2021, 7913950. [Google Scholar] [CrossRef]
- Deng, J.J.; Huang, W.Q.; Li, Z.W.; Lu, D.L.; Zhang, Y.; Luo, X.C. Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme Microb. Technol. 2018, 112, 35–42. [Google Scholar] [CrossRef]
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. Enhancement of Lytic Enzymes Activity and Antagonistic Traits of Trichoderma harzianum Using γ-Radiation Induced Mutation. J. Agric. Sci. Technol. 2019, 21, 1035–1048. Available online: http://jast.modares.ac.ir/article-23-15277-en.html (accessed on 10 April 2023).
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. The Role of Cell Wall Degrading Enzymes in Antagonistic Traits of Trichoderma virens Against Rhizoctonia solani. Iran. J. Biotechnol. 2020, 18, e2333. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R.Y. Mycoparasitic Trichoderma Viride as a Biocontrol Agent against Fusarium Oxysporum f. Sp. Adzuki and Pythium Arrhenomanes and as a Growth Promoter of Soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J. Plant Pathol. 2019, 101, 597–608. [Google Scholar] [CrossRef]
- Moreira, V.D.A.; Oliveira, C.E.D.S.; Jalal, A.; Gato, I.M.B.; Oliveira, T.J.S.S.; Boleta, G.H.M.; Giolo, V.M.; Vitória, L.S.; Tamburi, K.V.; Filho, M.C.M.T. Inoculation with Trichoderma harzianum and Azospirillum brasilense increases nutrition and yield of hydroponic lettuce. Arch. Microbiol. 2022, 204, 440. [Google Scholar] [CrossRef]
- Gaderer, R.; Lamdan, N.L.; Frischmann, A.; Sulyok, M.; Krska, R.; Horwitz, B.A.; Seidl-Seiboth, V. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015, 15, 2. [Google Scholar] [CrossRef]
- Sempere Ferre, F.; Santamarina, M.P. Efficacy of Trichoderma harzianum in suppression of Fusarium culmorum. Ann. Microbiol. 2010, 60, 335–340. [Google Scholar] [CrossRef]
- Mokhtar, H.; Dehimat, A. Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. Agri. Biol. J. N. Am. 2013, 4, 145–154. [Google Scholar] [CrossRef]
- Imran, M.; Abo-Elyousr, K.A.M.; Mousa, M.A.; Saad, M.M. Screening and biocontrol evaluation of indigenous native Trichoderma spp. against early blight disease and their field assessment to alleviate natural infection. Egypt. J. Biol. Pest Control 2022, 32, 40. [Google Scholar] [CrossRef]
- Sudantha, I.M.; Suwardji, S. Biodiversity of Trichoderma antagonist saprophytic fungi and its use for biocontrol of Fusarium wilt disease on shallots at Lombok Island, West Nusa Tenggara, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012123. [Google Scholar] [CrossRef]
- Modrzewska, M.; Błaszczyk, L.; Stepien, Ł.; Urbaniak, M.; Waskiewicz, A.; Yoshinari, T.; Bryła, M. Trichoderma versus Fusarium Inhibition of Pathogen Growth and Mycotoxin Biosynthesis. Molecules 2022, 27, 8146. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Kumar, M.; Ashraf, S. Role of Trichoderma spp. as a biocontrol agent of fungal plant pathogens. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 497–506. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.P.; Tiwari, R.; Sharma, N. Effect of different cultural variables on siderophores produced by Trichoderma spp. Int. J. Adv. Res. 2013, 1, 1–6. [Google Scholar]
- Dutta, S.; Kundu, A.; Chakraborty, M.R.; Ojha, S.; Chakrabarti, J.; Chatterjee, N.C. Production and optimization of Fe(III) specific ligand, the siderophore of soil inhabiting and wood rotting fungi as deterrent to plant pathogens. Acta Phytopathol. Entomol. Hung. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmstrom, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator-siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- De Santiago, A.; Quintero, J.M.; Avilés, M.; Delgado, A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant Soil 2011, 342, 97–104. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.A.; Rahman, M.M.; Brailey-Jones, P.; Lee, K.W.; Bennetzen, J.L. Mechanistic assessment of tolerance to iron deficiency mediated by Trichoderma harzianum in soybean roots. J. Appl. Microbiol. 2022, 133, 2760–2778. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plant. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Siderophore production by antagonistic fungi. J. Biopestic. 2017, 10, 105–112. [Google Scholar] [CrossRef]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, V.D.; Szucs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Munsterkotter, M.; Huynh, T.; et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef]
- Singh, R.; Anbazhagan, P.; Viswanath, H.S.; Tomer, A. Trichoderma Species: A Blessing for Crop Production. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Ed.; Springer: Cham, Switzerland, 2020; Volume 61, pp. 127–158. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostas, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turra, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; D’Errico, G.; et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1167. [Google Scholar] [CrossRef]
- Grzywacz, A. Gatunkowa różnorodność biologiczna grzybów terenów leśnych. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2015, 44, 239–253. [Google Scholar]
- Ramirez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a Model to Study Effector-like Molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, X.; Yang, X.; Ran, W.; Shen, Q. Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol. Biochem. 2014, 83, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Samolski, I.; Rincon, A.M.; Pinzon, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Huang, R. Auxin Controlled by Ethylene Steers Root Development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Hussein, K.A. Biological Control and Plant Growth Promotion Properties of Volatile Organic Compound-Producing Antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef]
- Kumar, K.; Manigundan, K.; Amaresan, N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J. Basic Microbiol. 2017, 57, 141–150. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, J.; Yang, X.; Cui, Y.; Chen, L.; Ran, W.; Shen, Q. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Hurley, J.F.; Taylor, J.T.; Puckhaber, L.; Lehner, S.; Druzhinina, I.; Schumacher, R.; Kenerley, C.M. Ferricrocin, the intracellular siderophore of Trichoderma virens, is involved in growth, conidiation, gliotoxin biosynthesis and induction of systemic resistance in maize. Biochem. Biophys. Res. Commun. 2018, 505, 606–611. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of Nutrients in Plant Growth and Development. In Contaminants in Agriculture; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2002; pp. 43–59. [Google Scholar] [CrossRef]
- Fathi, A. Role of nitrogen (N) in plant growth, photosynthesis pigments, and N use efficiency: A review. Agrisost 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.; Aggarwal, A.; Kaushish, S.; Chauhan, S. Interactive effect of AM fungi with Trichoderma viride and Pseudomonas fluorescens on growth and yield of broccoli. Plant Prot. Sci. 2013, 49, 137–145. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, H.B.; Singh, D.K.; Rakshit, A. Trichoderma-mediated enhancement of nutrient uptake and reduction in incidence of Rhizoctonia solani in tomato. Egypt J. Biol. 2014, 16, 29–38. [Google Scholar] [CrossRef]
- Kapri, A.; Tewari, L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmuga Arasu, V.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Promwee, A.; Issarakraisila, M.; Intana, W.; Chamswarng, C.; Yenjit, P. Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agric. Sci. 2014, 6, 8. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3-acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Illescas, M.; Moran-Diez, M.E.; Martinez de Alba, A.E.; Hermosa, R.; Monte, E. Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. Int. J. Mol. Sci. 2022, 23, 6782. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Yang, Y.; Ahammed, G.; Wu, C.; Fan, S.; Zhou, Y. Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U.; Szymczak, K.; Szczech, M. Involvement of metabolic components, volatile compounds, PR proteins and mechanical strengthening in multilayer protection of cucumber plants against Rhizoctonia solani activated by Trichoderma atroviride TRS25. Protoplasma 2018, 255, 359–373. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Wang, R.; Chen, D.; Khan, R.A.A.; Cui, J.; Hou, J.; Liu, T. A novel Trichoderma asperellum strain DQ-1 promotes tomato growth and induces resistance to gray mold caused by Botrytis cinerea. FEMS Microbiol. Lett. 2021, 368, fnab140. [Google Scholar] [CrossRef]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Sharma, P.K.; Saxena, A.K. Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolour L.) from Tarai and hill regions of India. Biol. Control 2020, 152, 104474. [Google Scholar] [CrossRef]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Urban, L.; Lauri, F.; Ben Hdech, D.; Aarrouf, J. Prospects for Increasing the Efficacy of Plant Resistance Inducers Stimulating Salicylic Acid. Agronomy 2022, 12, 3151. [Google Scholar] [CrossRef]
- Zaidi, N.W.; Dar, M.H.; Singh, S.; Singh, U.S. Trichoderma species as abiotic stress relievers in plants. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 38, pp. 515–525. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Tripathi, R.; Keswani, C.; Tewari, R. Trichoderma koningii enhances tolerance against thermal stress by regulating ROS metabolism in tomato (Solanum lycopersicum L.) plants. J. Plant Interact. 2021, 16, 116–126. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Calderón-Urrea, A.; John Tiika, R. Trichoderma longibrachiatum TG1 increases endogenous salicylic acid content and antioxidants activity in wheat seedlings under salinity stress. PeerJ 2022, 10, e12923. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agr. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-Dependent Response of Trichoderma harzianum in Improving Drought Tolerance in Rice Genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Devi, S.S.; Sreenivasulu, Y.; Rao, K.V.B. Protective Role of Trichoderma logibrachiatum (WT2) on Lead Induced Oxidative Stress in Helianthus annus L. Indian J. Exp. Biol. 2017, 55, 235–241. [Google Scholar]
- Vargas, J.T.; Rodriguez-Monroy, M.; Meyer, M.L.; Montes-Belmont, R.; Sepulveda-Jimenez, G. Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ. Exp. Bot. 2017, 136, 85–93. [Google Scholar] [CrossRef]
- Fu, J.; Liu, Z.; Li, Z.; Wang, Y.; Yang, K. Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS ONE 2017, 12, e0179617. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Gan, Y. Seed Treatment with Trichoderma Longibrachiatum T6 Promotes Wheat Seedling Growth under Nacl Stress through Activating the Enzymatic and Nonenzymatic Antioxidant Defense Systems. Int. J. Mol. Sci. 2019, 20, 3729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yang, T.; Ma, K.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef]
- Li, G.; Peng, X.; Wei, L.; Kang, G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 2013, 529, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Hu, Y.; Vandenbussche, F.; Van dDer Straeten, D. Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk. Planta 2017, 245, 467–489. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Mendez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Yusoff, W.M.W. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express 2014, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-Pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; d’Errico, G.; et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma Strains and Metabolites Enhances Soybean Productivity and Nutrient Content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.K.; Meena, M.; Upadhyay, R.S. Effect of different environmental conditions on growth and sporulation of some Trichoderma species. J. Environ. Biol. 2017, 38, 197–203. [Google Scholar] [CrossRef]
- Petrisor, C.; Paica, A.; Constantinescu, F. Influence of abiotic factors on in vitro growth of Trichoderma strains. Proc. Rom. Acad. Ser. B 2016, 18, 11–14. [Google Scholar]
- Sharma, P.K.; Gothalwal, R.; Tiwari, R.K.S. Isolation of cold tolerant antifungal strains of Trichoderma sp. from Northern Hilly Zones of Chhattisgarh. Int. J. Plant Prot. 2013, 6, 236–240. [Google Scholar]
- Kamo, M.; Tojo, M.; Yamazaki, Y.; Itabashi, T.; Takeda, H.; Wakana, D.; Hosoe, T. Isolation of growth inhibitors of the snow rot pathogen Pythium iwayamai from an arctic strain of Trichoderma polysporum. J. Antibiot. 2016, 69, 451–455. [Google Scholar] [CrossRef]
- Zhang, T.; Chaturvedi, V.; Chaturvedi, S. Novel Trichoderma polysporum Strain for the Biocontrol of Pseudogymnoascus destructans, the Fungal Etiologic Agent of Bat White Nose Syndrome. PLoS ONE 2015, 10, e0141316. [Google Scholar] [CrossRef]
- Montoya-Gonzalez, A.H.; Quijano-Vicente, G.; Morales-Maza, A.; Ortiz-Uribe, N.; Hernandez-Martinez, R. Isolation of Trichoderma spp. from Desert Soil, Biocontrol Potential Evaluation and Liquid Culture Production of Conidia Using Agricultural Fertilizers. J. Fertil. Pestic. 2016, 7, 163. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 641. [Google Scholar] [CrossRef]
- Andres, P.A.; Alejandra, P.M.; Benedicto, M.C.; Nahuel, R.I.; Clara, B.M. A Comparative Study of Different Strains of Trichoderma under Different Conditions of Temperature and pH for the Control of Rhizoctonia solani. Agric. Sci. 2022, 13, 702–714. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Raghu, K. Effect of temperature on antagonistic and biocontrol potential of shape Trichoderma sp. on Sclerotium rolfsii. Mycopathologia 1997, 139, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Malathi, P.; Doraisamy, S. Effect of temperature on growth and antagonistic activity of Trichoderma spp. against Macrophomina phaseolina. J. Biol. Control 2003, 17, 153–159. [Google Scholar]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef]
- Ali, H.Z.; Aboud, H.M.; Dheyab, N.S.; Musa, N.K.; Gasam, F.H. Effects of pH and ECW on Growth and Sporulation of Indigenous Trichoderma spp. Int. J. Phytopathol. 2015, 4, 15–20. [Google Scholar] [CrossRef]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol. Mycol. 2014, 3, 127. [Google Scholar] [CrossRef]
- Abeyratne, G.D.D.; Deshappriya, N. The effect of pH on the biological control activities of a Trichoderma sp. against Fusarium sp. isolated from the commercial onion fields in Sri Lanka. Trop. Plant Res. 2018, 5, 121–128. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.J. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Hu, Y.; Peng, Z.; Ren, S.; Xue, M.; Liu, Z.; Hou, J.; Xing, M.; Liu, T. A novel salt-tolerant strain Trichoderma atroviride HN082102.1 isolated from marine habitat alleviates salt stress and diminishes cucumber root rot caused by Fusarium oxysporum. BMC Microbiol. 2022, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) Enhances Wheat Seedlings Tolerance to Salt Stress and Resistance to Fusarium Pseudograminearum. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt stress, Its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotechnol. 2020, 8, 81–91. [Google Scholar]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Khakimov, A.A.; Omonlikov, A.U.; Utaganov, S.B.U. Current status and prospects of the use of biofungicides against plant diseases. GSC Biol. Pharm. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Meher, J.; Rajput, R.S.; Bajpai, R.; Teli, B.; Sarma, B.K. Trichoderma: A Globally Dominant Commercial Biofungicide. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Ed.; Springer: Cham, Switzerland, 2020; Volume 61, pp. 195–208. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Biopesticide Active Ingredients. Available online: https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients (accessed on 10 April 2023).
- Australian Pesticides and Veterinary Medicines Authority. Public Chemical Registration Information System Search. Available online: https://services.apvma.gov.au/web/guest/pubcris (accessed on 10 April 2023).
- Agrimm Technology—Effective, Safe and Environmentally Friendly Biological Crop Protection. Available online: https://agrimm.co.nz/ (accessed on 10 April 2023).
- CABI BioProtection Portal. Available online: https://bioprotectionportal.com/india (accessed on 10 April 2023).
- China MoARA Drafts Active Ingredient List to Facilitate Biopesticide Registration. Available online: https://agrochemical.chemlinked.com/news/china-moara-drafts-active-ingredient-list-facilitate-biopesticide-registration?referer=chemycal (accessed on 10 April 2023).
- Ministerstwo Rolnictwa i Rozwoju Wsi. Wyszukiwarka Środków Ochrony Roślin–Zastosowanie. Available online: https://www.gov.pl/web/rolnictwo/wyszukiwarka-srodkow-ochrony-roslin---zastosowanie (accessed on 10 April 2023).
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 10 April 2023).
- BPDB: Bio-Pesticides DataBase. Available online: https://sitem.herts.ac.uk/aeru/bpdb/atoz.htm (accessed on 10 April 2023).
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, R.A.; Huyop, F.; Rusli, M.H.; Yaacob, S.N.S.; Teo, H.L. An overview of the potential role of microbial metabolites as greener fungicides for future sustainable plant diseases management. J. Crop Prot. 2022, 11, 1–27. [Google Scholar]
- Czaja, K.; Goralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides—Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Puopolo, G.; Giovannini, O.; Angeli, D.; Sicher, C.; Perazzolli, M. Advantages and limitations involved in the use of microbial biofungicides for the control of root and foliar phytopathogens of fruit crops. Italus Hortus 2016, 23, 3–12. [Google Scholar]
- Abbey, L.; Abbey, J.; Leke-Aladekoba, A.; Iheshiulo, E.M.A.; Ijenyo, M. Biopesticides and Biofertilizers: Types, Production, Benefits, and Utilization. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldra, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 479–500. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]

| Species of Trichoderma | Pathogens | Secondary Metabolites | Reference |
|---|---|---|---|
| Trichoderma arundinaceum | Botrytis cinerea | aspinolide C | [64] |
| Trichoderma asperellum | Alternaria alternata Botrytis cinerea Colletotrichum gloeosporioides Fusarium oxysporum | trichotoxin | [65] |
| Trichoderma asperellum | Botrytis cinerea Sclerotinia sclerotiorum | volatile organic compounds: alkanes, alcohols, aldehydes, alkenes, amines, benzenes, ketone | [66] |
| Trichoderma asperellum | Corynespora cassiicola Curvularia aeria | volatile organic compounds: acids, alcohols, aldehydes, alkanes, pyrans, fatty acids | [67] |
| Trichoderma asperellum | Fusarium graminearum | hypomurocin, trichorzin, trichotoxin, trichovirin, 6-pentyl-α-pyrone | [68] |
| Trichoderma asperellum | Magnaporthiopsis maydis | 6-pentyl-α-pyrone | [69] |
| Trichoderma asperellum | Sclerotium rolfsii | butenolides, harzianolides, ferulic acid, cyclonerodiol, massoilactone, viridin, gliovirin, viridiofungin A, viridiol | [70] |
| Trichoderma asperelloides | Corynespora cassiicola Sclerotium rolfsii | volatile organic compounds: alcohols, fatty acids, pyrans, terpenes | [71] |
| Trichoderma atroviride | Phytophthora infestans | volatile organic compounds: alcohols, aldehydes, ketones, esters, alkenes, alkanes, alkynes, organic acids, benzenes, terpenes | [72] |
| Trichoderma brevicompactum | Botrytis cinerea Rhizoctonia solani Colletotrichum lindemuthianum | trichodermin | [73] |
| Trichoderma cremeum | Botrytis cinerea Fusarium oxysporum Rhizoctonia solani | cremenolide | [74] |
| Trichoderma harzianum | Colletotrichum gloeosporioides | trichoharzianol | [75] |
| Trichoderma harzianum | Sclerotinia sclerotiorum | harzianolide | [76] |
| Trichoderma harzianum | Fusarium oxysporum | volatile organic compounds: alcohols, acids, esters, ketones, sesquiterpenes | [77] |
| Trichoderma harzianum | Fusarium oxysporum Macrophomina phaseolina Rhizoctonia solani Sclerotium rolfsii | harzianopyridone, anthraquinones, stigmasterol | [78] |
| Trichoderma harzianum | Rhizoctonia solani Gaeumannomyces graminis | T22azaphilone | [79] |
| Trichoderma harzianum | Botrytis cinerea Rhizoctonia solani | harzianopyridone | [79] |
| Trichoderma harzianum | Rhizoctonia solani Sclerotinia sclerotiorum | harzianic acid | [61] |
| Trichoderma harzianum | Botrytis cinerea Rhizoctonia solani Gaeumannomyces graminis | harzianolide, T39butenolide | [61] |
| Trichoderma harzianum | Sclerotinia sclerotiorum | viridiofungin A | [60] |
| Trichoderma koningii | Botrytis cinerea Fusarium oxysporum Rhizoctonia solani | trichokonins VI, trichokonins VII, trichokonins VIII | [61] |
| Trichoderma koningiopsis | Botrytis cinerea Fusarium oxysporum | volatile organic compounds: alkenes, alkanes, esters | [80] |
| Trichoderma koningiopsis | Fusarium flocciferum Fusarium oxysporum | koninginins R, koninginins S | [81] |
| Trichoderma koningiopsis | Aspergillus fumigatus Botrytis cinera Pyricularia oryzae | trichodermin, volatile organic compounds: alcohols, esters, lactones, acids, furanes, lipids | [82] |
| Trichoderma longibrachiatum | Rhizoctonia solani | gliovirin | [61] |
| Trichoderma longibrachiatum | Fusarium oxysporum Sclerotinia sclerotiorum Sclerotium rolfsii | volatile organic compounds: alcohols, ketones, aldehydes, esters, acids, ethers, terpenes, hydrocarbons | [83] |
| Trichoderma pseudokoningii | Botrytis cinera Fusarium oxysporum | trichokonin VI | [84] |
| Trichoderma viride | Sclerotium rolfsii | viridepyronone | [79] |
| Trichoderma virens | Fusarium oxysporum | volatile organic compounds: alcohols, acids, esters, ketones, sesquiterpenes | [77] |
| Trichoderma virens | Macrophomina phaseolina Sclerotium rolfsii Rhizoctonia bataticola Rhizoctonia solani | gliotoxin | [85] |
| Types of Stress | Species of Trichoderma | Crops | Mechanisms of Action | Reference |
|---|---|---|---|---|
| High temperature | Trichoderma koningii | tomato |
| [168] |
| Low temperature | Trichoderma harzianum | tomato |
| [169] |
| Salinity | Trichoderma longibrachiatum | wheat |
| [170] |
| Salinity | Trichoderma asperellum | cucumber |
| [171] |
| Drought | Trichoderma harzianum | rice |
| [172] |
| Drought | Trichoderma harzianum | tomato |
| [173] |
| Presence of heavy metals | Trichoderma logibrachiatum | sunflower |
| [174] |
| Presence of heavy metals | Trichoderma asperellum | onion |
| [175] |
| Strain of Trichoderma | Name of Product and Manufacturer | Crops | Target Pathogens |
|---|---|---|---|
| Trichoderma afroharzianum strain T-22 | TRIANUM-P (Koppert) | tomato | Fusarium spp. Pythium spp. Rhizoctonia spp. |
| Trichoderma asperellum strain ICC012 | TRIANUM-P (Koppert) | carrot cucumber lettuce parsley strawberry tomato | Fusarium spp. Pythium spp. Rhizoctonia spp. |
| Trichoderma asperellum strain T25 | TUSAL (Certis) | tomato cucumber lettuce strawberry | Fusarium spp. Phythophthora spp. Pythium spp. Rhizoctonia solani Sclerotinia sclerotiorum |
| Trichoderma asperellum strain TV1 | VIRISAN (manufacturer unknown) | tomato | Fusarium spp. Pythium spp. Rhizoctonia spp. |
| Trichoderma asperellum strain T34 | ASPERELLO T34 (Biocontrol) | cabbage cucumber lettuce parsley pepper strawberry tomato | Fusarium spp. Phythium spp. |
| Trichoderma atrobrunneum strain ITEM 908 | TRIANUM-P (Koppert) | tomato | Fusarium spp. Pythium spp. Rhizoctonia spp. |
| Trichoderma atroviride strain I-1237 | ESQUIVE WP (Agrauxine) | grapevine | wood decay diseases |
| Trichoderma atroviride strain SC1 | VINTEC (Bi-PA NV/SA) | grapevine tomato | Diplodia seriata Eutypa lata Phaeoacremonium aleophilum Phaeomoniella chlamydospora |
| Trichoderma gamsii strain ICC080 | REMEDIER (Isagro) | tomato | Fusarium spp. Phoma spp. Phytophthora spp. Pythium spp. Rhizoctonia spp. Verticillium spp. |
| Trichoderma gamsii strain ICC080 Trichoderma asperellum strain ICC012 | REMEDIER (Isagro) | cabbage cereals grapevine pepper strawberry tomato | Armillaria spp. Fusarium spp. Phoma spp. Phytophthora spp. Pythium spp. Rosellinia spp. Rhizoctonia spp. Verticillium spp. Sclerotinia spp. Sclerotium rolfsii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Appl. Sci. 2023, 13, 6434. https://doi.org/10.3390/app13116434
Kubiak A, Wolna-Maruwka A, Pilarska AA, Niewiadomska A, Piotrowska-Cyplik A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Applied Sciences. 2023; 13(11):6434. https://doi.org/10.3390/app13116434
Chicago/Turabian StyleKubiak, Adrianna, Agnieszka Wolna-Maruwka, Agnieszka A. Pilarska, Alicja Niewiadomska, and Agnieszka Piotrowska-Cyplik. 2023. "Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture" Applied Sciences 13, no. 11: 6434. https://doi.org/10.3390/app13116434
APA StyleKubiak, A., Wolna-Maruwka, A., Pilarska, A. A., Niewiadomska, A., & Piotrowska-Cyplik, A. (2023). Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Applied Sciences, 13(11), 6434. https://doi.org/10.3390/app13116434








